Abstract
Background
Uveitis, or inflammatory eye disease, is a common extra-articular manifestation of many systemic autoinflammatory diseases involving the joints. Anakinra (recombinant interleukin (IL)-1 receptor antagonist (Ra)) is an effective therapy in several arthritic diseases; yet, few studies have investigated the extent to which IL-1 signalling or IL-1Ra influences the onset and/or severity of uveitis.
Objective
To seek possible links between arthritis and uveitis pathogenesis related to IL-1 signalling.
Methods
The eyes of IL-1Ra-deficient BALB/c mice were monitored histologically and by intravital videomicroscopy to determine if uveitis developed along with the expected spontaneous arthritis in ankles and knees. Expression levels of IL-1R and its negative regulators (IL-1Ra, IL-1RII, IL-1RAcP and single Ig IL-1R-related molecule) in eye and joint tissues were compared. Differences in uveitis induced by intraocular injection of lipopolysaccharide (LPS) in mice lacking IL-1R or IL-1Ra were assessed.
Results
Deficiency in IL-1Ra predisposes to spontaneous arthritis, which is exacerbated by previous systemic LPS exposure. The eye, however, does not develop inflammatory disease despite the progressive arthritis or LPS exposure. Organ-specific expression patterns for IL-1Ra and negative regulators of IL-1 activity were observed that appear to predict predisposition to inflammation in each location in IL-1Ra knockout mice. The eye is extremely sensitive to locally administered LPS, and IL-1Ra deficiency markedly exacerbates the resulting uveitis.
Conclusion
This study demonstrates that IL-1Ra plays an important role in suppressing local responses in eyes injected with LPS and that there is discordance between murine eyes and joints in the extent to which IL-1Ra protects against spontaneous inflammation.
INTRODUCTION
A recent clinical trial strongly implicates interleukin (IL)-1β in the pathogenesis of Behçet’s disease, with findings that anti-IL-1 therapy improves symptoms in patients with Behçet’s disease, with uveitis in particular being improved.1,2 Uveitis, or intraocular inflammation, is a leading cause of blindness worldwide and is comparable to diabetes or macular degeneration in terms of years of vision loss.3 Anterior uveitis, wherein the iris is consistently affected, is the most commonly diagnosed type of uveitis and is a common extra-articular manifestation of many systemic inflammatory diseases marked by arthritis such as Behçet’s disease or ankylosing spondylitis.4,5 The underlying mechanisms that predispose to ocular inflammation during these systemic diseases are not known, but inflammatory pathways or cytokines, such as IL-1β, have been hypothesised to be common pathogenic factors in eye and joint disease.
The importance of IL-1 has been documented in infection, tissue injury and an increasing number of systemic autoinflammatory diseases. Recent advances in our understanding of IL-1 production by inflammasomes have led to enhanced interest in IL-1 signalling and therapeutic agents targeting IL-1. Excessive IL-1 signalling as a consequence of inflammasome hyperactivity occurs in a defined set of autoinflammatory conditions6-8 commonly characterised by inflammation involving multiple organs, typically skin, joints and eyes. Anakinra (soluble IL-1R antagonist, IL-1 receptor antagonist (Ra)) is effective in ameliorating the symptoms in this group of disorders. In addition to these relatively rare diseases, polymorphisms in the IL-1 gene cluster (IL1A, IL1B, IL1RN) or IL-1RII affect susceptibility to more common inflammatory diseases wherein uveitis and arthritis coexist, such as ankylosing spondylitis and its associated spondyloarthropathies, sarcoidosis and Behçet’s disease.9-16 Very little is known, however, about the pathophysiological effects of IL-1 activities in the eye, and the extent to which IL-1Ra regulates ocular inflammation is poorly understood.
Numerous studies support a pathogenic role for IL-1 in experimental arthritis models.10,17 Conversely, BALB/c mice lacking IL-1Ra, which is encoded by the gene, IL-1RN, spontaneously develop arthritis and a psoriasis-like skin disorder.18-21 The consequences of dysregulated IL-1 signalling on the eye, however, have yet to be examined in IL-1Ra knockout (KO) mice. Here, we describe the contribution of IL-1 signalling in murine arthritis in comparison with uveitis.
METHODS
Mice
Female IL1R KO and IL1RN KO (IL-1Ra KO) mice on the BALB/c background have been described.18,22 All animal experiments complied with the ethics and animal experiment regulations of the Association of Assessment and Accreditation of Laboratory Animal Care International and our Institutional Animal Care and Use Committee. All animals had access to water and food ad libitum.
Administration of lipopolysaccharide (LPS) or IL-1β
Systemic LPS treatments consisted of an intraperitoneal injection of 25 μg LPS or saline. For local intraocular LPS injections, anaesthetised (1.7% isoflurane in oxygen) mice received 2 μl intravitreal injections with 250 ng LPS in one eye and saline in the contralateral eye.23 Mouse recombinant IL-1β (R&D Systems, Minneapolis, Minnesota, USA) was injected intravitreally at the indicated doses.
Intravital microscopy
Intraocular inflammation of the iris vasculature and tissue was observed in mice anaesthetised with 1.7% isoflurane in oxygen as previously described.23 Ten-second videos of three independent regions of the iris vasculature/tissue were recorded. Iris vessel and tissue measurements as well as the numbers of rolling, adherent and infiltrating cells were determined off-line.23,24
Histopathology
At the indicated times, mice were killed, and the eyes, ankles and knees were dissected, fixed in 10% neutral-buffered formalin, and prepared for paraffin embedding and sectioning as previously reported.25,26 Tissue sections (7 μm thick) were stained with H&E, and the severity of inflammatory changes was quantified by a masked observer.
