Abstract
Objective
In addition to inducing a self-limited myopathy, statin use is associated with an immune-mediated necrotizing (IMNM) myopathy with autoantibodies recognizing ~ 200 and ~100 kDa autoantigens. Identifying these molecules will clarify disease mechanism and facilitate diagnosis.
Methods
The effect of statin treatment on autoantigen expression was addressed by immunoprecipitation using patient sera. The identity of the ~100 kDa autoantigen was confirmed by immunoprecipitating in vitro-translated HMGCR protein. HMGCR expression in muscle was analyzed by immunofluorescence. A cohort of myopathy patients was screened for anti-HMGCR autoantibodies by ELISA and genotyped for the rs4149056 C allele, a predictor of self-limited statin myopathy.
Results
Statin exposure induced expression of the ~200/~100 kDa autoantigens in cultured cells. HMGCR was identified as the ~100 kDa autoantigen. Competition experiments demonstrated no distinct autoantibodies recognizing the ~200 kDa protein. In muscle biopsies from anti-HMGCR positive patients, HMGCR expression was up-regulated in cells expressing NCAM, a marker of muscle regeneration. Anti-HMGCR autoantibodies were found in 45 of 750 patients presenting to the Johns Hopkins Myositis Center (6%). Among patients age 50 or older, 92% were exposed to statins. The prevalence of the rs4149056 C allele was not increased in anti-HMGCR subjects.
Conclusion
Statins up-regulate expression of HMGCR, the major target of autoantibodies in statin-associated IMNM. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response even after statins are discontinued. These studies demonstrate a mechanistic link between an environmental trigger and the development of sustained autoimmunity. Detection of anti-HMGCR autoantibodies may facilitate diagnosis and direct therapy.
Statins lower cholesterol levels by specifically inhibiting HMGCR, a key enzyme in the cholesterol biosynthetic pathway. These drugs significantly reduce cardiovascular endpoints and are among the most commonly prescribed medications, with almost 30 million people in the U.S. prescribed a statin in 2005 (1). Musculoskeletal symptoms are a well-known complication of statin use and range from myalgias and cramps, which occur in 9–20% of statin users (2–4) to life-threatening rhabdomyolysis, a rare event occurring at a rate of ~0.4 per 10,000 patient years (5).
In most cases, statin-induced myopathic events are self-limited, with complete recovery in the weeks or months after the statin is discontinued (6). However, two recent studies have described 33 patients who developed an autoimmune myopathy following statin exposure, which did not abate after discontinuing statins (7, 8). Taking a different approach, we recently identified sixteen patients with a necrotizing myopathy who had a novel autoantibody recognizing ~200 and ~100 kDa proteins (9). Given that these patients had proximal muscle weakness, elevated CK levels, MHC I expression on non-necrotic muscle fibers, autoantibodies, and responded to immunosuppression, we concluded that these patients had an IMNM. Of the 12 anti-200/100 positive patients over the age of 50, 10 had prior statin exposure (83%). This was significantly higher than the frequency of statin exposure in age-matched controls with polymyositis (PM), dermatomyositis (DM), and inclusion body myositis (IBM).
We reasoned that statin-associated autoimmune myopathy with anti-200/100 autoantibodies provides a model system for defining the mechanistic relationship between drug exposure and developing a specific autoimmune response. Identification of the autoantigen(s) targeted by the immune response is a critical first step.
In this study, we demonstrated that statin exposure upregulates expression of the ~200 and ~100 kDa autoantigens. Given that statin exposure also increases expression of the ~97 kDa HMGCR (10, 11), we investigated whether this enzyme might be the ~100 kDa autoantigen. Serum from anti-200/100 positive patients specifically recognized the intracellular catalytic domain of HMGCR. Based on this finding, we developed an ELISA assay to rapidly screen patient sera for anti-HMGCR autoantibodies. We found that 45 of 750 patients with symptoms of muscle disease were anti-HMGCR positive (6%). Anti-HMGCR positive patients had an IMNM phenotype and greater than 90% of those over age 50 had prior statin exposure. Interestingly, HMGCR expression was upregulated in regenerating muscle fibers from anti-HMGCR positive patients. These studies suggest that statins trigger an autoimmune response against HMGCR by upregulating expression of this autoantigen. Even after discontinuing statins, the presence of high levels of HMGCR in regenerating muscle fibers may perpetuate the immune response.
MATERIALS and METHODS
Patients and genotyping
Between May 2002 and April 2010, 750 patients with suspected myopathy as defined by proximal muscle weakness, elevated CK levels, myopathic EMG findings, muscle edema on magnetic resonance imaging (MRI), and/or myopathic features on muscle biopsy were enrolled in a longitudinal study. Patients were defined as having DM if they had probable or definite disease by Bohan and Peter criteria (12, 13) and IBM if they met Grigg’s criteria for possible disease (14). Serum was available from each subject and DNA samples were available from 260 subjects. Serum samples from 20 healthy controls without prior statin exposure were also obtained. All subjects were enrolled in protocols approved by the Johns Hopkins Institutional Review Board. Genotyping of the rs4149046 C allele was performed using the appropriate verified TaqMan Drug Metabolism Genotyping Assay (Applied Biosystems) on all 17 anti-HMGCR positive patients for whom DNA samples were available (see Table 1 for detailed clinical information).
