Abstract
Objectives:
Duchenne muscular dystrophy (DMD) is a degenerative muscle wasting disease caused by mutations in the dystrophin gene. Dystrophic muscle is characterized by chronic inflammation, and inflammatory mediators could be promising targets for innovative therapeutic interventions. We analyzed muscle biopsy samples of DMD-affected children to characterize interleukin (IL)-17 and Forkhead box P3 (Foxp3) expression levels and to identify possible correlations with clinical status.
Methods:
Expression levels of IL-17, Foxp3, tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), IL-6, and transforming growth factor-β (TGF-β) were analyzed by real-time PCR in muscle biopsy samples from patients with DMD (n = 27) and juvenile dermatomyositis (JDM) (n = 8). Motor outcome of patients with DMD was evaluated by North Star Ambulatory Assessment score.
Results:
In DMD, we found higher levels of IL-17 and lower levels of Foxp3 mRNA compared with those for a typical inflammatory myopathy, JDM. Moreover, the IL-17/Foxp3 ratio was higher in DMD than in JDM biopsy samples. IL-17 mRNA levels appeared to be related to the expression levels of other proinflammatory cytokines (TNF-α and MCP-1) and significantly associated with clinical outcome of patients.
Conclusions:
The association of IL-17 expression with levels of other inflammatory cytokines and with the clinical course of DMD suggests a possible pathogenic role of IL-17.
Duchenne muscular dystrophy (DMD) is a degenerative muscle disease with a life expectancy of approximately 25 years.1,2 Dystrophic muscles show a prominent inflammatory infiltrate and, even before onset of symptoms, a generalized up-regulation of many inflammation-related genes is observed.1,3 Myofiber necrosis is the primary cause of inflammation in DMD muscles. However, chronic muscle inflammation itself is involved in the progressive loss of function contributing to muscle necrosis and atrophy and inhibiting regeneration. Glucocorticoids (GCs) are still the primary treatment for DMD, but it is well known that their long-term use causes severe side effects.1–2,4–6 Pivotal mediators of the chronic inflammation of DMD muscles may be promising therapeutic targets for more specific and effective anti-inflammatory therapies.
Interleukin (IL)-17 is involved in tissue chronic inflammation in several human autoimmune and inflammatory diseases and a vast body of evidence in animal models supports this pivotal role.7–13 Production of IL-17 characterizes proinflammatory T helper 17 lymphocytes (Th17) and innate immune cells.7,8 Th17 have been shown to have a reciprocal developmental pathway and opposing effects in the immune response from regulatory T cells (Treg),8,14,15, whose master gene is the transcription factor Forkhead box P3 (Foxp3).16–18 A fine balance between Th17 and Treg is believed to be crucial for tissue immune homeostasis.14
No information on the expression of Th17- or Treg-related genes in dystrophic muscle is available. In this study we characterized IL-17 and Foxp3 expression in muscle biopsy samples of patients with DMD and evaluated possible correlations with functional outcome.
METHODS
Patients.
Muscle biopsy samples at the time of diagnosis were obtained from 27 consecutive patients with DMD, who were referred to our center from 2001 to 2010. All patients had molecular genetic detection of a mutation in the DYS gene, predicting a marked dystrophin deficiency that we confirmed by immunofluorescence staining of muscle biopsy samples. Mean age at biopsy of patients with DMD was 4.1 years (SD 2.0 years). We also obtained muscle biopsy samples from patients with juvenile dermatomyositis (JDM) (n = 8) and from 2 healthy children. Mean age at biopsy of patients with JDM was 8.6 years (SD 3.9 years); mean age at biopsy of healthy children was 3.5 (SD 2.0 years). For patients with DMD and JDM, the time of biopsy coincided with the time of diagnosis. All patients were off therapy at the time of sampling. Muscle strength in DMD-affected children was assessed using the North Star Ambulatory Assessment (NSAA) functional scale. NSAA is one of the present gold standard functional tests to score DMD motor ability (from a value 34 for normal ambulation to a value 0 for the absence of ambulation).19 To evaluate the clinical outcome of this cohort, we chose to use the NSAA score obtained at the age of 6 years because at that age 1) all patients received the same GC treatment for 1 year and 2) although all of them were still ambulant, they could already present different degrees of muscle weakness.
