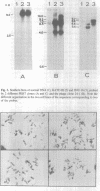
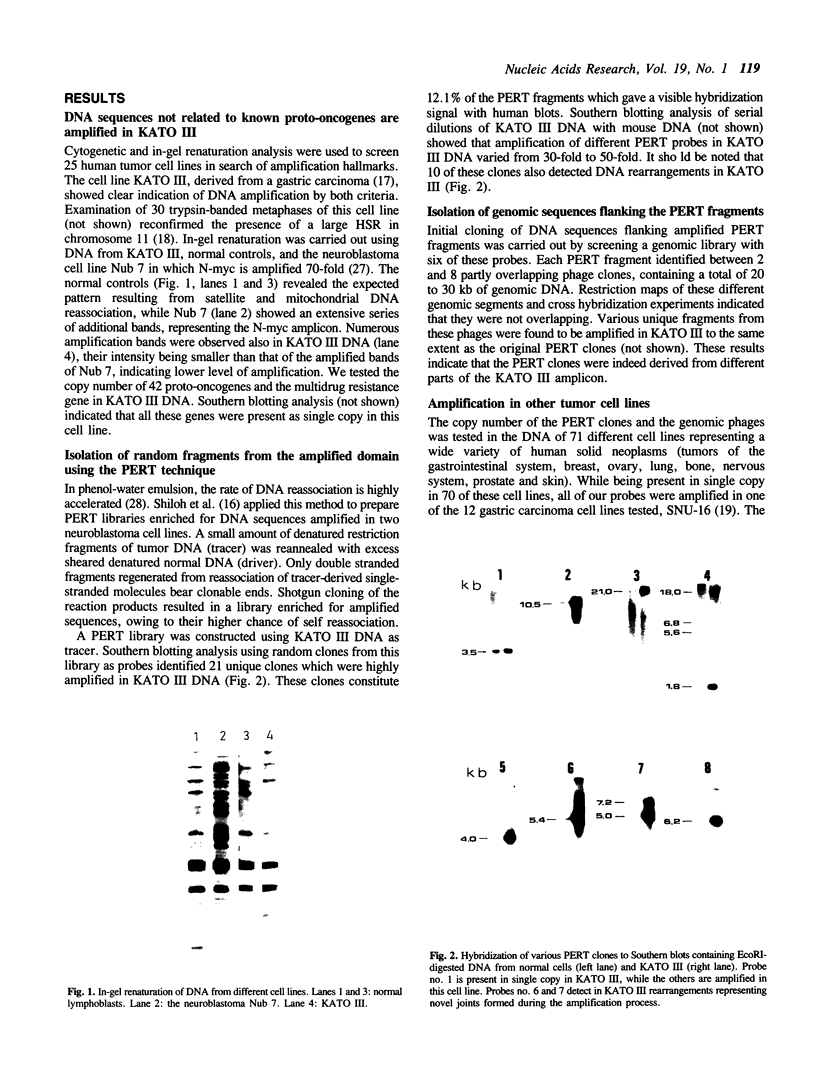
Abstract
Molecular cloning of genomic sequences altered in cancer cells is believed to lead to the identification of new genes involved in the initiation and progression of the malignant phenotype. DNA amplification is a frequent molecular alteration in tumor cells, and is a mode of proto-oncogene activation. The cytologic manifestation of this phenomenon is the appearance of chromosomal homogeneously staining regions (HSRs) or double minute bodies (DMs). The gastric carcinoma cell line KATO III is characterized by a large HSR on chromosome 11. In-gel renaturation analysis confirmed the amplification of DNA sequences in this cell line, yet none of 42 proto-oncogenes that we tested is amplified in KATO III DNA. We employed the phenol-enhanced reassociation technique (PERT) to isolate 21 random DNA fragments from the amplified domain, and used 6 of them to further clone some 150 kb from that genomic region. While in situ hybridization performed with some of these sequences indicated that in KATO III they are indeed amplified within the HSR on chromosome 11, somatic cell hybrid analysis and in situ hybridization to normal lymphocyte chromosomes showed that they are derived from chromosome 10, band q26. The same sequences were found to be amplified in another gastric carcinoma cell line, SNU-16, which contains DMs, but were not amplified in other 70 cell lines representing a wide variety of human neoplasms. One of these sequences was highly expressed in both KATO III and SNU-16. Thus, the cloned sequences supply a starting point for identification of novel genes which might be involved in the pathogenesis of gastric cancers, and are located in a relatively unexplored domain of the human genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K., Schwab M. Oncogene amplification in tumor cells. Adv Cancer Res. 1986;47:235–281. doi: 10.1016/s0065-230x(08)60201-8. [DOI] [PubMed] [Google Scholar]
- Amler L. C., Schwab M. Amplified N-myc in human neuroblastoma cells is often arranged as clustered tandem repeats of differently recombined DNA. Mol Cell Biol. 1989 Nov;9(11):4903–4913. doi: 10.1128/mcb.9.11.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker P. E. Double minutes in human tumor cells. Cancer Genet Cytogenet. 1982 Feb;5(1):81–94. doi: 10.1016/0165-4608(82)90043-7. [DOI] [PubMed] [Google Scholar]
- Brison O., Ardeshir F., Stark G. R. General method for cloning amplified DNA by differential screening with genomic probes. Mol Cell Biol. 1982 May;2(5):578–587. doi: 10.1128/mcb.2.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Hayes F. A., Green A. A., Casper J. T., Wasson J., Wallach S., Seeger R. C. Consistent N-myc copy number in simultaneous or consecutive neuroblastoma samples from sixty individual patients. Cancer Res. 1987 Aug 15;47(16):4248–4253. [PubMed] [Google Scholar]
- Brüderlein S., Gebhart E. Double minutes in prematurely condensed chromatin of human tumor cells. Cancer Genet Cytogenet. 1985 Mar 15;16(2):145–152. doi: 10.1016/0165-4608(85)90008-1. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Manuelidis L. Neuroblastoma double minutes isolated by pulsed-field gel electrophoresis without prior strand-cleaving treatments. Genomics. 1989 Apr;4(3):430–433. doi: 10.1016/0888-7543(89)90351-0. [DOI] [PubMed] [Google Scholar]