Near infrared (NIR) fluorescence imaging of joint inflammation
NIR imaging was performed as previously described26 with the NIR fluorescent probe ProSense 680 (ViseEn Medical, Woburn, Massachusetts, USA), which is a protease substrate that forms NIR fluorescent deposits when cleaved in inflamed tissues. ProSense (2 nmol/150 μl) was injected intravenously 24 h before the mice were killed. Images were analysed with LI-COR software for regions of interest in the knee or ankle, and the mean differences in fluorescence intensity were normalised to naïve, wild-type (WT) controls.
Immunoblotting
Dissected ankles, knees and eyes were homogenised in lysis buffer containing protease inhibitors.25,27 Protein concentrations were quantified by BCA assay (BioRad, Hercules, California, USA). The following primary antibodies were used: polyclonal anti-IL-1R and anti-(IL-1RII) (R&D Systems), anti-(single Ig IL-1R-related molecule (SIGIRR)) (ProSci, Woburn, Massachusetts, USA) anti-(IL-1RAcP) (Abcam, Cambridge, Massachusetts, USA) and monoclonal anti-(β-actin) (AC-15; Sigma Chemical, St Louis, Missouri, USA). The secondary antibodies were tagged with an NIR fluorophore (goat anti-rabbit IgG with IRDye 680 and goat anti-mouse IgG with IRDye 800CW; LI-COR, Lincoln, Nebraska, USA) and were detected with a LI-COR Odyssey scanner and software.
ELISA
IL-1β, IL-1α and IL-1Ra concentrations in plasma and tissue homogenates prepared as above were measured by DuoSet ELISA (R&D Systems) according to the manufacturer’s instructions. Results are represented as the ratio of pg/ml IL-1Ra to μg/ml total protein.
Statistical analysis
Results are expressed as mean±SEM. Statistical evaluation of differences between experimental groups was by analysis of variance and Student t test. Statistical significance was accepted at p<0.05.
RESULTS
Absence of IL-1Ra predisposes to spontaneous arthritis but not uveitis
Given the reported propensity of BALB/c mice lacking expression of IL-1Ra (ie, IL-1Ra KO mice) to exhibit increased circulating concentrations of IL-6 and acute-phase proteins coinciding with spontaneous onset of ankle joint inflammation,18,20,28 we ascertained if the absence of IL-1Ra predisposes to eye inflammation. IL-1Ra KO mice were examined for the onset of arthritis and uveitis as a function of age. Arthritis was assessed with a NIR fluorescence imaging method which indicates protease activation in the joint, which is an early event in the arthritic inflammatory cascade that closely predicts the extent of arthritis observed by histology.26,29
Naïve IL-1Ra KO mice develop inflammation in the ankle joints that is detectable by NIR imaging by 12 weeks of age (figure 1A). The progressive increase in NIR signal intensity over the next month coincides with increased histopathology in the ankle joints at 20 weeks, which is consistent with previous reports.28 Synovial and periarticular infiltration of inflammatory cells coincides with cartilage damage and destruction (arrows), which is prominent at 20 weeks of age (figure 1A, far right histology panel).
Figure 1.
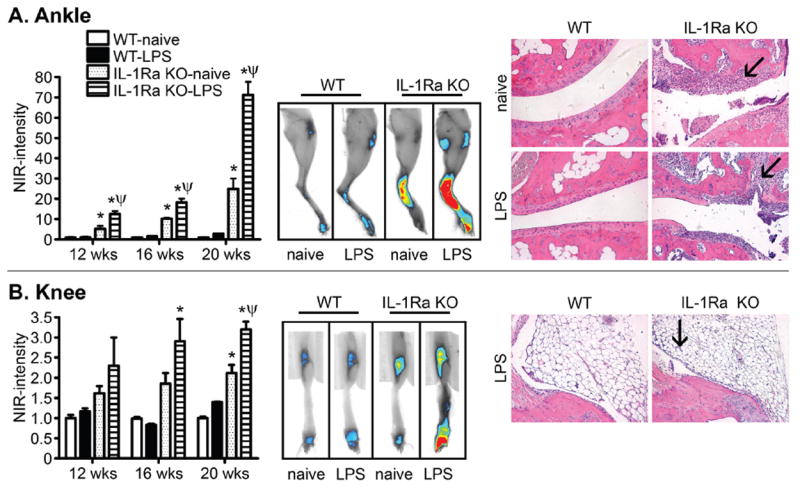
Interleukin 1 receptor antagonist (IL-1Ra) deficiency predisposes to more severe arthritis in the ankle than in the knee. The severity of inflammation in the ankle (A) or knee (B) was assessed in wild-type (WT) and IL-1Ra knockout (KO) mice with and without an intraperitoneal injection of 25 μg lipopolysaccharide (LPS) at 7 weeks of age. Left panels show quantification of the NIR fluorescence intensity from a ProSense inflammation marker. *p<0.05 for comparison between IL-1Ra KO and WT responses; ψp<0.05 for comparison between LPS-treated and naïve response within a genotype (n=8–10 mice/treatment/genotype/time). Middle panels show representative NIR fluorescence images of legs from the side to emphasise ankles or from the front to emphasise knees. The right panels show histological images of ankle or knee joints of mice at 20 weeks of age stained with H&E. Original magnification: ×200. Arrows indicate areas of signs of joint pathology in the ankle (ie, cell infi ltration, cartilage damage and destruction that is worsened by previous LPS exposure) or the knee (ie, minor proliferation of synovial membrane). Synovial and periarticular infiltration of inflammatory cells coincides with bone erosion (arrows).