Table 1.
| serum # |
Statin= | HMG ELISA¥ |
Age* | Sex | Race | Highest CK |
Prox. Weak. |
EMG | Muscle Bx | rs4149056 |
|---|---|---|---|---|---|---|---|---|---|---|
| 07039 | No | 0.969 | 49 | M | B | 20000 | Yes | not done | N+I | |
| 07056 | No | 0.749 | <40 | F | W | 6323 | Yes | IM | N | |
| 07090 | No | 1.304 | 57 | M | W | 10310 | Yes | IM | N | |
| 08024 | No | 1.123 | 32 | F | B | 7225 | Yes | NIM | N | |
| 08038 | No | 0.347 | 36 | M | W | 4071 | Yes | NIM | N+I | |
| 08050 | No | 1.260 | 21 | F | B | 17967 | Yes | IM | N | |
| 08109 | No | 0.849 | 68 | M | W | 3275 | Yes | IM | N | |
| 08126 | No | 1.378 | 40 | F | A | 13506 | Yes | IM | N+I | TT |
| 08196 | No | 1.524 | 42 | F | B | 35000 | Yes | IM | not done | TT |
| 08209 | No | 0.947 | 45 | F | W | 8500 | Yes | IM | N | CT |
| 09029 | No | 0.765 | 4 | F | B | 16000 | Yes | NIM | N | TT |
| 09063 | No | 0.982 | 20 | F | W | 2000 | Yes | n/a | N+I | |
| 09088 | No | 0.629 | 47 | F | B | 22733 | Yes | IM | n/a | |
| 10029 | No | 0.924 | 16 | F | A | 16000 | No | normal | N | |
| 09184 | No | 1.759 | 38 | M | W | 17976 | Yes | IM | N+I | |
| 03004 | Yes | 1.259 | 58 | M | B | 24714 | Yes | IM | N | TT |
| 05017 | Yes | 1.228 | 54 | M | W | 13600 | Yes | not done | N | |
| 06031 | Yes | 0.718 | 71 | M | W | 3052 | Yes | IM | N | |
| 06061 | Yes | 0.547 | 54 | F | W | 15000 | Yes | IM | N | |
| 07054 | Yes | 0.355 | 43 | M | W | 11427 | Yes | IM | N+I | |
| 07094 | Yes | 0.948 | 48 | F | W | 200 | Yes | n/a | not done | |
| 07109 | Yes | 0.942 | 44 | F | A | 11200 | Yes | NIM | N | |
| 08001 | Yes | 0.242 | 75 | F | W | 8602 | Yes | IM | N | |
| 08040 | Yes | 1.159 | 57 | F | B | 3993 | Yes | IM | N | |
| 08076 | Yes | 1.259 | 70 | M | W | 8800 | Yes | IM | N | CC |
| 08089 | Yes | 0.768 | 47 | F | B | 17000 | Yes | IM | N | TT |
| 08100 | Yes | 0.378 | 57 | F | W | 8000 | Yes | IM | N | |
| 08130 | Yes | 0.751 | 62 | M | W | 16500 | Yes | IM | N | |
| 08144 | Yes | 0.287 | 65 | M | W | 254 | No | not done | not done | |
| 08145 | Yes | 1.411 | 54 | F | W | 17000 | Yes | IM | N | |
| 08148 | Yes | 0.608 | 65 | M | W | 5800 | Yes | n/a | N+I | |
| 08176 | Yes | 1.142 | 66 | F | W | 6000 | Yes | IM | N | TT |
| 08227 | Yes | 0.966 | 49 | M | W | 7000 | Yes | NIM | N | |
| 09125 | Yes | 0.517 | 56 | F | W | 1876 | Yes | NIM | N+I | |
| 09135 | Yes | 0.746 | 58 | F | W | 3000 | Yes | NIM | N | TT |
| 09153 | Yes | 1.273 | 65 | M | W | 4197 | Yes | IM | N | TT |
| 09170 | Yes | 0.556 | 80 | F | W | 1200 | Yes | NIM | N | |
| 09172 | Yes | 1.495 | 53 | F | W | 6840 | Yes | IM | N | TT |
| 09176 | Yes | 1.000 | 70 | M | W | 8800 | Yes | IM | N | TT |
| 09188 | Yes | 1.996 | 65 | M | W | 4065 | Yes | IM | N | CT |
| 09190 | Yes | 1.486 | 49 | F | W | 3700 | Yes | n/a | N | TT |
| 10009 | Yes | 0.736 | 66 | M | W | 5000 | Yes | NIM | N | TT |
| 10044 | Yes | 1.810 | 62 | M | W | 11600 | Yes | IM | N+RV | TT |
| 10062 | Yes | 0.292 | 60 | F | W | 4000 | Yes | n/a | n/a | TT |
| 10072 | Yes | 1.169 | 54 | F | W | 4000 | Yes | IM | N |
N=necrotizing myopathy; N+ I = necrosis plus inflammation; N+RV= necrosis + rimmed vacuoles; IM=irritable myopathy; NIM=non-irritable myopathy = statin use prior to serum testing;
age at onset; ¥cut-off for a positive ELISA test was 0.215 (the mean value + 3 standard deviations of 20 control patients not on statins)
Immunoprecipitations from radiolabeled cell lysates
HeLa cells were cultured in the absence or presence of 10 µM mevinolin (Sigma) for 22 hours before radiolabeling with 100 µCi/mL 35S-methionine/cysteine (MP Biomedicals), lysing and immunoprecipitating with patient sera (9). Immunoprecipitates were reduced, boiled, electrophoresed on 10% SDS-polyacrylamide gels and visualized by fluorography.
