One of the principal priorities identified at the NCI summit for improving management of neuroendocrine tumors (NETs)[pancreatic endocrine tumors and carcinoids) 1 was the need to identify tumor markers that could be used for early diagnosis and management. All clinicians who manage these patients would agree with this 2. These are needed because the majority of NETs (especially early in their course) are nonfunctional 3 and they are usually discovered by endoscopic/imaging methods. Furthermore, their natural history is frequently long; they vary markedly in their aggressiveness and in their responses to various treatments, with the result that repeated imaging is required 1, 2. This is not only expensive, it is frequently inconvenient, and can in many cases, results in late diagnosis 1, 2. The ideal would be to have a tumor marker that could be easily assessed (serum/urine); that was sensitive and specific for NETs and whose magnitude correlated with the extent and rate of tumor growth, so that it could be used for both diagnosis and management. In this issue of Pancreas the specificity of a newly described serum assay for the CgA fragment, pancreastatin is reported by Raines et al 4 , that might be a useful candidate for fulfilling some of these requirements for the desired NET tumor marker. However, to understand why this could be an important step forward, it is important to summarize where we are at present.
Unfortunately at present, no current assay fulfills the above requirements for a generally useful tumor marker in patients with the majority of NETs. For the functional pancreatic endocrine tumors [PETs] (gastrinomas, insulinomas, VIPomas, etc), assessment of the specific hormone or related fragments allows diagnosis when coupled with the appropriate assessment of hormone excess (acid secretion, hypoglycemia, etc) and in some cases, their magnitude or changes in magnitude of their serum levels correlate with tumor growth/extent 5. Similarly, with carcinoid syndrome, associated in >95% of cases with metastatic disease in the liver, the assessment of serum serotonin/urinary breakdown down products (5-HIAA) allows diagnosis and has prognostic significance. However, in various series 50-75% of all PETs are not associated with a functional hormonal syndrome (i.e. nonfunctional PETs), as is the case in all early carcinoids and most advanced carcinoid disease that is non-midgut in location. Therefore, for up to 60-95% of NET patients in various series, no hormonal marker exists for any disease phase.
Chromogranins (Cg), particularly chromogranin A (CgA), is found in the neurosecretory granules of almost all well-differentiated NETs, whether functional or nonfunctional; is commonly used immunocytochemically to establish their diagnosis and circulates in nM concentrations in the serum, thus was originally hoped would fulfill all the needed requirements for a universal serum NET tumor marker 6-8. Unfortunately it has failed to fulfill this promise for a number of reasons. There is no standardization of CgA assays resulting in divergent values in different assays and many of the assays are not fully characterized, so it is unclear exactly what is being measured. CgA is a large peptide (MW-48,918) consisting of 439 amino acids and has an apparent molecular weight of 74,001 in tissues, because of glycosylation and other modifications (Fig. 1) 9. It has 10 dibasic cleavage amino sites 9 (Fig. 1), which results in the generation of a number of different fragments, both in the NET and serum (Fig. 1)6-10. A number of these fragments are reported to have biological activities such as inhibitory effects on secretion of various hormones (insulin, parathyroid hormone, catecholamine secretion); effects on intermediary metabolism and carbohydrate, lipid metabolism; regulation of cardiovascular function and regulation of inflammatory responses and reproduction 6, 7. If assay antibodies interact with varying degrees with the different CgA degradation products, then the assessment of serum concentrations between different assays becomes affected by many variables including the fact that different tumors may degrade CgA differently and can contribute to the non-uniformity of the results with different assays. Furthermore, it is further complicated by the fact that no one completely agrees on which of these products best reflects the needed diagnostic and management features of a good serum tumor marker. The full characterization of the antibody's specificity, even if the exact molecule needed to be measured is agreed on, is not a trivial consideration. This point is clearly demonstrated in a recent paper on the assessment of serum gastrin concentration, which is a widely used RIA in medicine. Numerous NH2 and COOH terminal extended gastrins circulate which are sulfated and non-sulfated. While there is general agreement that assessment of the biologically active amidated COOH terminus is clinically important, a recent study demonstrates 11 that 7 of 12 commercially available assays do not accurately measure the clinically important gastrin, because they are using not fully characterized antibodies. Therefore even if agreement is reached on the CgA molecule to be measured good characterization and standardization are need.
Figure 1.