We observed that knee joints are somewhat susceptible to inflammation as a consequence of IL-1Ra deficiency (figure 1B), but only at later ages (20 weeks). The ProSense NIR fluorescence intensity increases up to about twofold in naïve knees compared with ~20-fold in naïve ankles (figure 1B). Histologically, very little cellular inflammation is observed in naïve knees from IL-1Ra KO mice compared with WT controls even at 20 weeks (data not shown), albeit some proliferation of the synovial membrane is observed in IL-1Ra KO mice previously challenged with low-dose LPS at 20 weeks (figure 1B, arrow). These findings indicate that IL-1Ra deficiency renders the ankles more susceptible than the knee joints to disease.
Using intravital videomicroscopy, we are able to visualise ongoing cellular trafficking in the iris and quantify it on the basis of leucocyte trafficking steps: rolling and adhering along the endothelium of the iris vasculature and subsequent infiltration into the tissue.23 We examined the eyes of 12–20-week-old IL-1Ra KO mice for the presence of uveitis that might coincide with the progressive arthritis. As shown in figure 2, no significant ocular inflammatory response was observed in IL-1Ra KO mice. The numbers of intravascular rolling or adhering cells and the numbers of infiltrating extravascular cells were similar for the IL-1Ra KO mice and the WT controls (figure 2A–C). These data indicate that spontaneous joint inflammation did not predict the presence of eye disease. Histological examination further supported the lack of any ocular inflammation as a consequence of IL-1Ra deficiency (figure 2D, E).
Figure 2.
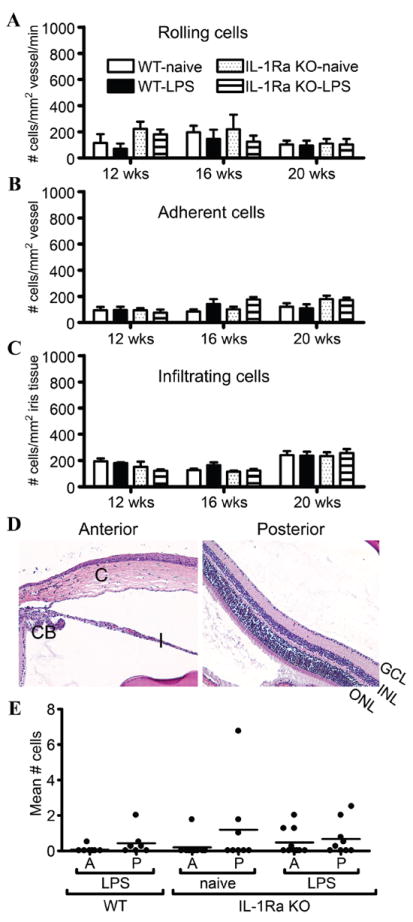
Interleukin 1 receptor antagonist (IL-1Ra) deficiency does not predispose to spontaneous uveitis. The onset and severity of uveitis was monitored over time in wild-type (WT) and IL-1Ra knockout (KO) mice with and without an intraperitoneal injection of 25 μg LPS at 7 weeks as in figure 1. The leucocyte trafficking response in the iris was assessed by intravital microscopy, and the numbers of rolling (A), adherent (B) and infiltrating (C) cells were quantified (n=14–17 mice/treatment/genotype/time). Results shown are combined from two independent experiments. (D) shows representative histological images of the naïve eyes of IL-1Ra KO mice at 20 weeks of age, and (E) displays the corresponding numbers of cells present in the aqueous humour (A) and vitreous body (P). CB, ciliary body; C, cornea; I, iris; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Original magnifi cation: ×200.
TLR4 is required for the spontaneous onset of murine arthritis in the absence of IL-1Ra, and exposure to a small dose of endotoxin (LPS) has long-lasting effects on arthritis severity,28,30,31 suggesting a gene–environment interaction in the pathogenesis. The data in figure 1 show that a low-dose exposure to LPS at 7 weeks of age significantly exacerbates the inflammation present several weeks later in the ankle and to a lesser extent in the knee. However, no comparable effect was observed in the eyes (figure 2). Intravital microscopy showed no differences in the numbers of rolling, adhering or sticking cells due to intraperitoneal LPS injection (figure 2A–C). Histological examination corroborated the intravital data at 12 and 16 weeks (data not shown) and at 20 weeks (shown in figure 2D,E); there was no significant cellular presence in the aqueous humour of the anterior segment or the vitreous of the posterior segment. These IL-1Ra KO eyes appeared phenotypically normal.
Investigation of the eye’s responsiveness to IL-1β
The absence of uveitis in the context of possible excessive IL-1 signalling due to the lack of IL-1Ra was somewhat surprising. However, the immunosuppressive environment in the eye may diminish the intraocular response to IL-1β. We tested the sensitivity of the eye to locally administered IL-1β. WT mice given intraocular injections of relatively low doses of recombinant IL-1β showed a rapid cellular response, as indicated by the significant increase in the numbers of rolling and adherent cells in the iris microvasculature and of infiltrating cells in the iris tissue (figure 3A). These findings indicate that the iris is responsive to exogenous IL-1β in vivo.
Figure 3.