Immunoprecipitations using 35S-methionine-labeled in vitro transcription/translated (IVTT) proteins
DNA encoding full-length human HMGCR was purchased (Invitrogen). DNA encoding the N-terminal piece (aa 1–377) was generated by mutating R377 to a stop codon. DNA encoding the C-terminus of HMGCR (aa 340–888) was made by PCR using the full-length DNA as a template. Constructs were sequence verified and used in IVTT reactions (Promega), generating 35S-methionine-labeled proteins. Immunoprecipitations using these products were performed {LCR Arth & Rheum 2001} with detection of the immunoprecipitates as described above.
Competition experiments
1 µL of each patient serum was preincubated (30 mins, 4°C in 50 µL) with the catalytic domain of human HMGCR (aa 426–888) expressed as a fusion protein with glutathione-S-transferase (hereafter referred to as “C-terminal HMGCR”; Sigma). Preincubated antibodies were subsequently used for immunoprecipitations with full-length IVTT HMGCR or radiolabeled lysates made from mevinolin-treated HeLa cells.
Anti-HMGCR ELISA
96 well ELISA plates were coated overnight at 4° C with 100 ng of C-terminal HMGCR (Sigma) diluted in PBS. Replicate wells were incubated with PBS alone. After washing the plates, human serum samples, diluted 1:400 in PBS with 0.05% Tween (PBS-T),were added to wells (1 hour, 37° C). After washing, HRP-labeled goat anti-human antibody (Pierce; 1:10,000) was added to each well (30 minutes, 37° C). Color development was performed using SureBlue™ peroxidase reagent (KPL) and absorbances at 450 nm were determined. For each sample, the background absorbance from the PBS-coated wells was subtracted from that of the corresponding C-terminal-HMGCR-coated well. Test sample absorbances were expressed as a proportion of an arbitrary positive control subject (#9176), a reference serum included in every ELISA.
Immunohistochemistry
The collection and use of human biopsy specimens was approved by the Johns Hopkins Institutional Review Board. Muscle biopsy specimens from six anti-HMGCR patients and three normal controls were studied. All biopsies were obtained from patients who had been off statins for >3 months. Paraffin section staining was performed as described (9). Antibody incubations comprised mixtures of rabbit anti-HMGCR (Millipore) and mouse anti-neuronal cell adhesion molecule (NCAM; Santa Cruz Biotechnology) primary antibodies followed by donkey anti-rabbit IgG Alexa Fluor 594 (to detect HMGCR) and donkey anti-mouse IgG Alexa Fluor 488 (to detect NCAM) secondary antibodies (Invitrogen).
RESULTS
200 and 100 kDa autoantigen expression is up-regulated by statins
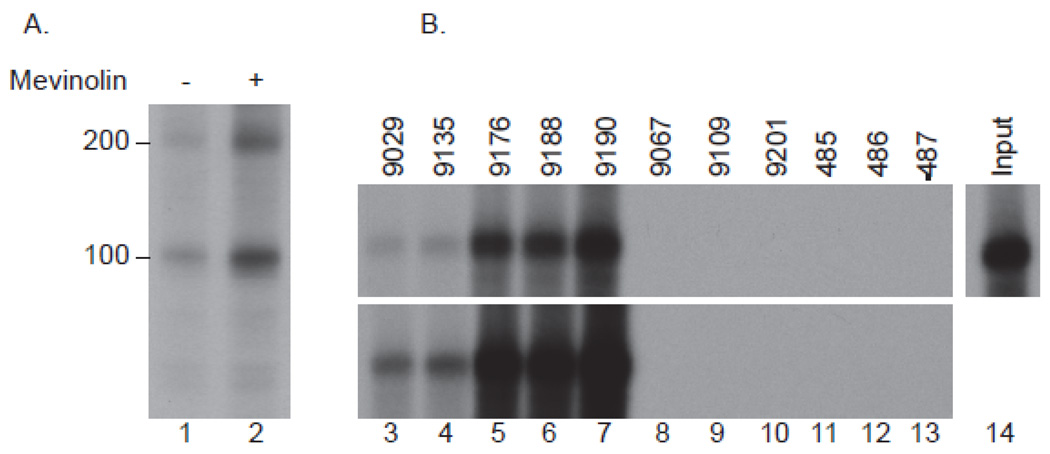
We previously demonstrated that sera from a group of patients with immune-mediated necrotizing myopathy immunoprecipitate ~200 and ~100 kDa proteins from radiolabeled HeLa extracts (9). Given the strong-association of statin use with development of these anti-200/100 autoantibodies, we labeled HeLa cells with 35S-methionine/cysteine after pre-treatment for 24 hours with either 10 µM mevinolin or vehicle alone (DMSO). To validate the protein equivalence of these lysates, immunoprecipitations were performed using antibodies against Mi-2 or PM/ScL. As anticipated, equal amounts of Mi-2 and the 5 protein components of the PM/ScL complex were detected in each lysate type (not shown). In contrast, three-fold increased levels of both 200 and 100 kDa proteins were immunoprecipitated from the mevinolin-treated cells, demonstrating that levels of these autoantigens are up-regulated by statins (figure 1A).
Figure 1.
Expression of the 200 and 100 kDa autoantigens is up-regulated by statins (A) and the 100 kDa autoantigen is HMGCR (B). A: Radiolabeled lysates generated from HeLa cells treated in the absence (lane 1) or presence (lane 2) of 10 µM mevinolin for 24 hours were immunoprecipitated with patient serum 9190 as described in the Methods section. B: 35S-methionine-labeled full-length IVTT HMGCR was immunoprecipitated using sera from anti-100/200 kDa positive patients (lanes 3–7; representative of 16 anti-200/100 positive serum samples tested), anti-100/200 kDa negative DM patients (lanes 8–10) or healthy controls (lanes 11–13). The input IVTT product is shown in lane 14. The data shown in A and B are representative of similar results obtained in at least 3 separate experiments.