- Emanuel B. S., Balaban G., Boyd J. P., Grossman A., Negishi M., Parmiter A., Glick M. C. N-myc amplification in multiple homogeneously staining regions in two human neuroblastomas. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3736–3740. doi: 10.1073/pnas.82.11.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gebhart E., Brüderlein S., Augustus M., Siebert E., Feldner J., Schmidt W. Cytogenetic studies on human breast carcinomas. Breast Cancer Res Treat. 1986;8(2):125–138. doi: 10.1007/BF01807701. [DOI] [PubMed] [Google Scholar]
- Haluska F. G., Tsujimoto Y., Croce C. M. Oncogene activation by chromosome translocation in human malignancy. Annu Rev Genet. 1987;21:321–345. doi: 10.1146/annurev.ge.21.120187.001541. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Milbrandt J. D., Heintz N. H., Azizkhan J. C. DNA sequence amplification in mammalian cells. Int Rev Cytol. 1984;90:31–82. doi: 10.1016/s0074-7696(08)61487-4. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hubbell H. R., Quinn L. A., Dolby T. W. Cloning of a non-c-myc DNA fragment from the double minutes of a human colon carcinoid cell line. Cancer Genet Cytogenet. 1987 Jan;24(1):17–31. doi: 10.1016/0165-4608(87)90080-x. [DOI] [PubMed] [Google Scholar]
- Hyrien O., Debatisse M., Buttin G., de Saint Vincent B. R. A hotspot for novel amplification joints in a mosaic of Alu-like repeats and palindromic A + T-rich DNA. EMBO J. 1987 Aug;6(8):2401–2408. doi: 10.1002/j.1460-2075.1987.tb02518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K. W., Ruppert J. M., Bigner S. H., Vogelstein B. The GLI gene is a member of the Kruppel family of zinc finger proteins. Nature. 1988 Mar 24;332(6162):371–374. doi: 10.1038/332371a0. [DOI] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Monaco A. P., Middlesworth W., Ochs H. D., Latt S. A. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney J. E., Ma C., Leu T. H., Flintoff W. F., Troutman W. B., Hamlin J. L. The dihydrofolate reductase amplicons in different methotrexate-resistant Chinese hamster cell lines share at least a 273-kilobase core sequence, but the amplicons in some cell lines are much larger and are remarkably uniform in structure. Mol Cell Biol. 1988 Dec;8(12):5268–5279. doi: 10.1128/mcb.8.12.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Looney J. E., Leu T. H., Hamlin J. L. Organization and genesis of dihydrofolate reductase amplicons in the genome of a methotrexate-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1988 Jun;8(6):2316–2327. doi: 10.1128/mcb.8.6.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery K. T., Biedler J. L., Spengler B. A., Melera P. W. Specific DNA sequence amplification in human neuroblastoma cells. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5724–5728. doi: 10.1073/pnas.80.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. G., Frucht H., LaRocca R. V., Bliss D. P., Jr, Kurita Y., Chen T. R., Henslee J. G., Trepel J. B., Jensen R. T., Johnson B. E. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990 May 1;50(9):2773–2780. [PubMed] [Google Scholar]