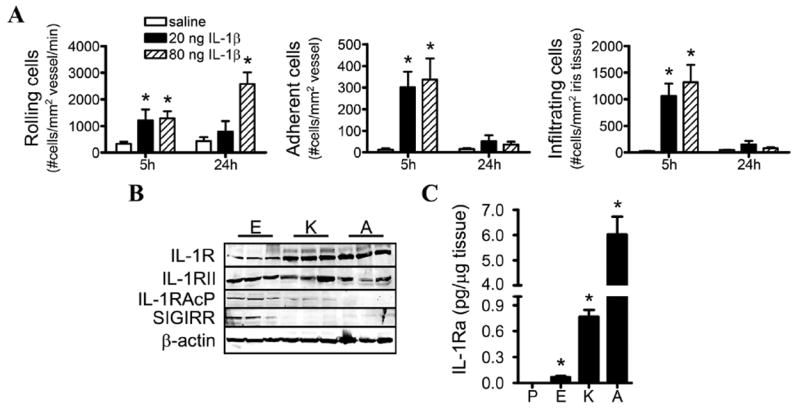
Investigation of the eye’s responsiveness to interleukin (IL)-1β. (A) Wild-type (WT) mice were administered an intraocular injection of the indicated concentrations of recombinant IL-1β or saline, and the cellular inflammatory responses in the iris were assessed by intravital microscopy. The numbers of rolling, adhering and infiltrating cells were quantified (*p<0.05 for comparison between saline and IL-1β injections; n=8–10 mice/treatment/time). (B) Immunoblotting of tissue homogenates from naïve, WT mice comparing expression levels of IL-1R and the indicated negative regulators of IL-1 across the three tissues. Shown are immunoblots of three mice; a total of nine mice were examined. (C) The concentrations of IL-1 receptor antagonist (IL-1Ra) in naïve mice were measured by ELISA. *p<0.05 for comparison with plasma concentrations. P, plasma; E, eye; K, knee; A, ankle. IL-1RAcP, IL-1 receptor accessory protein; SIGIRR, single Ig IL-1R-related molecule.
The inflammatory actions of IL-1 depend on its interaction with its receptor, IL-1R, and this can be negatively controlled by several endogenous inhibitors such as IL-1Ra, membrane-bound IL-1R type II (IL-1RII), SIGIRR and the soluble form of IL-1 receptor accessory protein (IL-1RAcP).10 These molecules act to avert excessive inflammatory responses by interfering with IL-1R activation. We asked whether expression of any of these negative regulators differed in eyes compared with ankles and knees (figure 3B). Expression of IL-1R and IL-1RII appeared to be constitutively expressed to a comparable extent across all organs, albeit IL-1R expression appears slightly less within eyes. Baseline expression of IL-1RAcP and SIGIRR was greater in the eyes than the knee or ankle. Interestingly, the opposite difference was observed for that of IL-1Ra (figure 3C), for which baseline concentrations were the greatest in the ankle joint compared with the knee joint or eyes and directly correlate with disease susceptibility in each tissue.
IL-1R signalling does not play a critical role in acute inflammatory uveitis induced by locally administered LPS
In contrast with systemic LPS, the mouse eye is extremely responsive to locally administered LPS. Intraocular injection of a low dose of LPS triggers an acute inflammatory uveitis, which has been well described and is historically referred to as endotoxin-induced uveitis (EIU).32 Consistent with previous reports,27 mouse eyes with EIU have elevated concentrations of the IL-1R ligand, IL-1β, and we further demonstrate elevated concentrations of a second IL-1R agonist, IL-1α (figure 4A). Using IL-1R KO mice, we tested whether a lack of IL-1 signalling impairs EIU. Based on intravital microscopy of leucocytes (figure 4B) and quantification of the number of cells present in the aqueous humour and vitreous body (figure 4C, D), we concluded that deficiency in IL-1R had no significant impact on the onset or severity of inflammation in the iris tissue. No other pathological differences were noted. This observation indicates that IL-1 signalling is not an essential mediator of ocular responses to locally administered LPS.
Figure 4.
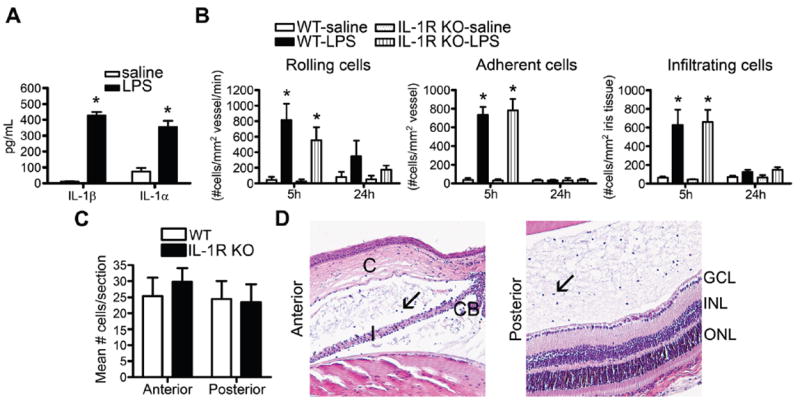
Interleukin 1 receptor (IL-1R) deficiency does not alter the severity of endotoxin-induced uveitis (EIU). (A) IL-1β and IL-1α concentrations in eye homogenates of wild-type (WT) mice were analysed by ELISA to compare responses to intraocular injection of 250 ng LPS vs saline 5 h after injection. WT and IL-1R knockout (KO) mice were administered an intraocular injection of 250 ng LPS or saline, and the severity of uveitis was assessed by intravital microscopy as a function of time (B) or histologically (C, D) at 24 h after LPS injection. (D) depicts images of the anterior and posterior eye segments of IL-1R KO mice. Arrows indicate leucocytes in the aqueous humour of the anterior chamber or the vitreous body of the posterior chamber. CB, ciliary body; C, cornea; I, iris; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. *p<0.05 for comparison between LPS and saline (n=14–17 mice/treatment/genotype). No significant difference among genotypes was observed. Results are combined data from two independent experiments.