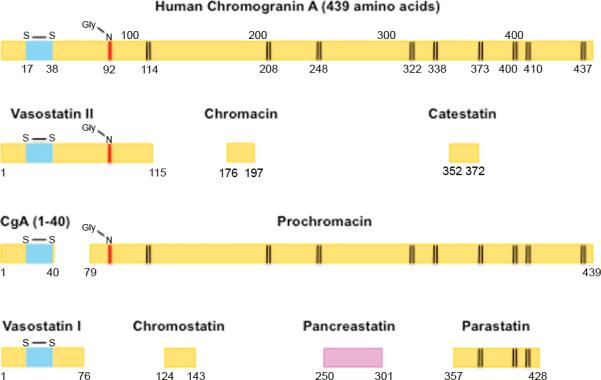
Schematic representation of Chromogranin A and its postulated biologically active sequences with the sites of proteolytic cleavage at dibasic amino acids indicated (vertical lines). Abbreviations. Gly-N refers to the putative N-Linked glycosylation site; S-S-indicates the sites of a disulfide linkage. The numbers below each sequence refer to the location in chromogranin A.
There is disagreement in the literature whether the serum CgA levels correlate with the extent of tumor or growth of the tumor and are useful for the management of NETs 5, 6, 12, 13. Furthermore, serum CgA levels are frequently decreased by the use of somatostatin analogues which are commonly used in treatment of functional and nonfunctional NETs either alone or in combination with other drugs, and it is unclear whether their effect is variable, making it difficult to perform assessments when these commonly used drugs are administered. Assessment of CgA levels has also failed as a tumor marker for the early detection of NETs. This has primarily occurred because CgA levels are affected by not only the NET, but numerous other processes including inflammatory disorders, other endocrine diseases, GI disorders, cardiovascular diseases, renal function and particularly important, the use of potent acid suppressant drugs such as proton pump inhibitors (PPIs) 6, 14. PPIs are a particular problem because they increase serum CgA in 90-100% of patients with protracted use; they are widely used and available now over-the-counter so their use is often not even mentioned in the medical history; they increase serum CgA after only 5 days of use and they can lead to CgA levels 5-10 fold normal, which overlap with that seen in many patients with early NETs 15.
It is in this latter context that the study of Raines 4 reporting results of a recently described specific RIA for pancreastatin 12 in patients taking PPIs, is of particular interest. Human pancreastatin is a 52 amino acid peptide corresponding to CgA (250-301)9 (Fig. 1) and its generation from CgA depends to a large part on the activity of prohormone convertase-1 16. Pancreastatin is present in NETs 17, 18, is present in pM concentrations in normal serum 12, 19 primarily as the CgA (250-301) form and in higher molecular weight forms 19. The results of Raines study 4 are particularly important because they show no increase in serum pancreastatin levels in patients chronically taking PPIs, whereas serum gastrin is increased in all and serum CgA levels in almost 70% 4. These results are of particular interest because they raise the possibility that serum pancreastatin could be a more sensitive and specific assay than CgA in detecting early NETs as well as nonfunctional NETs and possibly useful for management. Is there any evidence to support these speculations? The principal finding of this study is supported by results of a early study by Syversen 20 using a specific pancreastatin assay in patients with gastrinomas, where they found serum pancreastatin levels correlated closely with gastrin levels, but not with serum CgA levels, leading them to propose the there is little or no processing of CgA to pancreastatin in ECL cells, while in gastrinoma cells it is extensive. Will this assay be sensitive enough to be useful? That remains to be proven. In one study 20 using a different antibody from that used in Raines study 4, serum pancreastatin levels were normal in 44% of patients with gastrinomas 20 and in another study in 27% of patients with NETs and 100% of patients with NETs with only lymph node metastases 21. Will it be specific enough for NETs? At present this is also not proven.
Hopefully, lessons will be learned from the experience with CgA and these questions will be answered rapidly. Particularly important will be in all assays for serum pancreastatin the complete characterization of all antibodies generated for cross-reactivity with the various CgA products that could react within the assay (Fig. 1). Next, the inclusion of patients with a wide vary of conditions that lead to false positives, as seen in the CgA assay, needs to be specifically done, as well as a clear determination of sensitivity. Lastly, correlations with important clinical changes in tumor growth/size will need to be done. The present study by Raines 4 is important because their data suggest the new assay could be a significant advance.
Footnotes
Disclosure: We have no conflicts of interest.