The 100 kDa autoantigen is HMGCR
Brown and Goldstein originally demonstrated that the expression of HMGCR is up-regulated by statin treatment (10). Morikawa and colleagues extended these findings to muscle cells. They used DNA microarray analysis to demonstrate that statins induce the expression of 19 genes in a human skeletal muscle cell line, most of which are related to cholesterol biosynthesis (11). Among these, we selected HMGCR as a candidate for the ~100 kDa autoantigen because of its ~97 kDa molecular weight.
35S-methionine-labeled HMGCR was generated by IVTT and used in an immunoprecipitation assay with serum from 16 patients with anti-200/100 autoantibodies, as well as negative controls consisting of 3 DM patients and 3 normal individuals without statin exposure. We found that serum from anti-200/100 positive patients immunoprecipitated HMGCR, while serum from the control groups did not (figure 1B).
Human anti-HMGCR autoantibodies recognize the C-terminus of HMGCR
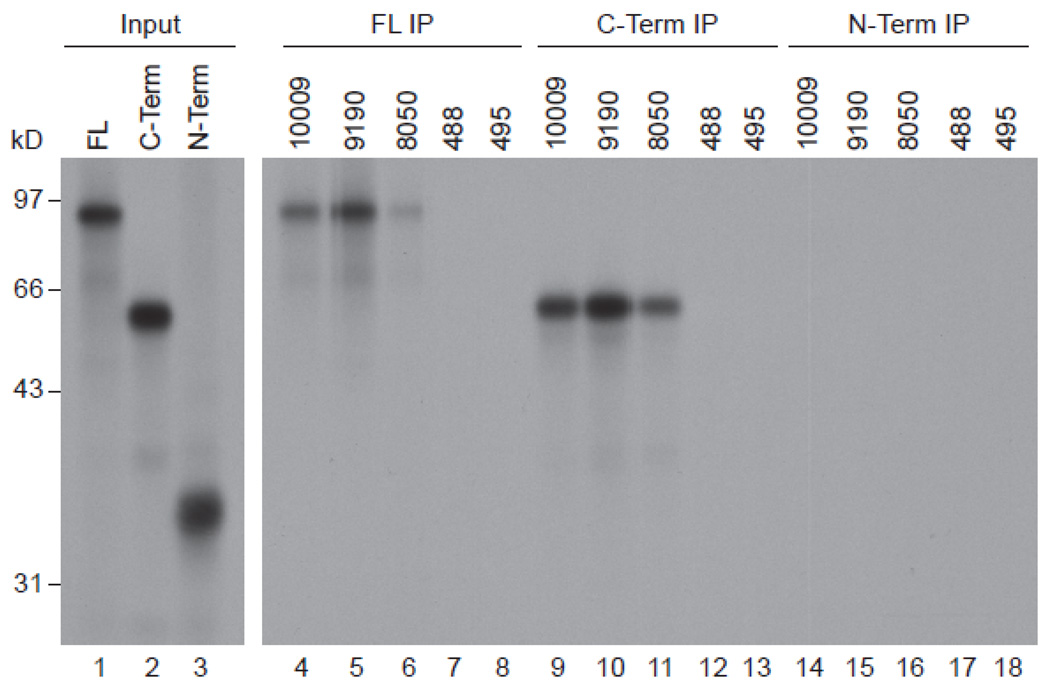
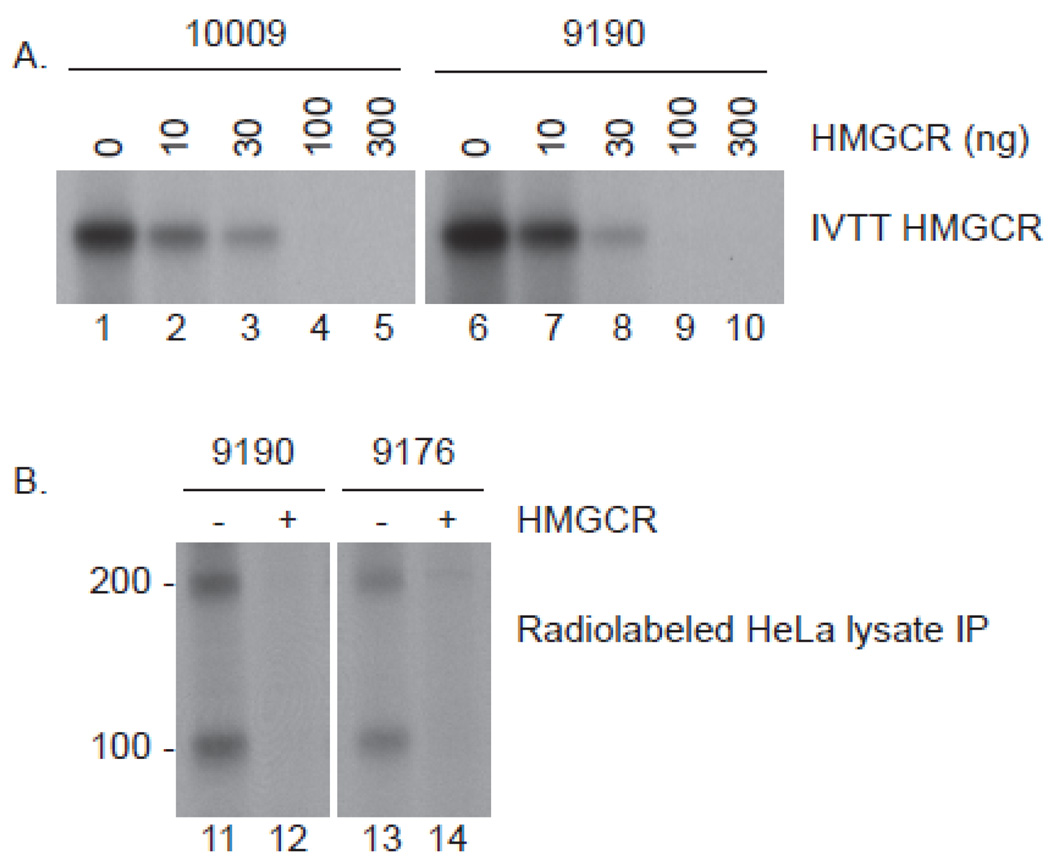
HMGCR is a membrane protein with a small extracellular domain, seven membrane spanning domains, and an intracellular catalytic domain. To define the region(s) of the protein recognized by anti-HMGCR patient sera, we synthesized 35S-labeled full-length HMGCR protein, an N-terminal fragment including the extracellular and membrane spanning domains (aa 1–377), and a C-terminal fragment including the intracellular portion of the molecule (aa 340–888). Serum from anti-HMGCR positive subjects consistently immunoprecipitated full-length HMGCR and the C-terminal fragment, but not the N-terminal fragment (figure 2). When anti-HMGCR positive sera were pre-incubated with increasing concentrations of unlabeled C-terminal HMGCR prior to immunoprecipitation of 35S-methionine-labeled full-length HMGCR protein, immunoprecipitation was abolished (figure 3A). Taken together, these findings demonstrate that the anti-HMGCR autoantibodies recognize the intracellular C-terminal portion of this enzyme.
Figure 2.
Human anti-HMGCR antibodies immunoprecipitate full-length HMGCR and a piece corresponding to the C-terminus (aa 340–888). Immunoprecipitations were performed using 3 different 35S-methionine labeled HMGCR products: full-length (lanes 4–8), C-terminus (lanes 9–13) and N-terminus (lanes 14–18). Sera 10009, 9190 and 8050 are all from anti-100/200 kDa patients, while 488 and 495 are normal controls. Lanes 1–3 show the input IVTT products, which, in each case, was 0.4 times the amount used for the immunoprecipitation. Data shown are representative of those obtained in 2–8 separate experiments.
Figure 3.