Expression of IL-1R and IL-1 negative regulators during EIU
We examined the expression of IL-1R and potential IL-1 negative regulators in the eyes of mice challenged with intraocular LPS. Expression of IL-1R, IL-1RII, IL-1AcP and SIGIRR remained at baseline concentrations during EIU (figure 5A). We found that concentrations of IL-1Ra significantly increased over twofold (figure 5B).
Figure 5.
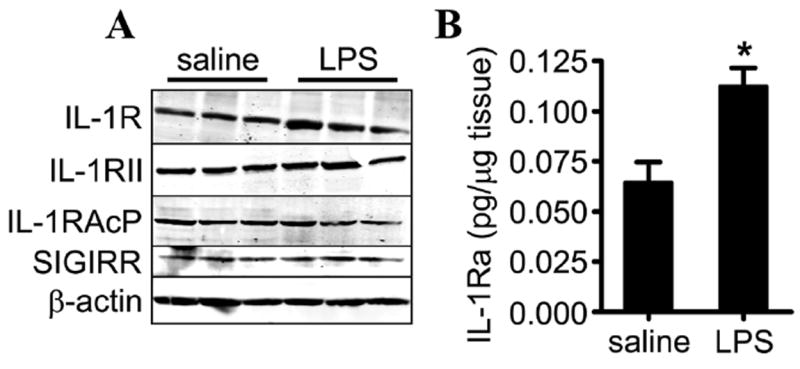
Expression of interleukin 1 receptor (IL-1R) and its negative regulators during endotoxin-induced uveitis. (A) depicts the protein expression of IL-1R and the indicated negative regulators of IL-1 signalling in eye tissue in response to intraocular injection of 250 ng LPS versus saline at 5 h after injection. Shown are three individual wild-type (WT) mice (a total of nine individual mice were examined). (B) IL-1Ra concentrations in eye homogenates of WT mice were analysed by ELISA to compare responses to intraocular injection of 250 ng LPS vs saline at 5 h after injection. *p<0.05 for comparison between LPS and saline (n=8 mice/treatment).
IL-1Ra deficiency renders mice more susceptible to locally administered LPS
IL-1Ra is considered to be an important immunosuppressive factor in the eye and may block the action of IL-1β secreted during EIU. Consistent with this postulate, our previous report27 and the data in figure 3C show that the concentration of IL-1Ra in the eye at baseline is about fivefold greater than in plasma and, as noted above, is increased during EIU (figure 5B). To investigate the role of IL-1Ra further, we compared EIU in IL-1Ra KO and WT mice.
Intravital videomicroscopy of iris revealed interesting differences in IL-1Ra KO and WT mice injected with saline or LPS. At the 5 h time point, saline injection activated a stronger cell trafficking response in the IL-RA KO eyes than in the WT eyes (figure 6A). The number of rolling leucocytes was comparable to that seen in LPS-injected eyes of WT mice, and the numbers of adhering cells were also significantly elevated. The saline response was not sustained and no longer evident at 24 h. At 5 h after LPS injection, the IL-1Ra KO and WT irises had similar numbers of rolling and adhering leucocytes, but the IL-1Ra KO mice had more infiltrating cells. The number of rolling cells was sustained for 24 h in only the IL-1Ra KO mice. In both genotypes, concentrations of adhering and infiltrating cells were approaching baseline values at this time.
Figure 6.
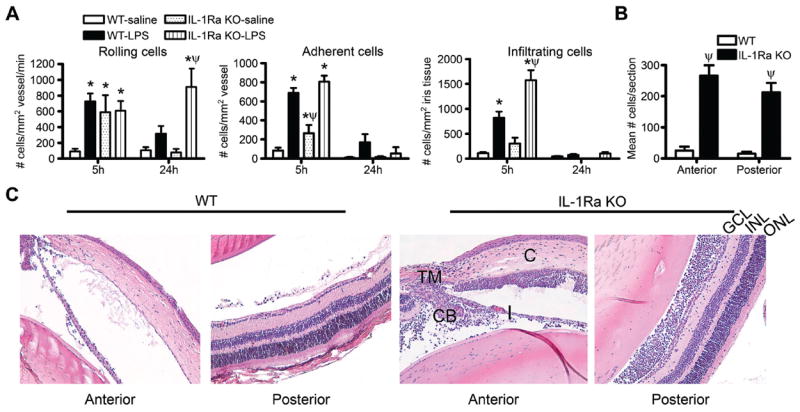
Interleukin 1 receptor antagonist (IL-1Ra) deficiency renders mice more susceptible to locally administered lipopolysaccharide (LPS). Wild-type (WT) or IL-1Ra knockout (KO) mice were administered an intraocular injection of 250 ng LPS or saline, and the cellular inflammatory response in the iris was assessed by intravital microscopy. The numbers of rolling versus adherent versus infiltrating cells were quantified as a function of time (A; *p<0.05 for comparison between LPS responses vs saline within a genotype; ψp<0.05 for comparison between LPS response in WT vs IL-1Ra KO). The extent to which IL-1Ra regulates uveitis was assessed histologically at 24 h after injection, and the numbers of infiltrating leucocytes in the anterior or posterior eye segments after LPS injection was quantifi ed (B; ψp<0.05 for comparison between LPS response in WT vs IL-1Ra KO; both WT and IL-1Ra KO mice showed significant responses over saline-injection controls). (C) shows representative histological images of WT versus IL-1Ra KO eyes at 24 h after LPS injection. C, cornea; I, iris; TM, trabecular meshwork; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. n=10–16 mice/genotype/treatment/time. Results are combined data from two independently performed experiments.
