Abstract
A novel MCAP-cycloaddition sequence has been applied to the facile synthesis of β-carboline intermediates to gain rapid access to novel derivatives of yohimbine-like and corynanthe-like compounds that may be easily diversified by cross-coupling reactions and N-derivatizations to generate small compound libraries.
Keywords: multicomponent assembly process, imine, dipolar cycloaddition, Suzuki reaction, N-Derivatization
The pentacyclic and tetracyclic ring systems characteristic of the Yohimbine and Corynanthe alkaloids, respectively, are exemplified in indole alkaloids such as yohimbine (1), tetrahydroalstonine (2) and geissoschizine (3), and alkaloids belonging to these families exhibit a remarkable range of biological activities.1 For example, owing to their high affinity for α1–adrenergic receptor, some of these alkaloids may be used to treat hypertension, depression and diabetes.2 Accordingly, derivatives of these alkaloids are promising targets as bioactive compounds in drug discovery, and some analogs having substructures of these natural products have been found to exhibit useful biological activities.3 In this context, it is significant that the synthesis of functionalized frameworks found in natural products has emerged as a useful strategy, which is known as biology oriented synthesis (BIOS),4 for discovering biologically active compounds. We thus became interested in preparing 4 and 5, as novel analogs of the Yohimbine and Corynanthe alkaloids.
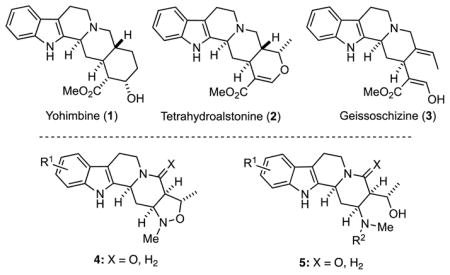
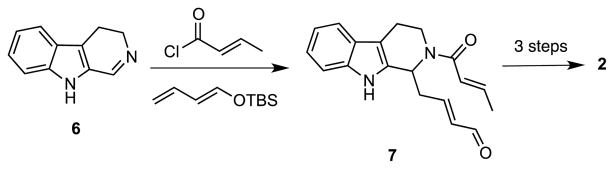
We have recently been interested in the design and development of effective strategies for preparing functionalized heterocyclic scaffolds that can be easily diversified by cross-coupling and N-derivatization reactions.5 Indeed, a three-component reaction of 6 to give 7, a key step in our concise synthesis of tetrahydroalstonine (2) (Scheme 1),6 served as the inspiration for a novel multicomponent assembly process (MCAP) that featured the use of Mannich-type reactions to give substituted aryl methylamine derivatives. These adducts can be subjected to various cyclization reactions that are enabled by selective functional group pairing to construct substituted heterocyclic ring systems.7 We have demonstrated the utility of this general approach to diversity oriented synthesis (DOS) by applying it to preparation of small libraries of substituted benzodiazepines,8 norbenzomorphans,9 aryl piperidines,10 tetrahydroisoquinolines,11 as well as several conformationally-constrained benzoxazocines and benzazocines.12 We now report the extension of this useful methodology to the facile preparation of compounds derived from the yohimbine and corynanthe scaffolds.
Scheme 1.