We analyzed the correlation between the NSAA score at 6 years of age and muscle inflammatory parameters in 20 of 27 patients with DMD (2 patients were lost at follow-up, 1 patient had not yet reached 6 years of age at the time of the evaluation, and 4 patients were excluded because biopsy samples were obtained at age older than 5 years). In these 20 patients with DMD, the mean time lag between muscle biopsy and 6 years of age was 2.1 years. The NSAA score ranged from 12 to 34.
Standard protocol approvals and patient consents.
We obtained written parental informed consent for each patient. The use of the muscle biopsy for this study was approved by the local ethics committee.
RNA extraction, reverse transcription, and real-time PCR.
RNA extraction and reverse transcription.
Muscle biopsy samples were snap-frozen in liquid nitrogen and immediately stored at −80°C. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For each sample, 2.5 μg of total RNA was reverse-transcribed using a SuperScript Vilo kit (Invitrogen).
Real-time PCR.
All reagents were purchased from Applied Biosystems (Foster City, CA). The following gene expression assays were used in the real-time PCRs: Hs00174383_m1, IL-17; Hs00203958_1, Foxp3; Hs00174128_m1, tumor necrosis factor-α (TNF-α); Hs00234140_m1, monocyte chemoattractant protein-1 (MCP-1); Hs00998133_m1, transforming growth factor-β (TGF-β); and Hs00985639_m1, IL-6. All PCRs were run in duplicate on a 7700 ABI Prism instrument (Applied Biosystems). Gene expression data were normalized using human HPRT1 (Applied Biosystems, cat. no. 4326321E) as an endogenous control and analyzed using the comparative ΔCt method.
Statistical analysis.
We showed levels of cytokine expression as box-and-whiskers plots (median, interquartile range, and minimum and maximum values are shown). We performed group comparisons using the nonparametric Mann-Whitney U test. To assess the correlations between variables, we calculated the Spearman rank correlation coefficient (r). For both tests, we set significance levels at p < 0.05.
RESULTS
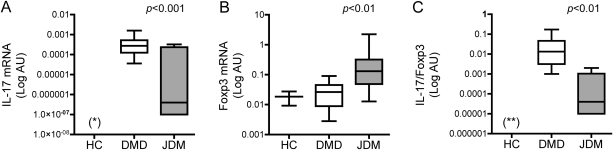
In control muscles (n = 2), IL-17 mRNA was undetectable, whereas we observed detectable levels of Foxp3 in both samples. In both DMD and JDM muscles, mRNA of IL-17 and Foxp3 were measurable, suggesting that IL-17 and Foxp3 are up-regulated in these conditions characterized by chronic inflammation albeit of different causes (figure 1). However, DMD muscles showed markedly higher levels of IL-17 mRNA and markedly lower levels of Foxp3 mRNA compared with JDM samples (figure 1, A and B). Because the balance between proinflammatory and anti-inflammatory pathways is believed to be crucial for tissue homeostasis, we calculated the IL-17/Foxp3 ratio for each patient. We found that the IL-17/Foxp3 ratio was markedly higher in DMD than in JDM biopsy samples (figure 1C).
Figure 1. Interleukin (IL)-17 and Forkhead box P3 (Foxp3) mRNA levels in Duchenne muscular dystrophy (DMD) and juvenile dermatomyositis (JDM).
(A) IL-17, (B) Foxp3 mRNA levels, and (C) IL-17/Foxp3 ratio in control muscle (HC n = 2), DMD (n = 27), and JDM (n = 8) muscles. Gene expression values are expressed in log scale of arbitrary units (AU). *Below the limit of detection; **<0.000001.