Marked quantitative differences in the ocular inflammatory response were observed histologically (figure 6B, C) in that the cellular influx into both the aqueous and vitreous humour was exacerbated in IL-1Ra KO mice, resulting in ~10-fold increase in the mean number of infiltrating cells per section in IL-1Ra KO mice compared with WT mice at 24 h after LPS injection (figure 6B). In the IL-1Ra KO mice, a massive cellular infiltrate was observed in the aqueous humour, most notably in the anterior chamber around the iris (I) and ciliary body (CB), and a cellular infiltrate was present in the trabecular meshwork (TM) and cornea (C) as well (depicted in figure 6C). Typically the posterior segment of the eye is minimally affected by locally administered LPS; yet in the absence of IL-1Ra, a massive cellular infiltrate is observed in the vitreous body and there is some cellular infiltrate in the retinal ganglion cell layer (GCL) and inner plexiform layer. Examination 72 h after LPS injection in IL-1Ra KO mice showed no significant difference in leucocyte rolling, adherence or infiltration in the iris (not shown) compared with saline-injected or WT controls. Histological examination at 72 h after LPS injection demonstrated abundant cells in the posterior segment (32.9±9.2) of IL-1Ra KO mice, whereas WT mice had few cells remaining (4.3±1.0). Moreover, we noted sustained tissue damage to the stroma of the cornea and vacuolisation of the iris in the IL-1Ra KO mice at this point in time. These findings support the notion of a critical immunosuppressive role for IL-1Ra in the eye.
DISCUSSION
Uveitis is a common extra-articular manifestation of several multisystem inflammatory diseases involving the joints. IL-1 is one of the best studied cytokines in arthritis, yet, in comparison, its potential to influence uveitis is poorly understood. Given the propensity of mice lacking IL-1Ra to exhibit features of systemic inflammation and spontaneous onset of arthritis, we tested the hypothesis that lack of IL-1Ra predisposes to uveitis. We found that the eye is less prone than joints to adverse effects from the loss of the IL-1Ra, and this observation is not altered by systemic LPS exposure. Interestingly, while deletion of IL-1R had no impact on EIU, IL-1Ra deficiency had a profound effect on the severity of EIU. This is consistent with the hypothesis that IL-1Ra has a critical regulatory role in preventing excessive IL-1 signalling in ocular inflammatory responses.
One contributing factor to the development of spontaneous arthritis in IL-1Ra KO mice is TLR4 activation by microbial flora.28 We verified previous reports28 that augmentation of TLR4 activation in IL-1Ra KO mice by a low dose of LPS at 7 weeks of age leads to exacerbated arthritis several weeks later. The eyes, however, remained unaffected despite the exacerbated arthritis. These observations are similar to the characteristics of patients with deficiency of the interleukin-1 receptor antagonist (DIRA), which is an autosomal recessive disease caused by an IL-1RN deletion mutation,33,34 which manifests as inflammation of the skin and joints. We are not aware of any reported eye involvement in these patients. The extent to which systemic inflammatory diseases affect the eye varies, and discordant mechanisms of disease in the eye versus joints have presented therapeutic challenges. For example, the tumour necrosis factor α (TNFα) inhibitor, etanercept, is used effectively for treating the arthritic symptoms in patients with ankylosing spondylitis, but it does not always improve uveitis in the same patients35 and will sometimes worsen uveitis.36 Although the presence of uveitis in several autoinflammatory diseases manifesting as arthritis suggests activation of common pathogenic mediators, such as IL-1β, in the eye and joint, our data would indicate that organ-specific responses must be considered.
The immunosuppressive milieu of the aqueous humour is thought to influence uveitogenesis37 by contributing to the immune privileged state of the eye.38 Resistance to the actions of IL-1β may be an underlying protective mechanism in the eye, and there are several possible mechanisms for regulating IL-1β actions. We demonstrated that IL-1R is expressed in eyes at concentrations comparable to susceptible joints and that it is functional because uveitis can be induced with exogenous IL-1β. Furthermore, the concentrations of IL-1β and IL-1α were increased in eyes with EIU. Given the presence of the receptor and the increased agonist concentrations, the finding that IL-1R deficiency did not significantly affect the severity of uveitis was somewhat surprising, but not completely unexpected. This observation is consistent with previous reports of EIU in rabbits,39 but is not in complete agreement with a report demonstrating that IL-1Ra overexpression in mouse eyes infected with a lentiviral expression vector suppresses EIU.40 Methods for the latter report differed substantially from ours; those authors used four times the amount of LPS and performed multiple LPS injections, suggesting their observation may be dependent on the high doses of LPS. Other cytokines, such TNFα and IL-8, may have somewhat redundant roles to IL-1 in EIU41,42 and reduce potential dependency on IL-1. Another aspect of IL-1 regulation is the production of negative regulators. We did not detect remarkably different expression of IL-1RII among the tissues. Of note, however, is that expression of the receptor variants, soluble IL-1RAcP and SIGIRR, which impede the activities of IL-1, was greater in the eye than in the knee or ankles and may contribute to the anti-inflammatory milieu controlling IL-1-mediated responses in the eye. Future investigation into how the actions of IL-1β may be inhibited by IL-1RAcP, SIGIRR or additional IL-1 family members such as IL-3743 will be important to our understanding of uveitis.