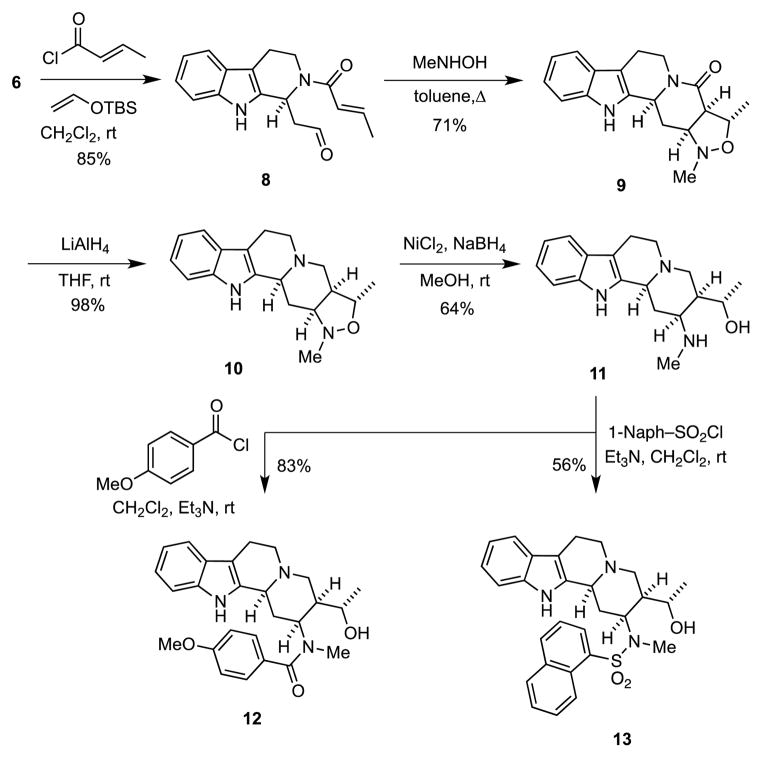
We envisioned that compounds generally related to 4 could be accessed by a sequential MCAP of a dihydro-β-carboline such as 6 followed by a dipolar cycloaddition of a nitrone derived from the adduct.7 Toward this end, treatment of 6 with crotonyl chloride and tert-butyldimethyl(vinyloxy)silane in CH2Cl2 afforded the amide 8 in 85% yield. Heating 8 with N-methylhydroxylamine hydrochloride and triethylamine in refluxing toluene then provided an intermediate nitrone that underwent a spontaneous [3+2] dipolar cycloaddition to give 9 as a single diastereoisomer. When 9 was treated with LiAlH4, the amine 10 was obtained in nearly quantitative yield. Subsequent reductive scission of the N–O bond in 10 using Ni2B, which was generated in situ,13 delivered the amino alcohol 11 in 64% yield. The amino group in 11 could then be derivatized by a number of selective N-functionalizations. For example, N-acylation provides amides such as 12, whereas reaction with isocyanates gives ureas like 13.
In order to establish the versatility of our approach to yohimbine analogs as 9 and 10 and corynanthe-like compounds like 11–13, we queried whether representative bromo substituted carbolines might be employed as starting materials. We reasoned that a bromo group would be a convenient functional handle for performing a variety of cross-coupling reactions to introduce other substituents.
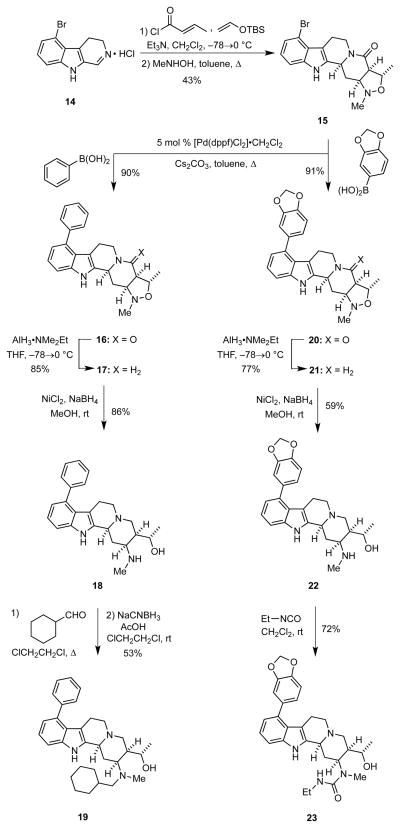
Accordingly, the known 5-bromo-β-carboline (14),14 which was prepared in four steps from 4-bromoindole was treated with tert-butyldimethyl(vinyloxy)silane and crotonyl chloride to provide an intermediate adduct that was then heated with N-methylhydroxylamine to afford the isoxazolidine 15 in 43% overall yield (Scheme 3). The intramolecular dipolar cycloaddition reaction was highly stereoselective as 15 was the only isomer detected. After exploring a number of conditions for performing a Suzuki cross-coupling reaction, we found the best yield of 16 was obtained by heating phenylboronic acid and 15 in the presence of 5 mol % [Pd(dppf)Cl2] CH2Cl2 and Cs2CO3 in refluxing toluene for 17 h. Use of other catalysts including Pd(OAc)2, Pd(PPh3)4, and Pd(t-Bu3P)2 gave lower yields. We performed exploratory experiments to use 15 as a reaction partner in Buchwald-Hartwig type cross-couplings with secondary amines, but these preliminary reactions were low yielding. Reduction of the lactam moiety in 16 with alane gave the amine 17 in 85% yield. Subsequent reductive cleavage of the N,O–bond in 17 using nickel boride as before produced the desired amino alcohol 18 in excellent yield. Selective N-functionalization of 18 was then achieved in several ways. For example, reductive amination of 18 with cyclohexanecarboxaldehyde, which proceeded via a relatively stable intermediate N,O-acetal, using NaCNBH3 under mildly acidic conditions to furnish 19 in 53% yield. Similarly, the cycloadduct 15 was converted into the amino alcohol 22 in good overall yield via sequential Suzuki cross-coupling, lactam reduction, and, N–O bond scission. Reaction of the secondary amine in 22 with ethylisocyanate gave the urea 23 in 72% yield.