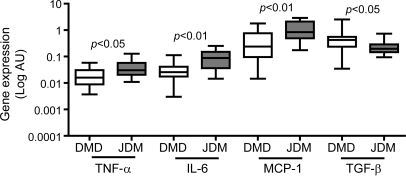
To obtain a quantitative measure of the inflammatory state in DMD, we evaluated expression levels of TNF-α, IL-6, and MCP-1, classic proinflammatory cytokines, and of the fibrogenic cytokine TGF-β. These are well known to be elevated in dystrophic muscle. In DMD muscles, mRNA levels of TNF-α, IL-6, and MCP-1 were lower compared with those in JDM, a classic autoimmune inflammatory muscle disease. Conversely, the expression of the fibrogenic cytokine TGF-β was higher in DMD than in JDM muscles, underscoring the intense tissue fibrosis that is characteristic of DMD (figure 2).
Figure 2. Tumor necrosis factor-α (TNF-α), interleukin (IL)-6, monocyte chemoattractant protein-1 (MCP-1), and transforming growth factor-β (TGF-β) mRNA levels in Duchenne muscular dystrophy (DMD) and juvenile dermatomyositis (JDM).
TNF-α, IL-6, MCP-1, and TGF-β mRNA levels in DMD (n = 27) and JDM (n = 8) muscle biopsy samples. Gene expression values are expressed in log scale of arbitrary units (AU).
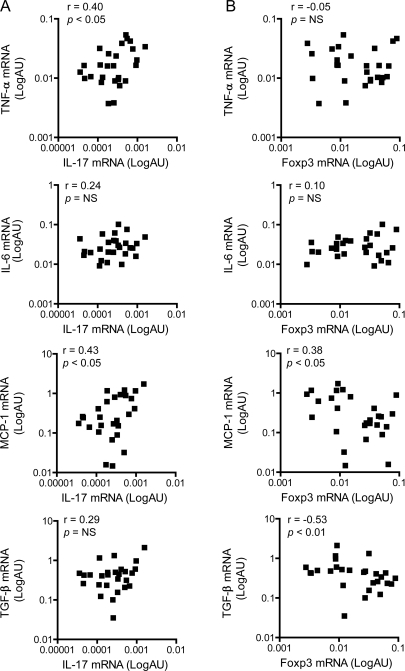
IL-17 is believed to be classically proinflammatory, whereas Foxp3 drives the differentiation and function of Treg and the subsequent development of anti-inflammatory mechanisms. To verify potential proinflammatory and anti-inflammatory roles of IL-17 and Foxp3 in DMD, we evaluated the correlation of IL-17 and Foxp3 levels with expression of TNF-α, IL-6, MCP-1, and TGF-β. In DMD muscles, high IL-17 mRNA levels were associated with high levels of the inflammatory cytokines TNF-α and MCP-1, whereas there was no association between IL-17 and IL-6 or TGF-β expression (figure 3A). Conversely, low Foxp3 mRNA levels were associated with high MCP-1 and TGF-β mRNA levels, whereas there was no association with the expression of IL-6 or TNF-α (figure 3B).
Figure 3. Cytokine correlations in Duchenne muscular dystrophy (DMD) muscle biopsy samples.
Correlations of (A) interleukin (IL)-17 and (B) Forkhead box P3 (Foxp3) mRNA levels with expression levels of tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1) IL-6, and transforming growth factor-β (TGF-β) in DMD muscles (n = 27). Gene expression values are expressed in log scale of arbitrary units (AU). NS = not significant.
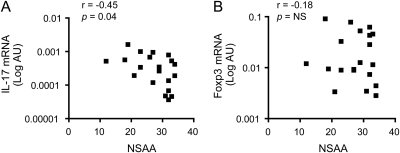
Because of the known role of chronic inflammation in promoting tissue damage and muscle loss in DMD, we investigated the possible relationship between IL-17 expression in muscle biopsy samples and motor outcome at 6 years of age as assessed by the NSAA score. We observed that patients characterized by higher IL-17 expression in their muscle biopsy samples at the time of diagnosis had lower NSAA scores at 6 years of age (figure 4A, table), suggesting that IL-17 mRNA levels and functional outcome were associated. No association between Foxp3 expression and motor outcome was found (figure 4B, table). No association of TNF-α, IL-6, MCP-1, or TGF-β expression levels with NSAA at 6 years was found (table).
