Abstract
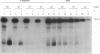
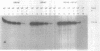
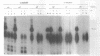
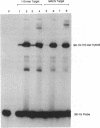
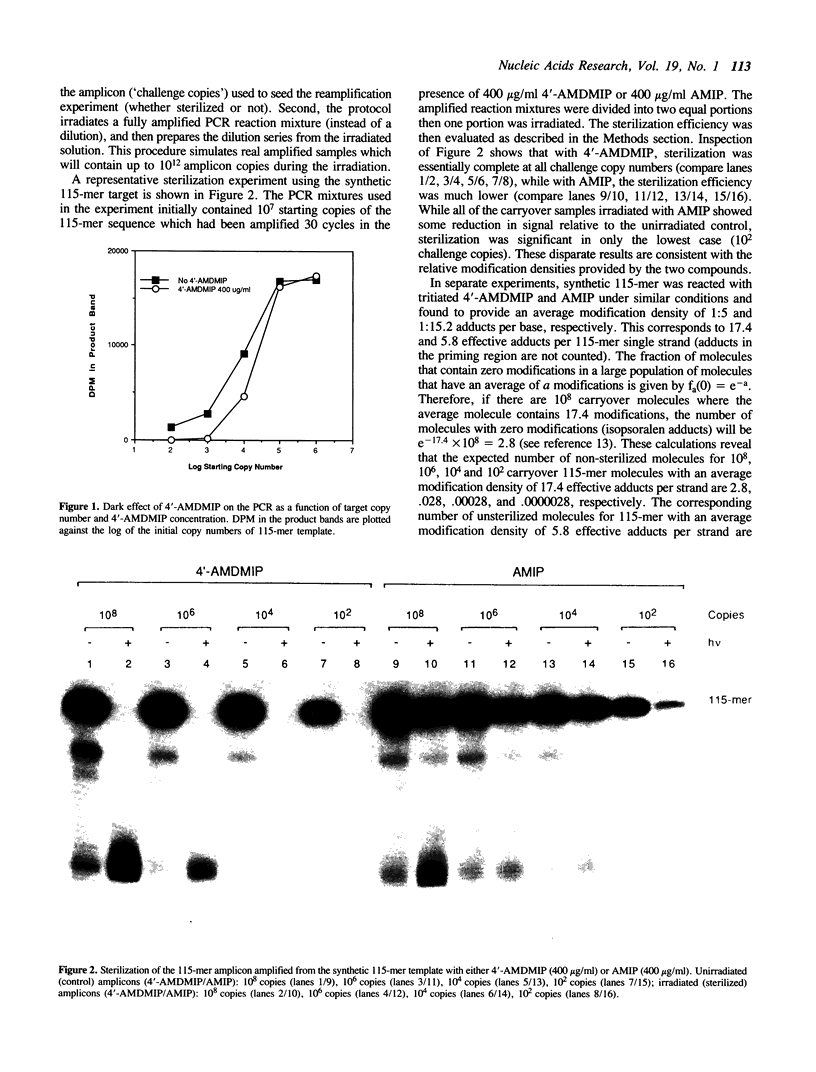
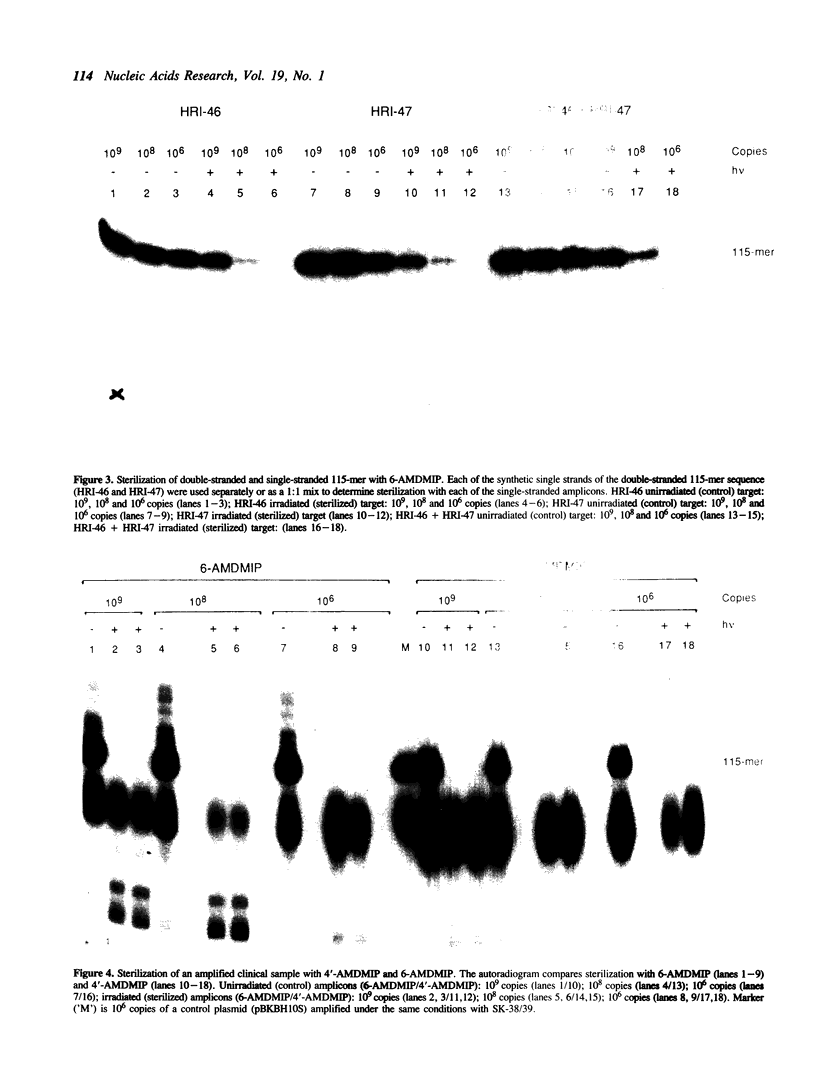
We have developed an effective post-PCR sterilization process and have applied the procedure to a diagnostic assay for HIV-1. The method, which is based on isopsoralen photochemistry, satisfies both the inactivation and hybridization requirements of a practical sterilization process. The key feature of the technique is the use of isopsoralen compounds which form covalent photochemical adducts with DNA. These covalent adducts prevent subsequent extension of previously amplified sequences (amplicons) by Taq polymerase. Isopsoralens have minimal inhibitory effect on the PCR, are activated by long wavelength ultraviolet light, provide sufficient numbers of covalent adducts to impart effective sterilization, modify the amplified sequence such that it remains single-stranded, and have little effect on subsequent hybridization. The sterilization procedure can be applied to a closed system and is suitable for use with commonly used detection formats. The photochemical sterilization protocol we have devised is an effective and pragmatic method for eliminating the amplicon carryover problem associated with the PCR. While the work described here is limited to HIV-1, proper use of the technique will relieve the concern associated with carryover for a wide variety of amplicons, especially in the clinical setting.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Metchette K., Isaacs S. T., Zhu Y. S. More false-positive problems. Nature. 1990 Jun 28;345(6278):773–774. doi: 10.1038/345773b0. [DOI] [PubMed] [Google Scholar]
- Clewley J. P. The polymerase chain reaction, a review of the practical limitations for human immunodeficiency virus diagnosis. J Virol Methods. 1989 Aug;25(2):179–187. doi: 10.1016/0166-0934(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Vedaldi D., Caffieri S., Guiotto A., Rodighiero P., Baccichetti F., Carlassare F., Bordin F. New monofunctional reagents for DNA as possible agents for the photochemotherapy of psoriasis: derivatives of 4,5'-dimethylangelicin. J Med Chem. 1981 Feb;24(2):178–184. doi: 10.1021/jm00134a010. [DOI] [PubMed] [Google Scholar]