Competition immunoprecipitations confirm that human anti-HMGCR antibodies detect the C-terminus (panel A) and that the 200 kDa protein is not recognized by a unique autoantibody (panel B). A: Sera 10,009 and 9190 were preincubated with the indicated amounts of unlabeled C-terminal HMGCR and subsequently used to immunoprecipitate full-length 35S-methionine labeled HMGCR. B: Sera 9190 and 9176 were preincubated in the absence (lanes 11 & 13) or presence (lanes 12 & 14) of 300 ng unlabeled C-terminal HMGCR. They were subsequently added to radiolabeled lysates generated from HeLa cells treated with 10 µM mevinolin for 24 hours. The resulting immunoprecipitates were processed as described in the methods section. Identical data were obtained in 2 separate experiments using 4 (A) or 6 (B) different patient sera.
The 200 kDa protein is not recognized by a unique autoantibody
To determine whether serum from anti-HMGCR positive patients includes distinct autoantibodies recognizing the 200 kDa protein, we performed immunoprecipitations from 35S-methionine-labeled, mevinolin-treated HeLa cell extracts, again pre-incubating with purified C-terminal HMGCR protein (figure 3B). This inhibited the immunoprecipitation of both HMGCR and the ~ 200 kDa protein, suggesting that the ~200 kDa protein is either co-immunoprecipitated with HMGCR or is an HMGCR dimer.
Validation of a new ELISA assay to detect anti-HMGCR autoantibodies in patient sera
To screen patients rapidly for anti-HMGCR autoantibodies, we developed an ELISA assay. We defined a serum sample as positive for anti-HMGCR if the relative absorbance value was 3 standard deviations or higher than the mean value of 20 control subjects who had never used statins. Using this method, we found that all 16 of the anti-200/100 patient serum samples previously identified by immunoprecipitation from HeLa cell extracts, were anti-HMGCR positive. In contrast, 0 of 33 subjects with DM (including 5 with prior statin exposure) and 0/31 subjects with IBM (including 11 with prior statin exposure) were anti-HMGCR positive (data not shown).
Next, we used the HMGCR ELISA to screen serum samples from all 750 patients enrolled in our longitudinal study of patients at the Johns Hopkins Myositis Center between May 2002 and April 2010. 45 patients (~6%) were anti-HMGCR positive by ELISA (Table 1). To validate the ELISA, we compared ELISA and IVTT immunoprecipitation data obtained using a subset of sera from this cohort, collected from 307 consecutive unique patients between January 2009 and April 2010. In this subgroup, 17 anti-HMGCR patients were identified by both methods. The ELISA identified one additional anti-HMGCR positive serum that was negative by immunoprecipitation (#10029). Since this patient had a necrotizing myopathy with elevated CK levels, we concluded that this was a true anti-HMGCR positive patient and not a false-positive. These results demonstrate a very high correlation between these two methods and validate the ELISA test as a reliable, efficient screen for detecting anti-HMGCR autoantibodies.