The final regulator we considered is the competitive inhibitor, IL-1Ra.44 Our data suggest that IL-1Ra successfully blocks the activity of IL-1 present in eyes during acute EIU, as mice deficient in IL-1Ra were extremely sensitive to local administration of LPS. We also noticed that IL-1Ra KO mice have an enhanced leucocyte trafficking response to intravitreally administered saline, which may reflect lack of inhibition of IL-1β released because of the injection injury. Collectively, these results underscore the importance of IL-1Ra in modulating ocular inflammation. Interestingly, differences in normal IL-1Ra concentrations among eyes, knees and ankles are inversely related to spontaneous disease predisposition in the IL-1Ra KO mice. Ankles, which had the highest basal concentrations of IL-1Ra, were the most inflamed in the IL-1Ra KO mice, whereas eyes, which had the lowest basal concentration, did not develop spontaneous inflammation. Perhaps ankles depend on IL-1Ra because they make more IL-1β, whereas eyes have less IL-1Ra because they make relatively low amounts of IL-1β and also have more IL-1RAcP and SIGIRR as discussed above. One caveat is that direct comparison of negative regulators and IL-1Ra concentrations among the organs is somewhat limited by variations in tissue composition and by the fact that we did not directly compare IL-1Ra concentrations in aqueous humour and synovial fluid because both fluids are extremely scant in the mouse. Nonetheless, the functional studies clearly support protective roles for IL-1Ra in arthritis and uveitis. Our results indicate that the actions of IL-1 appear to have a non-essential role in EIU because deficiency in IL-1R did not reduce inflammation. We would like to emphasise that the converse, however, is not true. The importance of proper regulation of IL-1 signalling is clearly evidenced by the severely exacerbated EIU that occurs in IL-1Ra KO mice when the effects of IL-1 are amplified. The latter would presumably relate to diseases such as Behçet’s wherein dysregulation of IL-1 is implicated. The efficacy of anti-IL-1 therapy of uveitis in patients with arthritis should be informative regarding pathogenesis of particular diseases. Interestingly, a preliminary trial suggests marked benefit of anti-IL-1 therapy in treatment of uveitis in patients with Behçet’s disease.1,2
Despite a strong link between uveitis and several arthritic diseases, few studies have attempted to explain the common coexistence of inflammation in the two organ systems. Here we sought to elucidate how dysregulation of the actions of a single cytokine (ie, IL-1β) affects experimental models of uveitis and arthritis. Our findings underscore the complexity of the determinants that influence the eye’s susceptibility to uveitis and show differences not only between the eye and joints but between the knee and the ankle. Collectively, the data indicate the importance of proper regulation of the actions of IL-1 in the eye, as worsened uveitis ensues in the absence of IL-1Ra.
Acknowledgments
This work was funded by NIH grants EY019020, EY010572 and EY019604. We are grateful for support from the Stan and Madelle Rosenfeld Family Trust, the William and Mary Bauman Foundation, and the Research to Prevent Blindness Foundation (to HLR and the CEI). Funding for this project was also provided by American College of Rheumatology Research and Education Foundation Health Professional New Investigator Award (to HLR).
Footnotes
Provenance and peer review Not commissioned; externally peer reviewed.
Competing interests None.
References
- 1.Deal watch. XOMA and Servier to develop anti-IL-1β antibody for inflammatory diseases. Nat Rev Drug Discov. 2011;10:166. doi: 10.1038/nrd3390. [DOI] [PubMed] [Google Scholar]
- 2.Gul A, Artim-Esen B, Solinger AM, et al. Safe, rapid-onset, and sustained biological activity of IL-1 regulating antibody XOMA 052 in resistant uveitis of Behcet’s idsease: results of a pilot trial. Arth Rheum. 2010;62:1308. [Google Scholar]
- 3.Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10:263–79. doi: 10.1076/ocii.10.4.263.15592. [DOI] [PubMed] [Google Scholar]
- 4.Reveille JD. The genetic basis of spondyloarthritis. Ann Rheum Dis. 2011;70:i44–50. doi: 10.1136/ard.2010.140574. [DOI] [PubMed] [Google Scholar]
- 5.Ali A, Samson CM. Seronegative spondyloarthropathies and the eye. Curr Opin Ophthalmol. 2007;18:476–80. doi: 10.1097/ICU.0b013e3282f0fda2. [DOI] [PubMed] [Google Scholar]
- 6.Goldbach-Mansky R, Kastner DL. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124:1141–9. doi: 10.1016/j.jaci.2009.11.016. quiz 1150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masters SL, Simon A, Aksentijevich I, et al. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annu Rev Immunol. 2009;27:621–68. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson C, Goldbach-Mansky R. Monogenic autoinflammatory diseases: new insights into clinical aspects and pathogenesis. Curr Opin Rheumatol. 2010;22:567–78. doi: 10.1097/BOR.0b013e32833ceff4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGarry F, Neilly J, Anderson N, et al. A polymorphism within the interleukin 1 receptor antagonist (IL-1Ra) gene is associated with ankylosing spondylitis. Rheumatology (Oxford) 2001;40:1359–64. doi: 10.1093/rheumatology/40.12.1359. [DOI] [PubMed] [Google Scholar]
- 10.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–41. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djouadi K, Nedelec B, Tamouza R, et al. Interleukin 1 gene cluster polymorphisms in multiplex families with spondylarthropathies. Cytokine. 2001;13:98–103. doi: 10.1006/cyto.2000.0795. [DOI] [PubMed] [Google Scholar]
- 13.Maksymowych WP, Reeve JP, Reveille JD, et al. High-throughput single-nucleotide polymorphism analysis of the IL1RN locus in patients with ankylosing spondylitis by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Arthritis Rheum. 2003;48:2011–18. doi: 10.1002/art.11037. [DOI] [PubMed] [Google Scholar]