Figure 4. IL-17 mRNA levels at biopsy and motor outcome of patients with Duchenne muscular dystrophy (DMD).
Correlation of (A) interleukin (IL)-17 and (B) Forkhead box P3 (Foxp3) mRNA levels at biopsy with North Star Ambulatory Assessment (NSAA) score at 6 years of age in patients with DMD (n = 20). Gene expression values are expressed in log scale of arbitrary units (AU). NS = not significant.
Table.
NSAA score and muscle inflammation in patients with DMDa
Abbreviations: DMD = Duchenne muscular dystrophy; Foxp3 = Forkhead box P3; IL = interleukin; MCP-1 = monocyte chemoattractant protein-1; NSAA = North Star Ambulatory Assessment; TGF-β = transforming growth factor-β; TNF-α = tumor necrosis factor-α.
Spearman rank correlation coefficients (r) for correlations between NSAA score of 6-year-old patients with DMD (n= 20) and mRNA levels of IL-17, Foxp3, TNF-α, MCP-1, IL-6, and TGF-β at biopsy are shown.
DISCUSSION
DMD muscle was characterized by increased expression of IL-17, which was significantly associated with functional outcome at 6 years of age.
It is worth noting that we evaluated cytokine expression in biopsy samples obtained at the time of diagnosis, when all patients were off therapy, therefore ruling out any possible influence of GC treatment. In agreement with previous observations, in both humans and mice, we observed a complex inflammatory milieu with a prominent CD45+ mononuclear cell infiltrate (data not shown) and up-regulation of inflammatory molecules including MCP-1, TNF-α, and IL-6. Recently, IL-17–producing cells have been reported to be present in muscles of patients with JDM.20 The presence of IL-17–producing cells has also been reported in adult dermatomyositis,20–22 suggesting that IL-17 production occurs locally in muscle autoimmune inflammation. Moreover, serum IL-17 levels have been reported to correlate with disease activity in JDM.23 These data in humans are consistent with a possible role of IL-17 in chronic muscle inflammation. It should be noted that IL-17 appears to be dispensable in the only experimental model of polymyositis in which IL-17-null mice have been used.24 However, this single observation is in apparent contradiction with a vast body of evidence supporting a major role of IL-17 in several models of chronic autoimmune inflammation, including collagen-induced arthritis, experimental autoimmune encephalomyelitis, and experimental autoimmune uveoretinitis.8,22,25–32
We did not find any detectable levels of IL-17 in control muscles. Consistent with the above-mentioned observations in JDM, IL-17 mRNA was detectable in all except 2 JDM muscle biopsy samples tested, albeit with a wide range of expression levels. Notably, IL-17 mRNA levels in DMD muscles were consistently higher than those observed in JDM. Although IL-17 production is the functional characteristic feature of Th17, it may also be produced by other cell types including mast cells, natural killer cells, and neutrophils.7 The above-mentioned studies on biopsy samples from patients with JDM, although identifying IL-17–producing cells, did not address the issue of which cell type was expressing IL-17. Because in this study we extracted RNA from whole tissue, it was impossible to determine the cellular source(s) of IL-17. Therefore, we cannot conclude whether muscle IL-17 expression reflects the amount of infiltrating Th17 cells or is the result of the production by other cell types.