Scheme 3.
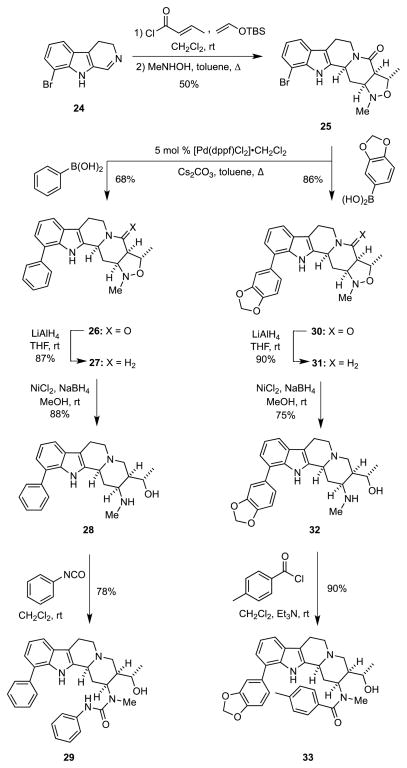
We then extended this chemistry in a straightforward fashion to the syntheses of 8-substituted analogs as outlined in Scheme 4. In the event the known 8-bromocarboline 24,14 which was prepared in four steps from 7-bromoindole, tert-butyldimethyl(vinyloxy)silane, and crotonyl chloride were combined in a multicomponent assembly process to provide an intermediate adduct, heating of which with N-methylhydroxylamine generated the isoxazolidine 25 in 50% yield. When 25 was subjected to cross-coupling reactions with aryl boronic acids in the presence of 5 mol % [Pd(dppf)Cl2] CH2Cl2 and Cs2CO3 in refluxing toluene, 26 and 30 were obtained in 68% and 86% yields, respectively. The products of these Suzuki cross-couplings were invariably contaminated with about 10% of the debrominated material 9. Although we conducted several exploratory experiments we were unable to identify conditions under which debromination did not occur. Fortunately, compound 9 could be largely removed from the coupled product by exploiting the lower solubility of 9 in ethyl acetate or by column chromatography. Reduction of the lactam moiety of 26 and 30 with LiAlH4, followed by cleavage of the N–O bond with nickel boride provided the amino alcohols 28 and 32; alane could also be used to reduce the lactam. The amino alcohol 28 was then converted into the urea 29 upon reaction with phenylisocyanate, whereas 32 was transformed into the amide 33 with p-toluoyl chloride.
Scheme 4.
In summary, we have extended our general approach for diversity oriented synthesis7 and applied a MCAP/cyclization sequence to various dihydro-β-carbolines to gain facile access to novel compounds having scaffolds related to the Yohimbine and Corynanthe alkaloids. Intermediates are functionalized to enable a number of different derivatization reactions, including Suzuki cross-coupling reactions and N-functionalization by reductive alkylation, acylation, urea and thiourea formation, and sulfonylation. The application of this general plan for DOS to the synthesis of small libraries of biaryl compounds for biological screening is in progress, and the results of these and related investigations will be reported in due course.
Supplementary Material
Scheme 2.
Acknowledgments
We thank the National Institutes of Health (GM 24539 and GM 86192) and the Robert A. Welch Foundation (F-0652) for their generous support of this work.