Clinical features of anti-HMGCR positive patients
Out of 45 anti-HMGCR positive patients, 30 subjects had prior statin exposure (66.6%; Table 1). Among those who presented to our clinic at age 50 or older, 24 of 26 subjects had used statin medications (92.3 %). Thus, the prevalence of statin use in anti-HMGCR patients is significantly higher than what we and others have previously reported in age-matched patients (50 years-old or greater) with other myopathies including DM (25%), PM (36.8%), and IBM (33.3%) (8, 9).
Anti-HMGCR positive patients were characterized by proximal muscle weakness (95.6%), elevated CK levels (9718 +/− 7383 IU/L), and myopathic EMG findings (97.3%; Table 2). 40 of 40 available muscle biopsies were reported to have prominent degenerating, regenerating, and/or necrotic fibers (100%). Significant inflammatory infiltrates were noted in 8 of 40 muscle biopsies (20%) and rimmed vacuoles were visualized in 1 of 40 biopsy specimens (2.5%); this patient had predominantly proximal muscle weakness and did not have clinical features typical of IBM. Patients without statin exposure were clinically indistinguishable from those with prior statin use except for their younger age (37 +/− 17 vs. 59 +/− 9 years old), higher CK levels (13,392 +/− 8839 vs. 7881 +/− 5875 IU/L), and race (46.7% vs. 86.7% white; Table 2).
Table 2.
| All Patients | |||
|---|---|---|---|
| Characteristics | # | Total N | % |
| White | 33 | 45 | 73.3 |
| Male | 19 | 45 | 42.2 |
| Mean age + sd | 52+/−16 | 45 | |
| Mean CPK + sd | 9718+/−7383 | 45 | |
| Myopathic EMG | 36 | 37 | 97.3 |
| Irritable myopathy | 27 | 37 | 72.9 |
| Non-irritable myopathy | 9 | 37 | 24.3 |
| Proximal weakness | 43 | 45 | 95.6 |
| Necrosis on biopsy | 40 | 40 | 100 |
| Inflammation on biopsy | 8 | 40 | 20 |
| Statin-Naïve Patients | |||
|---|---|---|---|
| Characteristics | # | Total N | % |
| White | 7 | 15 | 46.7 |
| Male | 5 | 15 | 33.3 |
| Mean age + sd | 37+ /− 17 | 14 | |
| Mean CPK + sd | 13392+/− 8839 | 15 | |
| Myopathic EMG | 12 | 13 | 92.3 |
| Irritable myopathy | 9 | 13 | 69.2 |
| Non-irritable myopathy | 3 | 13 | 23.1 |
| Proximal weakness | 14 | 15 | 93.3 |
| Necrosis on biopsy | 13 | 13 | 100 |
| Inflammation on biopsy | 5 | 13 | 38.5 |
| Statin- Exposed Patients | ||||
|---|---|---|---|---|
| Characteristics | # | Total N | % | P-Value* |
| White | 26 | 30 | 86.7 | 0.012 |
| Male | 14 | 30 | 46.7 | ns |
| Mean age + sd | 59+/− 9 | 30 | <0.0001 | |
| Mean CPK + sd | 7881 +/− 5875 | 30 | 0.0164 | |
| Myopathic EMG | 24 | 24 | 100 | ns |
| Irritable myopathy | 18 | 24 | 75 | ns |
| Non-irritable myopathy | 6 | 24 | 25 | ns |
| Proximal weakness | 29 | 30 | 96.7 | ns |
| Necrosis on biopsy | 27 | 27 | 100 | ns |
| Inflammation on biopsy | 3 | 27 | 11.1 | 0.11 |
statin-exposed vs. statin-naive
While 43 of 45 anti-HMGCR positive patients had no other systemic autoimmune disease (95.6%), patient #8196 had Jo-1 antibodies with interstitial lung disease. Another patient (#l8038) had scleroderma with positive anti-Pm/Scl titers and interstitial lung disease. Neither of these patients was exposed to statins prior to developing muscle symptoms.
The vast majority of anti-HMGCR positive patients had clinical features consistent with an immune-mediated myopathy. However, a single patient presented with only persistent myalgias after statin use, normal subjective and objective strength, unremarkable bilateral thigh MRI findings, normal EMG, and a CK of only 254 IU/L (#8144).
Anti-HMGCR positive patients do not have an increased prevalence of the SNP associated with statin myopathy
A recent study published by the SEARCH collective demonstrated that the presence of a specific polymorphism in the SLCO1B1 gene (i.e, the rs4149056 C allele) is strongly associated with the development of statin myopathy (15). This gene encodes the organic anion-transporting polypeptide OATP1B1, which regulates the hepatic uptake of statins. While the prevalence of the C allele in their population of ~12,000 participants (mostly of European ancestry) was 0.15, its prevalence in those who developed a statin myopathy within one year of starting simvastatin at 80 mg per day was 0.54.
DNA samples were available from 17 anti-HMGCR positive patients and the frequency of the rs4149056 C allele in this population was 0.12. When the 6 subjects without statin exposure and/or with non-European ancestry were excluded, the prevalence of the C allele in the remaining 11 subjects was 0.14. Although the number of subjects genotyped was small, the prevalence of the rs4149056 C allele in these anti-HMGCR positive subjects is consistent with the range of 0.14 to 0.22 previously reported among those of European ancestry (15).
