Abstract
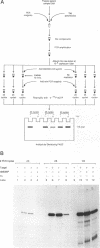
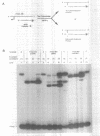
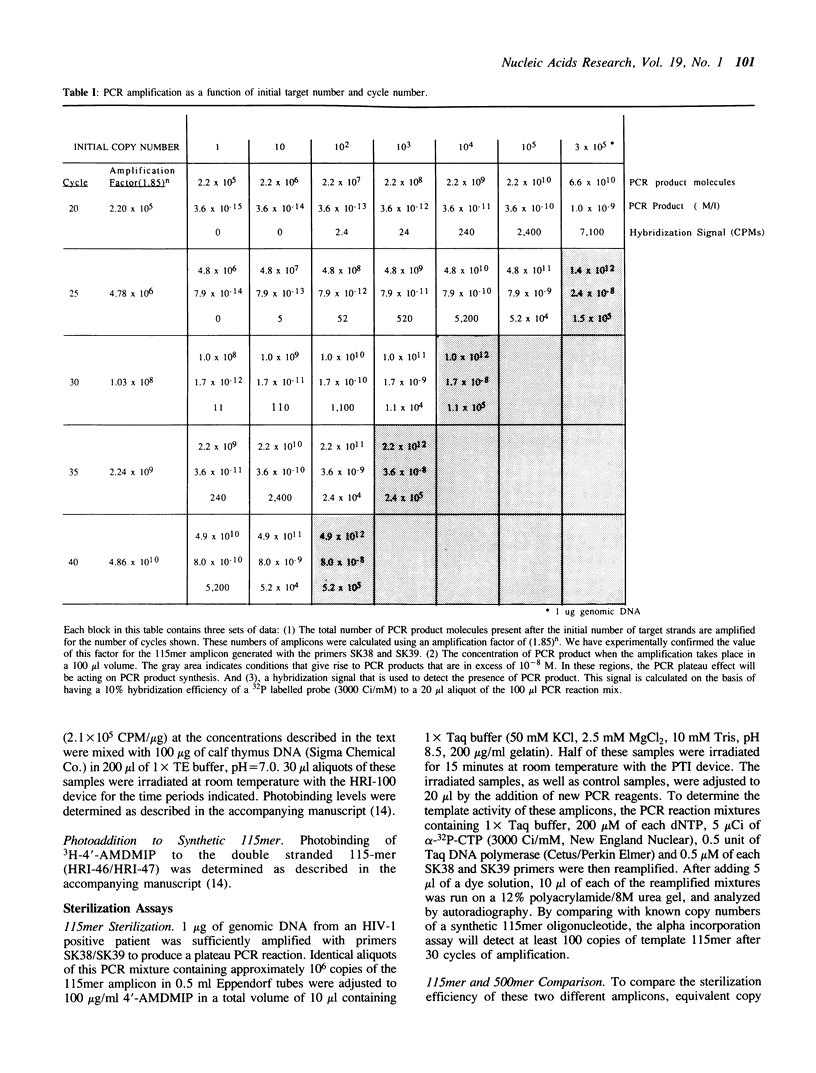
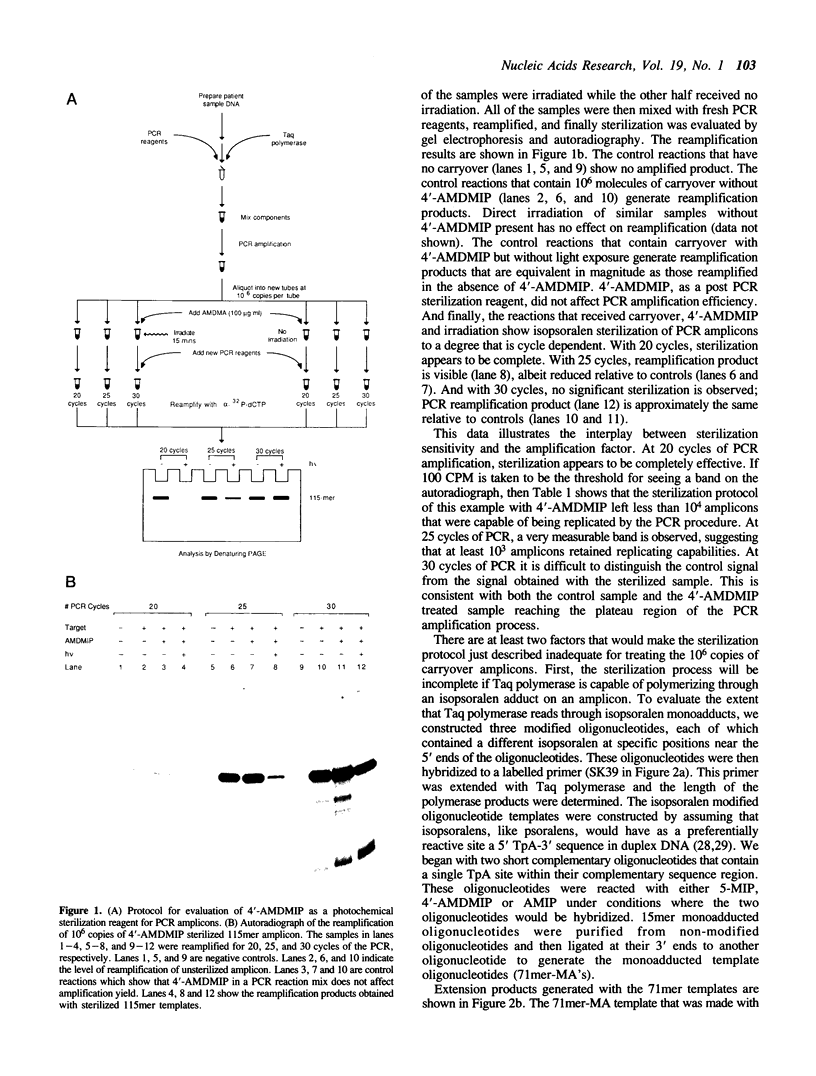
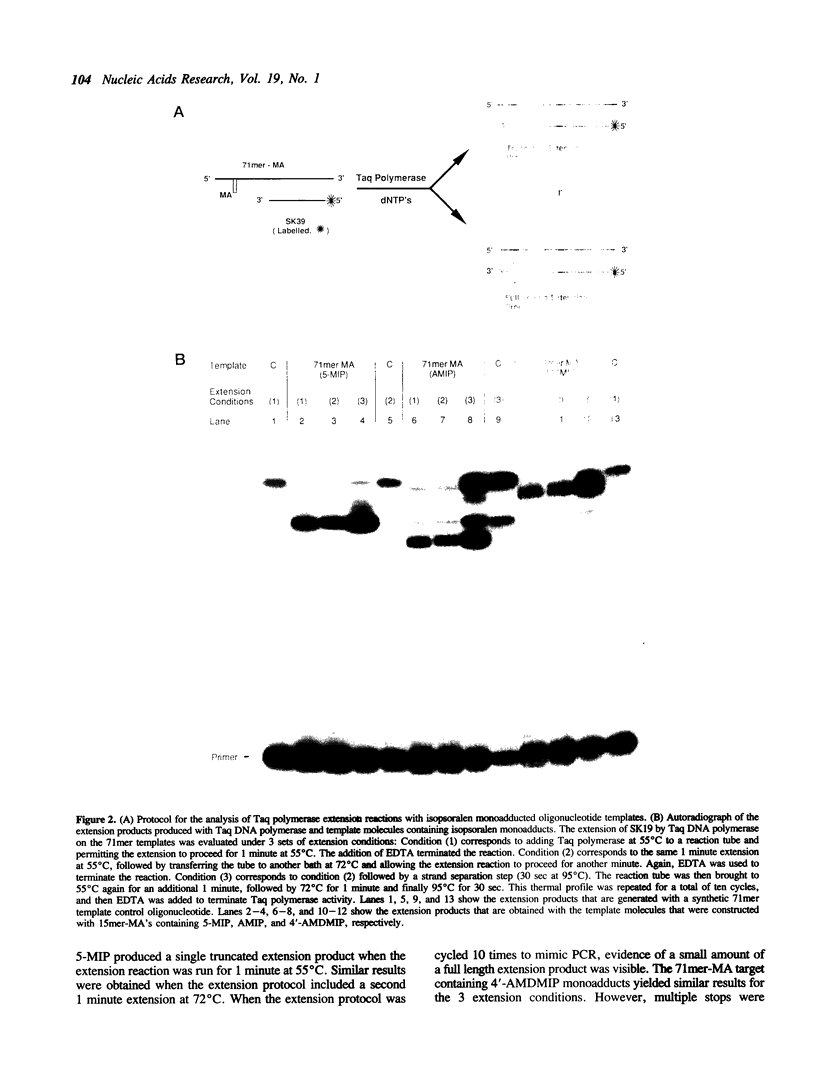
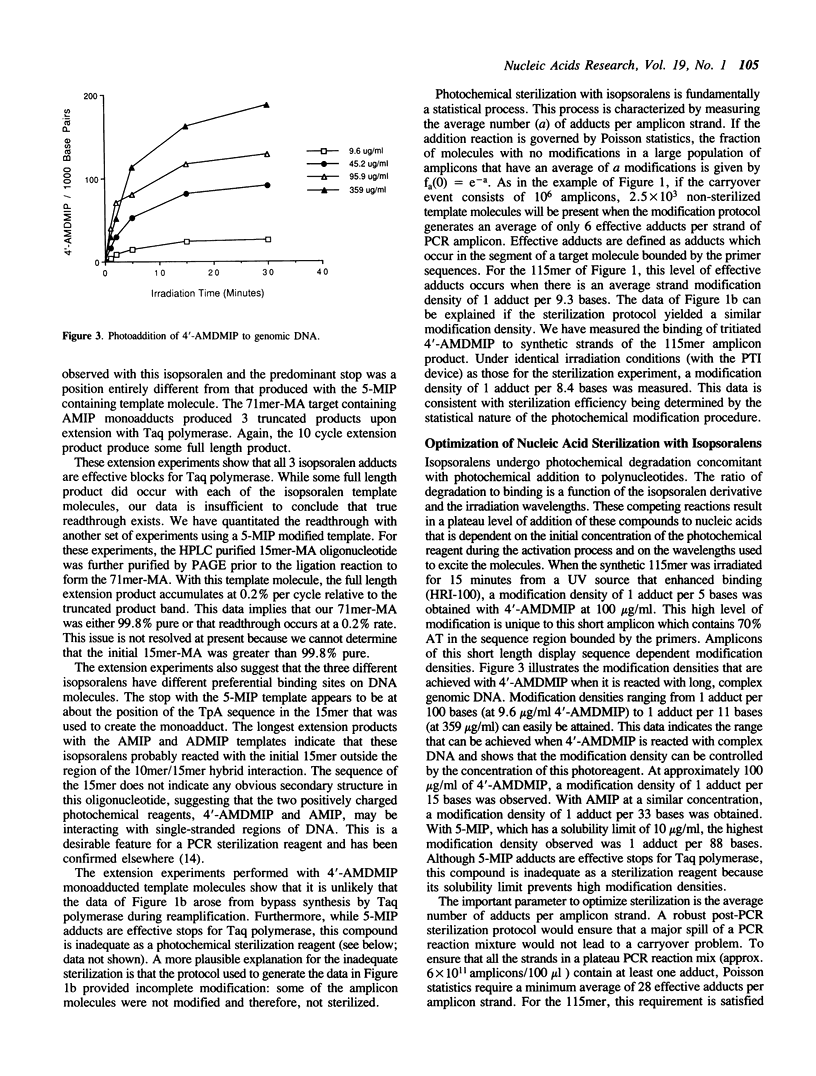
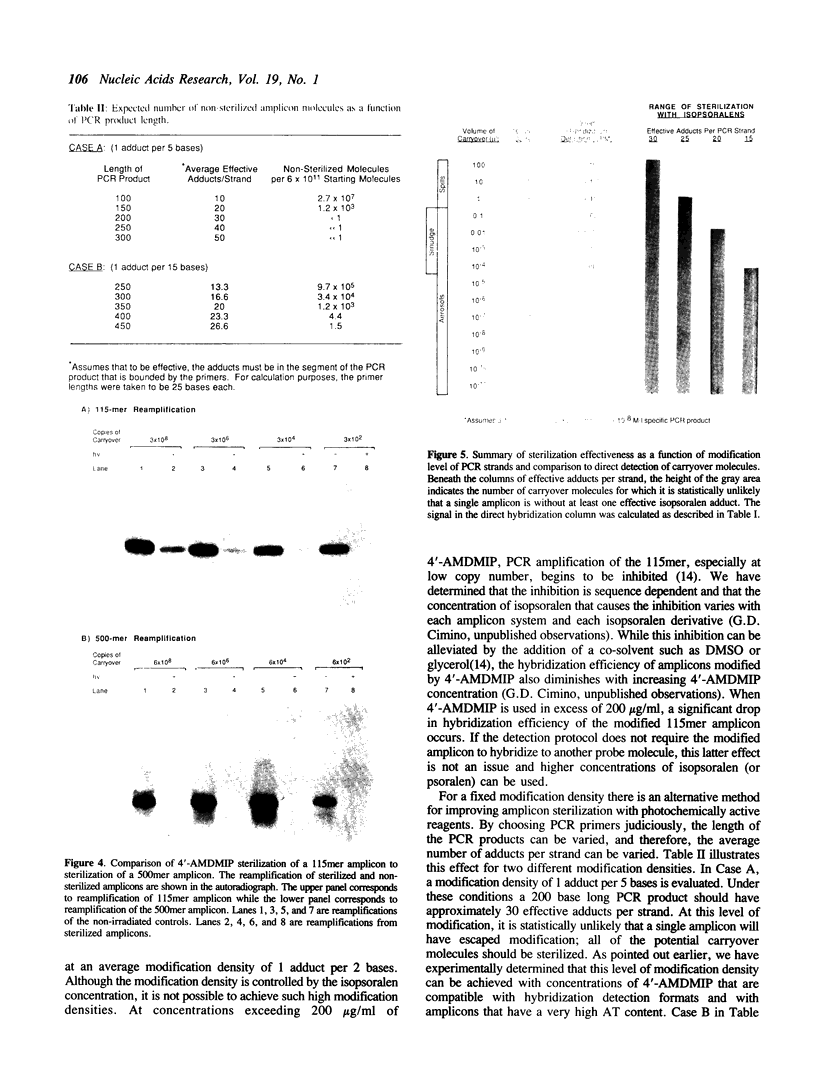
We describe a photochemical procedure for the sterilization of polynucleotides that are created by the Polymerase Chain Reaction (PCR). The procedure is based upon the blockage of Taq DNA polymerase when it encounters a photochemically modified base in a polynucleotide strand. We have discovered reagents that can be added to a PCR reaction mixture prior to amplification and tolerate the thermal cycles of PCR, are photoactivated after amplification, and damage a PCR strand in a manner that, should the damaged strand be carried over into a new reaction vessel, prevent it from functioning as a template for the PCR. These reagents, which are isopsoralen derivatives that form cyclobutane adducts with pyrimidine bases, are shown to stop Taq polymerase under conditions appropriate for the PCR process. We show that effective sterilization of PCR products requires the use of these reagents at