- Feorino P. M., Jaffe H. W., Palmer E., Peterman T. A., Francis D. P., Kalyanaraman V. S., Weinstein R. A., Stoneburner R. L., Alexander W. J., Raevsky C. Transfusion-associated acquired immunodeficiency syndrome. Evidence for persistent infection in blood donors. N Engl J Med. 1985 May 16;312(20):1293–1296. doi: 10.1056/NEJM198505163122005. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Cimino G. D., Isaacs S. T., Ferguson M., Hearst J. E. Reverse Southern hybridization. Nucleic Acids Res. 1986 Dec 22;14(24):9943–9954. doi: 10.1093/nar/14.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Kwok S. Y., Hopsicker J. S., Sannerud K. J., Sninsky J. J., Edson J. R., Balfour H. H., Jr Absence of HIV-1 infection in antibody-negative sexual partners of HIV-1 infected hemophiliacs. Transfusion. 1989 Mar-Apr;29(3):265–267. doi: 10.1046/j.1537-2995.1989.29389162735.x. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. False-positive results and the polymerase chain reaction. Lancet. 1988 Sep 17;2(8612):679–679. doi: 10.1016/s0140-6736(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Walsh P. S., Levenson C. H., Erlich H. A. Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6230–6234. doi: 10.1073/pnas.86.16.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Shedding light on PCR contamination. Nature. 1990 Jan 4;343(6253):27–27. doi: 10.1038/343027a0. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Ou C. Y., Jones W. K. Polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1154–1157. doi: 10.1093/infdis/158.6.1154. [DOI] [PubMed] [Google Scholar]
- Shibata D., Brynes R. K., Nathwani B., Kwok S., Sninsky J., Arnheim N. Human immunodeficiency viral DNA is readily found in lymph node biopsies from seropositive individuals. Analysis of fixed tissue using the polymerase chain reaction. Am J Pathol. 1989 Oct;135(4):697–702. [PMC free article] [PubMed] [Google Scholar]
- Ward J. W., Grindon A. J., Feorino P. M., Schable C., Parvin M., Allen J. R. Laboratory and epidemiologic evaluation of an enzyme immunoassay for antibodies to HTLV-III. JAMA. 1986 Jul 18;256(3):357–361. [PubMed] [Google Scholar]