HMGCR is up-regulated in regenerating muscle fibers
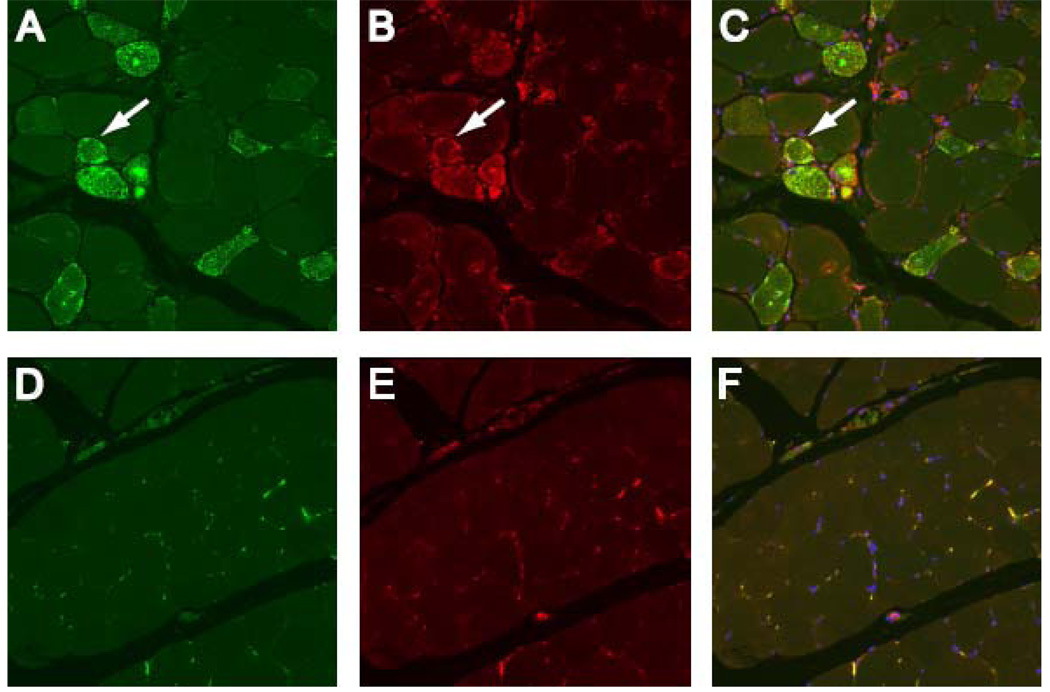
To directly examine HMGCR expression in vivo, we stained muscle biopsy sections with polyclonal HMGCR antibodies. Because other myositis-associated autoantigens are expressed at high levels in muscle cells with features of regeneration (16, 17), we co-stained sections with NCAM, an established marker of muscle regeneration. In normal muscle biopsy specimens, HMGCR and NCAM are expressed at relatively low levels (figure 4: D–F). In contrast, NCAM positive fibers were prominent in muscle biopsies obtained from anti-HMGCR positive subjects (who had been off statins for months to years). Interestingly, most of these NCAM positive fibers also expressed high levels of HMGCR (figure 4; A–C). These findings provide in vivo confirmation that regenerating muscle fibers from anti-HMGCR positive patients express high levels of HMGCR.
Figure 4.
HMGCR expression is up-regulated in regenerating myofibers expressing NCAM. Muscle biopsies from anti-HMGCR positive (A, B, and C) and control subjects (D, E, and F) were co-stained with anti-NCAM (A and D; green), anti-HMGCR antibodies (B and E; red) and DAPI (blue) to stain nuclei. Overlay (C and F) demonstrates HMGCR and NCAM are frequently co-expressed at high levels in the same myofibers in anti-HMGCR positive biopsies (white arrows) but not control muscle tissue. To ensure comparability, images A–C and D–F were obtained using identical exposure settings for each channel. These results are representative of staining seen in 6 anti-HMGCR positive and 3 normal muscle biopsies. (20X objective).
DISCUSSION
Statins are a widely prescribed class of medications with known adverse effects on muscles, usually mild. We recently described novel autoantibodies recognizing ~200 and ~100 kDa proteins associated with autoimmune myopathy and statin use (9). In this report, we demonstrate a plausible causal link between statin exposure and this distinct form of IMNM through identification of the autoantigen as HMGCR. Immunoprecipitation assays demonstrated the specificity of the autoantibodies for the carboxy terminus of this enzyme while competition experiments confirmed that anti-HMGCR autoantibodies immunoprecipitated both HMGCR and the ~200 kDa protein. The larger protein may be an associated protein or a multimer of HMGCR. The latter possibility is supported by other studies showing that HMGCR can be immunoprecipitated as a 97 kDa monomer and as a ~200 kDa dimer.(18).
Having identified HMGCR as the relevant autoantigen, we developed an ELISA assay to rapidly screen patient sera. Using this ELISA, we found the prevalence of anti-HMGCR autoantibodies to be 6% among patients with suspected myopathy who presented to the Johns Hopkins Myositis Center. Extending our previous studies, we found that anti-HMGCR autoantibodies are preferentially found in patients with a necrotizing myopathy on muscle biopsy and were not found in patients with IBM, DM or normal controls (9). Thus, anti-HMGCR autoantibodies are one of the most frequent “myositis specific antibodies” in our cohort, second only to anti-Jo-1 (19). Since necrotizing myopathy is not always immune mediated, the detection of anti-HMGCR by ELISA may be diagnostically helpful to identify those patients with this form of IMNM, the majority of whom respond to immunosuppressive therapy (9).
Among the 45 anti-HMGCR positive subjects, one had Jo-1-positive antisynthetase syndrome (2.2%) and another had scleroderma with anti-Pm/Scl autoantibodies (2.2%). Therefore, as with other forms of autoimmune muscle disease, patients with anti-HMGCR autoantibodies may, in rare cases, have an overlap syndrome with another connective tissue disease.