Footnotes
Supplementary data (representative experimental procedures and characterization data of compounds 14, 16, 20–23, 24, 26, 27, 33, and 36) can be found in the online version at doi:10.1016/j.tetlet.2011.xx.xxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.For general reviews of the structure, bioactivity, and synthesis of indole alkaloids of the corynanthe and yohimbine groups, see: Brown RT. In: Indoles. Saxton JE, editor. Chapter 3 Wiley-Interscience; New York: 1983. Part Four (The Monoterpenoid Indole Alkaloids)Szántay C, Blaskó C, Honty K, Dörnyei G. The Alkaloids. In: Brossi A, editor. Chemistry and Physiology. Vol. 27. Academic Press; New York: 1986. p. 131.Lounasmaa M, Tolvanen A. In: Monoterpenoid Indole Alkaloids. Saxton JE, editor. Vol. 25. Wiley-Interscience; New York: 1994. Part 4, Chapter 3.
- 2.(a) Wink Michael, Roberts MW. Alkaloids: biochemistry, ecology, and medicinal applications. Chapter 2 New York: Plenum Press; 1998. [Google Scholar]; (b) Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, Nagaraj V, Reinbothe TM, et al. Science. 2010;327:217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- 3.(a) Klioze SS, Ehrgott FJ, Jr, Wilker JC, Woodward DL. J Med Chem. 1979;22:1497–504. doi: 10.1021/jm00198a013. [DOI] [PubMed] [Google Scholar]; (b) Clark RD, Repke DB, Berger J, Nelson JT, Kilpatrick AT, Brown CM, MacKinnon AC, Clague RU, Spedding M. J Med Chem. 1991;34:705–717. doi: 10.1021/jm00106a036. [DOI] [PubMed] [Google Scholar]; (c) Clark RD, Nelson JT, Repke, David B. J Heterocyclic Chem. 1993;30:829–831. [Google Scholar]; (d) Boehringer M, Kuhn B, Luebbers T, Mattei P, Narquizian R, Wessel HP. 2004259902 A1. US Pat Appl Publ. 2004:23.; (e) Boehringer M, Kuhn B, Mattei P, Narquizian R. 2004259903 A1. US Pat Appl Publ. 2004:28.
- 4.For leading references, see: Koch MA, Schuffenhauer A, Scheck M, Wetzel S, Casaulta Ma, Odermatt A, Ertl P, Waldmann H. Proc Nat Acad Sci (USA) 2005;102:17272–17277. doi: 10.1073/pnas.0503647102.Wetzel S, Schuffenhauer A, Roggo S, Ertl P, Waldmann H. Chimia. 2007;61:355–360.
- 5.For a review of such strategies, see: Sunderhaus JD, Martin SF. Chem Eur J. 2009;15:1300–1308. doi: 10.1002/chem.200802140.
- 6.Martin SF, Benage B, Hunter JE. J Am Chem Soc. 1988;110:5925–5927. [Google Scholar]
- 7.(a) Sunderhaus JD, Dockendorff C, Martin SF. Org Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357. [DOI] [PubMed] [Google Scholar]; (b) Sunderhaus JD, Dockendorff C, Martin SF. Tetrahedron. 2009;65:6454–6469. doi: 10.1016/j.tet.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donald JR, Martin SF. Org Lett. 2011;13:852–855. doi: 10.1021/ol1028404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahn JJ, Su JY, Martin SF. Org Lett. 2011;13:2590–2593. doi: 10.1021/ol200709h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy S, Martin SF. Org Lett. 2011;13:3102–3105. doi: 10.1021/ol201010s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granger BA, Kaneda K, Martin SF. Org Lett. 2011;13:4542–4545. doi: 10.1021/ol201739u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahn JJ, Martin SF. Tetrahedron Lett. doi: 10.1016/j.tetlet.2011.10.022. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones AD, Knight DW, Thornton SR. J Chem Soc, Perkin Trans. 1999;1:3337–3344. [Google Scholar]
- 14.Glennon RA, Grella B, Tyacke RJ, Lau A, Westaway J, Hudson AL. Bioorg Med Chem Lett. 2004;14:999–1002. doi: 10.1016/j.bmcl.2003.11.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.