References
- 1.Modlin IM, Moss SF, Chung DC, et al. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J Natl Cancer Inst. 2008;100:1282–1289. doi: 10.1093/jnci/djn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 3.Scherubl H, Jensen RT, Cadiot G, et al. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011;3:133–139. doi: 10.4253/wjge.v3.i7.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raines D, Chester M, Diebold A, et al. A prospective evaluation of the effect of chronic proton pump inhibitor use on plasma biomarker levels in humans. Pancreas. doi: 10.1097/MPA.0b013e318243a0b6. In press. [DOI] [PubMed] [Google Scholar]
- 5.Goebel SU, Serrano J, Yu F, et al. Prospective study of the value of serum chromogranin A or serum gastrin levels in assessment of the presence, extent, or growth of gastrinomas. Cancer. 1999;85:1470–1483. [PubMed] [Google Scholar]
- 6.Modlin IM, Gustafsson BI, Moss SF, et al. Chromogranin A-Biological Function and Clinical Utility in Neuro Endocrine Tumor Disease. Ann Surg Oncol. 2010;17:2427–43. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 7.Helle KB, Corti A, Metz-Boutigue MH, et al. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64:2863–2886. doi: 10.1007/s00018-007-7254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portela-Gomes GM, Grimelius L, Wilander E, et al. Granins and granin-related peptides in neuroendocrine tumours. Regul Pept. 2010;165:12–20. doi: 10.1016/j.regpep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Konecki DS, Benedum UM, Gerdes HH, et al. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987;262:17026–17030. [PubMed] [Google Scholar]
- 10.Tartaglia A, Portela-Gomes GM, Oberg K, et al. Chromogranin A in gastric neuroendocrine tumours: an immunohistochemical and biochemical study with region-specific antibodies. Virchows Arch. 2006;448:399–406. doi: 10.1007/s00428-005-0113-1. [DOI] [PubMed] [Google Scholar]
- 11.Rehfeld JF, Gingras MH, Bardram Lm, et al. The Zollinger-Ellison Syndrome and Mismeasurement of Gastrin. Gastroenterology. 2011;140:1444–1453. doi: 10.1053/j.gastro.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 12.O'Dorisio TM, Krutzik SR, Woltering EA, et al. Development of a highly sensitive and specific carboxy-terminal human pancreastatin assay to monitor neuroendocrine tumor behavior. Pancreas. 2010;39:611–616. doi: 10.1097/MPA.0b013e3181c68d7a. [DOI] [PubMed] [Google Scholar]
- 13.Arnold R, Wilke A, Rinke A, et al. Plasma chromogranin a as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol. 2008;6:820–827. doi: 10.1016/j.cgh.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 14.Jensen RT. Consequences of long-term proton pump blockade: Highlighting insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol. 2006;98:4–19. doi: 10.1111/j.1742-7843.2006.pto_378.x. [DOI] [PubMed] [Google Scholar]
- 15.Pregun I, Herszenyi L, Juhasz M, et al. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion. 2011;84:22–28. doi: 10.1159/000321535. [DOI] [PubMed] [Google Scholar]
- 16.Udupi V, Lee HM, Kurosky A, et al. Prohormone convertase-1 is essential for conversion of chromogranin A to pancreastatin. Regul Pept. 1999;83:123–127. doi: 10.1016/s0167-0115(99)00061-0. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt WE, Siegel EG, Kratzin H, et al. Isolation and primary structure of tumor-derived peptides related to human pancreastatin and chromogranin A. Proc Natl Acad Sci USA. 1988;85:8231–8235. doi: 10.1073/pnas.85.21.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura W, Jimi A, Miyasaka K, et al. Immunohistochemical study of the distribution of pancreastatin in endocrine tumors of the pancreas and in normal pancreatic tissue: analysis of autopsy cases. Pancreas. 1991;6:688–693. doi: 10.1097/00006676-199111000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Kitayama N, Tateishi K, Funakoshi A, et al. Pancreastatin molecular forms in normal human plasma. Life Sci. 1994;54:1571–1578. doi: 10.1016/0024-3205(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 20.Syversen U, Mignon M, Bonfils S, et al. Chromogranin A and pancreastatin-like immunoreactivity in serum of gastrinoma patients. Acta Oncol. 1993;32:161–165. doi: 10.3109/02841869309083906. [DOI] [PubMed] [Google Scholar]
- 21.Stronge RL, Turner GB, Johnston BT, et al. A rapid rise in circulating pancreastatin in response to somatostatin analogue therapy is associated with poor survival in patients with neuroendocrine tumours. Ann Clin Biochem. 2008;45:560–566. doi: 10.1258/acb.2008.008033. [DOI] [PubMed] [Google Scholar]