Importantly, we have demonstrated that muscle expression of HMGCR is increased not only with statin exposure (11), but in regenerating muscle cells marked by NCAM expression. This suggests that immune-mediated muscle damage initiated in the presence of statins and associated with anti-HMGCR autoantibodies may be sustained even after the statin is discontinued, through persistently increased HMGCR expression associated with muscle repair.
Since most patients on statins do not develop an immune-mediated myopathy, other factors, including genetic susceptibility, must also play a role. The most common genetic factor predisposing subjects to self-limited statin myopathy is the presence of the rs4149056 C allele, which accounts for up to 60% of statin myopathies in patients taking 80 mg of simvastatin daily (15). This polymorphism most likely increases the risk of myopathy by decreasing the hepatic uptake of statins by the OATP1B1 transporter. However, this genetic alteration was not over-represented in anti-HMGCR subjects, suggesting that other genetic susceptibilities or environmental co-exposures are required to develop the autoimmune response.
Interestingly, 33% of anti-HMGCR positive patients were not previously exposed to statins. Although these patients were younger at the time of disease onset and had higher CK levels, they also had an apparently immune-mediated myopathy and were otherwise indistinguishable from those with statin exposure. We hypothesize that other genetic and/or environmental factors may cause high levels of HMGCR expression in these patients.
Because our clinic patients are referred with weakness and other prominent features of myopathy, this study does not address how prevalent anti-HMGCR autoantibodies are among patients taking statins who have milder symptoms. However, we did identify one anti-HMGCR positive subject with persistent statin-induced myalgias who had no other compelling clinical evidence of myopathy. This suggests that an autoimmune response may also be associated with low-grade myopathic symptoms in some patients. Future studies will determine how frequently patients with self-limited statin myopathy and statin-induced myalgias develop anti-HGMCR autoantibodies.
Acknowledgments
This work was supported NIH grants K08-AR-054783 (A.M.), K23-AR-053197 (L.C.-S.), and R01-AR-044684 (L.C.-R.). These studies were also supported by the Passano Foundation (A.M.), the Ira Fine Discovery Fund, and the Dorothy and Donald Stabler Foundation. We thank the Johns Hopkins University Rheumatic Diseases Research Core Center (P-30-AR-053503) for assays. We thank Kimberly Doering for her insightful suggestions, Dr. Jennifer Mammen for editing the manuscript, Dr. Katherine Pak for technical assistance, and Drs. Thomas Lloyd and Sonye Danoff for enrolling patients.
REFERENCES
- 1.Statistical brief #205. Trends in statins utilization and expenditures for the U.S. civilian noninstitutionalized population, 2000 and 2005. [July 26, 2010]; Available at: http://www.meps.ahrq.gov/mepsweb/data_files/publications/st205/stat205.pdf.
- 2.de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]) Am J Cardiol. 2002 Jul 15;90(2):181–184. doi: 10.1016/s0002-9149(02)02449-9. [DOI] [PubMed] [Google Scholar]
- 3.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005 Dec;19(6):403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 4.Franc S, Dejager S, Bruckert E, Chauvenet M, Giral P, Turpin G. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther. 2003 Sep–Nov;17(5–6):459–465. doi: 10.1023/b:card.0000015861.26111.ab. [DOI] [PubMed] [Google Scholar]
- 5.Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004 Dec 1;292(21):2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 6.Soininen K, Niemi M, Kilkki E, Strandberg T, Kivisto KT. Muscle symptoms associated with statins: a series of twenty patients. Basic Clin Pharmacol Toxicol. 2006 Jan;98(1):51–54. doi: 10.1111/j.1742-7843.2006.pto_193.x. [DOI] [PubMed] [Google Scholar]
- 7.Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord. 2007 Feb;17(2):194–200. doi: 10.1016/j.nmd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2009 Oct 7; doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 9.Christopher-Stine L, Hong G, Casciola-Rosen LA, Corse AM, Chung T, Mammen AL. A novel autoantibody recognizing 200 and 100 kDa proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010 May 23; doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 11.Morikawa S, Murakami T, Yamazaki H, Izumi A, Saito Y, Hamakubo T, et al. Analysis of the global RNA expression profiles of skeletal muscle cells treated with statins. J Atheroscler Thromb. 2005;12(3):121–131. doi: 10.5551/jat.12.121. [DOI] [PubMed] [Google Scholar]
- 12.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975 Feb 13;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 13.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975 Feb 20;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 14.Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995 Nov;38(5):705–713. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 15.SEARCH Collaborative Group. Link E, Parish S, Armitage J, Bowman L, Heath S, et al. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008 Aug 21;359(8):789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 16.Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005 Feb 21;201(4):591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mammen AL, Casciola-Rosen LA, Hall JC, Christopher-Stine L, Corse AM, Rosen A. Expression of the dermatomyositis autoantigen Mi-2 in regenerating muscle. Arthritis Rheum. 2009 Dec;60(12):3784–3793. doi: 10.1002/art.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker RA, Miller SJ, Gibson DM. Phosphorylation of native 97-kDa 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Impact on activity and degradation of the enzyme. J Biol Chem. 1989 Mar 25;264(9):4877–4887. [PubMed] [Google Scholar]
- 19.Sarkar K, Miller FW. Autoantibodies as predictive and diagnostic markers of idiopathic inflammatory myopathies. Autoimmunity. 2004 Jun;37(4):291–294. doi: 10.1080/08916930410001710839. [DOI] [PubMed] [Google Scholar]