concentrations that are tailored to the length and sequence of the PCR product and the level of amplification of the PCR protocol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Metchette K., Isaacs S. T., Zhu Y. S. More false-positive problems. Nature. 1990 Jun 28;345(6278):773–774. doi: 10.1038/345773b0. [DOI] [PubMed] [Google Scholar]
- Clewley J. P. The polymerase chain reaction, a review of the practical limitations for human immunodeficiency virus diagnosis. J Virol Methods. 1989 Aug;25(2):179–187. doi: 10.1016/0166-0934(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Vedaldi D., Caffieri S., Guiotto A., Rodighiero P., Baccichetti F., Carlassare F., Bordin F. New monofunctional reagents for DNA as possible agents for the photochemotherapy of psoriasis: derivatives of 4,5'-dimethylangelicin. J Med Chem. 1981 Feb;24(2):178–184. doi: 10.1021/jm00134a010. [DOI] [PubMed] [Google Scholar]
- Dattagupta N., Rae P. M., Huguenel E. D., Carlson E., Lyga A., Shapiro J. A., Albarella J. P. Rapid identification of microorganisms by nucleic acid hybridization after labeling the test sample. Anal Biochem. 1989 Feb 15;177(1):85–89. doi: 10.1016/0003-2697(89)90018-3. [DOI] [PubMed] [Google Scholar]