- 14.Hutyrová B, Pantelidis P, Drábek J, et al. Interleukin-1 gene cluster polymorphisms in sarcoidosis and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;165:148–51. doi: 10.1164/ajrccm.165.2.2106004. [DOI] [PubMed] [Google Scholar]
- 15.Coskun M, Bacanli A, Sallakci N, et al. Specific interleukin-1 gene polymorphisms in Turkish patients with Behçet’s disease. Exp Dermatol. 2005;14:124–9. doi: 10.1111/j.0906-6705.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 16.Karasneh J, Hajeer AH, Barrett J, et al. Association of specific interleukin 1 gene cluster polymorphisms with increased susceptibility for Behcet’s disease. Rheumatology (Oxford) 2003;42:860–4. doi: 10.1093/rheumatology/keg232R. [DOI] [PubMed] [Google Scholar]
- 17.Ferraccioli G, Bracci-Laudiero L, Alivernini S, et al. Interleukin-1ß and interleukin-6 in arthritis animal models: roles in the early phase of transition from acute to chronic inflammation and relevance for human rheumatoid arthritis. Mol Med. 2010;16:552–7. doi: 10.2119/molmed.2010.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicklin MJ, Hughes DE, Barton JL, et al. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horai R, Saijo S, Tanioka H, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–20. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepherd J, Little MC, Nicklin MJ. Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. J Invest Dermatol. 2004;122:665–9. doi: 10.1111/j.0022-202X.2004.22305.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima A, Matsuki T, Komine M, et al. TNF, but not IL-6 and IL-17, is crucial for the development of T cell-independent psoriasis-like dermatitis in Il1rn-/- mice. J Immunol. 2010;185:1887–93. doi: 10.4049/jimmunol.1001227. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd J, Nicklin MJ. Elastic-vessel arteritis in interleukin-1 receptor antagonist-deficient mice involves effector Th1 cells and requires interleukin-1 receptor. Circulation. 2005;111:3135–40. doi: 10.1161/CIRCULATIONAHA.104.519132. [DOI] [PubMed] [Google Scholar]
- 23.Becker MD, Nobiling R, Planck SR, et al. Digital video-imaging of leukocyte migration in the iris: intravital microscopy in a physiological model during the onset of endotoxin-induced uveitis. J Immunol Methods. 2000;240:23–37. doi: 10.1016/s0022-1759(00)00165-4. [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig HL, Martin TM, Jann MM, et al. NOD2, the gene responsible for familial granulomatous uveitis, in a mouse model of uveitis. Invest Ophthalmol Vis Sci. 2008;49:1518–24. doi: 10.1167/iovs.07-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kezic J, Taylor S, Gupta S, et al. Endotoxin-induced uveitis is primarily dependent on radiation-resistant cells and on MyD88 but not TRIF. J Leukoc Biol. 2011;90:305–11. doi: 10.1189/jlb.0111036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenzweig HL, Jann MJ, Vance EE, et al. Nucleotide-binding oligomerization domain 2 and Toll-like receptor 2 function independently in a murine model of arthritis triggered by intraarticular peptidoglycan. Arthritis Rheum. 2010;62:1051–9. doi: 10.1002/art.27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenzweig HL, Martin TM, Planck SR, et al. Activation of NOD2 in vivo induces IL-1beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J Leukoc Biol. 2008;84:529–36. doi: 10.1189/jlb.0108015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–16. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenzweig HL, Clowers JS, Nunez G, et al. Dectin-1 and NOD2 mediate cathepsin activation in zymosan-induced arthritis in mice. Inflamm Res. 2011;60:705–14. doi: 10.1007/s00011-011-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56:2957–67. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 31.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, et al. Local interleukin-1-driven joint pathology is dependent on toll-like receptor 4 activation. Am J Pathol. 2009;175:2004–13. doi: 10.2353/ajpath.2009.090262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum JT, McDevitt HO, Guss RB, et al. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–13. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 33.Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–37. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438–44. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JR, Levinson RD, Holland GN, et al. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum. 2001;45:252–7. doi: 10.1002/1529-0131(200106)45:3<252::AID-ART257>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? A registry-based study. Arthritis Rheum. 2007;56:3248–52. doi: 10.1002/art.22918. [DOI] [PubMed] [Google Scholar]
- 37.Verma MJ, Lloyd A, Rager H, et al. Chemokines in acute anterior uveitis. Curr Eye Res. 1997;16:1202–8. doi: 10.1076/ceyr.16.12.1202.5034. [DOI] [PubMed] [Google Scholar]
- 38.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–30. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 39.Rosenbaum JT, Boney RS. Activity of an interleukin 1 receptor antagonist in rabbit models of uveitis. Arch Ophthalmol. 1992;110:547–9. doi: 10.1001/archopht.1992.01080160125049. [DOI] [PubMed] [Google Scholar]
- 40.Trittibach P, Barker SE, Broderick CA, et al. Lentiviral-vector-mediated expression of murine IL-1 receptor antagonist or IL-10 reduces the severity of endotoxin-induced uveitis. Gene Ther. 2008;15:1478–88. doi: 10.1038/gt.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith JR, Hart PH, Coster DJ, et al. Mice deficient in tumor necrosis factor receptors p55 and p75, interleukin-4, or inducible nitric oxide synthase are susceptible to endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1998;39:658–61. [PubMed] [Google Scholar]
- 42.Brito BE, O’Rourke LM, Pan Y, et al. Murine endotoxin-induced uveitis, but not immune complex-induced uveitis, is dependent on the IL-8 receptor homolog. Curr Eye Res. 1999;19:76–85. doi: 10.1076/ceyr.19.1.76.5339. [DOI] [PubMed] [Google Scholar]
- 43.Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–22. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–40. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]