- Roninson I. B. Detection and mapping of homologous, repeated and amplified DNA sequences by DNA renaturation in agarose gels. Nucleic Acids Res. 1983 Aug 25;11(16):5413–5431. doi: 10.1093/nar/11.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Kanda N., Shiloh Y., Donlon T., Schreck R., Shipley J., Dryja T., Chaum E., Chaganti R. S., Latt S. Molecular and cytologic analysis of DNA amplification in retinoblastoma. Cancer Genet Cytogenet. 1985 Jun;17(2):95–112. doi: 10.1016/0165-4608(85)90020-2. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Sakakibara K., Fujii G. Establishment of cultured cell lines derived from a human gastric carcinoma. Jpn J Exp Med. 1978 Feb;48(1):61–68. [PubMed] [Google Scholar]
- Shiloh Y., Rose E., Colletti-Feener C., Korf B., Kunkel L. M., Latt S. A. Rapid cloning of multiple amplified nucleotide sequences from human neuroblastoma cell lines by phenol emulsion competitive DNA reassociation. Gene. 1987;51(1):53–59. doi: 10.1016/0378-1119(87)90473-2. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Shipley J., Brodeur G. M., Bruns G., Korf B., Donlon T., Schreck R. R., Seeger R., Sakai K., Latt S. A. Differential amplification, assembly, and relocation of multiple DNA sequences in human neuroblastomas and neuroblastoma cell lines. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3761–3765. doi: 10.1073/pnas.82.11.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A., Haley L. L., Byers M. G., Eddy R. L., Cooper E. S., Goggin A. P. Assignment of the beta-glucuronidase structural gene to the pter leads to q22 region of chromosome 7 in man. Cytogenet Cell Genet. 1978;21(1-2):99–104. doi: 10.1159/000130882. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Sakaguchi A. Y., Naylor S. L. Mapping the human genome, cloned genes, DNA polymorphisms, and inherited disease. Adv Hum Genet. 1982;12:341–452. doi: 10.1007/978-1-4615-8315-8_5. [DOI] [PubMed] [Google Scholar]
- Shows T., Eddy R., Haley L., Byers M., Henry M., Fujita T., Matsui H., Taniguchi T. Interleukin 2 (IL2) is assigned to human chromosome 4. Somat Cell Mol Genet. 1984 May;10(3):315–318. doi: 10.1007/BF01535253. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Wahl G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989 Mar 15;49(6):1333–1340. [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Weith A., Winking H., Brackmann B., Boldyreff B., Traut W. Microclones from a mouse germ line HSR detect amplification and complex rearrangements of DNA sequences. EMBO J. 1987 May;6(5):1295–1300. doi: 10.1002/j.1460-2075.1987.tb02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeger H., Baumal R., Pawlin G., Tonin P., Nissen L., Kaplinsky C., Phillips M. J. Phenotypic and molecular characterization of inducible human neuroblastoma cell lines. Differentiation. 1988 Dec;39(3):216–227. doi: 10.1111/j.1432-0436.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Zhou D. J., Casey G., Cline M. J. Amplification of human int-2 in breast cancers and squamous carcinomas. Oncogene. 1988 Mar;2(3):279–282. [PubMed] [Google Scholar]