- Esposito F., Brankamp R. G., Sinden R. R. DNA sequence specificity of 4,5',8-trimethylpsoralen cross-linking. Effect of neighboring bases on cross-linking the 5'-TA dinucleotide. J Biol Chem. 1988 Aug 15;263(23):11466–11472. [PubMed] [Google Scholar]
- Gamper H. B., Cimino G. D., Isaacs S. T., Ferguson M., Hearst J. E. Reverse Southern hybridization. Nucleic Acids Res. 1986 Dec 22;14(24):9943–9954. doi: 10.1093/nar/14.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper H., Piette J., Hearst J. E. Efficient formation of a crosslinkable HMT monoadduct at the Kpn I recognition site. Photochem Photobiol. 1984 Jul;40(1):29–34. doi: 10.1111/j.1751-1097.1984.tb04549.x. [DOI] [PubMed] [Google Scholar]
- Kitchin P. A., Szotyori Z., Fromholc C., Almond N. Avoidance of PCR false positives [corrected]. Nature. 1990 Mar 15;344(6263):201–201. doi: 10.1038/344201a0. [DOI] [PubMed] [Google Scholar]
- Kwoh D. Y., Davis G. R., Whitfield K. M., Chappelle H. L., DiMichele L. J., Gingeras T. R. Transcription-based amplification system and detection of amplified human immunodeficiency virus type 1 with a bead-based sandwich hybridization format. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1173–1177. doi: 10.1073/pnas.86.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. False-positive results and the polymerase chain reaction. Lancet. 1988 Sep 17;2(8612):679–679. doi: 10.1016/s0140-6736(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Peckler S., Graves B., Kanne D., Rapoport H., Hearst J. E., Kim S. H. Structure of a psoralen-thymine monoadduct formed in photoreaction with DNA. J Mol Biol. 1982 Nov 25;162(1):157–172. doi: 10.1016/0022-2836(82)90166-8. [DOI] [PubMed] [Google Scholar]
- Piette J. G., Hearst J. E. Termination sites of the in vitro nick-translation reaction on DNA that had photoreacted with psoralen. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5540–5544. doi: 10.1073/pnas.80.18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Hearst J. Sites of termination of in vitro DNA synthesis on psoralen phototreated single-stranded templates. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Sep;48(3):381–388. doi: 10.1080/09553008514551381. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Shedding light on PCR contamination. Nature. 1990 Jan 4;343(6253):27–27. doi: 10.1038/343027a0. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Ou C. Y., Jones W. K. Polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1154–1157. doi: 10.1093/infdis/158.6.1154. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Hearst J. E. The effects of covalent additions of a psoralen on transcription by E. coli RNA polymerase. Nucleic Acids Res. 1987 Sep 11;15(17):6843–6854. doi: 10.1093/nar/15.17.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Van Houten B., Hearst J. E. Interaction of Escherichia coli RNA polymerase with DNA in an elongation complex arrested at a specific psoralen crosslink site. J Mol Biol. 1988 Jan 20;199(2):277–293. doi: 10.1016/0022-2836(88)90314-2. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Griffith J., Gamper H., Hearst J. E. Evidence for structural deformation of the DNA helix by a psoralen diadduct but not by a monoadduct. Nucleic Acids Res. 1988 Sep 26;16(18):8945–8952. doi: 10.1093/nar/16.18.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Hearst J. E. Thermostability of double-stranded deoxyribonucleic acids: effects of covalent additions of a psoralen. Biochemistry. 1986 Oct 7;25(20):5895–5902. doi: 10.1021/bi00368a009. [DOI] [PubMed] [Google Scholar]