Whichever the producing cells, our data show that IL-17 was more prominently expressed in a muscle inflammatory condition in which inflammation is triggered and amplified by myofiber necrosis, compared with JDM, a classic chronic autoimmune inflammatory condition. We would have expected, based on the present understanding of the regulation of Th17 and Treg balance, that, compared with inflammation in JDM, inflammation triggered by necrosis in the absence of autoimmune reactivity, as in DMD, would have led to lower IL-17 expression and higher Foxp3 expression, the latter being a measure of Treg. To the best of our knowledge, no information is available on the possible effect of cell necrosis in inducing IL-17. Interestingly, however, IL-17 has recently been shown to be up-regulated at early time points in an experimental burn model.33
The possible pathogenic role of IL-17 in inflammatory myositis and in muscle damage remains to be established. In this respect, we found that in DMD muscle increased levels of IL-17 were associated with higher expression levels of the inflammatory cytokines TNF-α and MCP-1. Based on these significant correlations, we cannot rule out the possibility that IL-17 expression in DMD muscle might be merely a consequence rather than a cause of inflammation. However, based on the known proinflammatory role of IL-17, this observation is consistent with the hypothesis that IL-17 could contribute to maintaining chronic tissue inflammation. In further support of this hypothesis, we found that patients with a more pronounced functional impairment at 6 years of age had higher levels of IL-17 mRNA in muscle biopsy samples.
Additional support for the potential role of IL-17 is provided by the recent observations linking osteopontin (OPN) to IL-17. OPN has recently been suggested to play a role in DMD based on reduced infiltration of inflammatory cells in muscle, increased muscle strength, reduced muscle fibrosis, and increased numbers of Foxp3+ Treg in muscle, as well as reduced TGF-β levels, in OPN-deleted dystrophic mice.34 Moreover, in DMD, OPN levels are elevated in serum and muscle,34 and OPN polymorphisms have been shown to be genetic modifiers of disease severity.35 Interestingly, it has been demonstrated in vitro that the intracellular isoform of OPN promotes Th17 responses36 and that OPN induces IL-17 expression from human T cells.37 It is therefore tempting to speculate that the high levels of IL-17 and possibly also the low levels of Foxp3 observed in this study might be induced by OPN and that these may mediate at least part of the detrimental effects of OPN in muscles.
Because Foxp3 is specifically expressed by Treg, it could be argued that Foxp3 mRNA levels represent a measure of Treg activity in a given tissue. In DMD, low Foxp3 levels were associated with high levels of MCP-1 and TGF-β. Although TGF-β is produced by Treg, the great portion of TGF-β present in a tissue is produced by stromal cells and is probably related to the amount of tissue fibrosis, which is a consequence of chronic inflammation. The fact that Foxp3 levels reflect anti-inflammatory mechanisms is also supported by the observation that its expression was directly related to the number of CD163+ M2c infiltrating macrophages (data not shown). The latter are known to have an anti-inflammatory role in dystrophin-deficient mice.38
The association of IL-17 expression with levels of other inflammatory cytokines and with the clinical course of DMD suggests a possible pathogenic role of IL-17. Studies on a larger number of patients are needed to define a prognostic value of IL-17. In addition, preclinical studies in dystrophic (mdx) mice are needed to demonstrate a causative role of IL-17 in DMD and, therefore, to identify IL-17 itself as a potential therapeutic target.
GLOSSARY
- DMD
Duchenne muscular dystrophy
- Foxp3
Forkhead box P3
- GC
glucocorticoid
- IL
interleukin
- JDM
juvenile dermatomyositis
- MCP-1
monocyte chemoattractant protein-1
- NSAA
North Star Ambulatory Assessment
- OPN
osteopontin
- TGF-β
transforming growth factor-β
- Th17
T helper 17 lymphocytes
- TNF-α
tumor necrosis factor-α
- Treg
T regulatory cells
AUTHOR CONTRIBUTIONS
L. De Pasquale: RNA extraction, real-time PCR, data collection and interpretation, statistical analysis, drafting of the manuscript. Dr. D'Amico: patient inclusion criteria assessment, DMD patient clinical evaluation. M. Verardo: muscle sample collection and provision, immunofluorescence staining of muscle samples for molecular diagnosis of patients. Dr. Petrini: immunofluorescence staining of muscle samples for molecular diagnosis of patients, confocal microscopy observations. Dr. Bertini: patient inclusion criteria assessment, DMD patient clinical evaluation, revision of the manuscript. Dr. De Benedetti: study design, patient inclusion criteria assessment, data analysis and interpretation, statistical analysis, revision of the manuscript.
DISCLOSURE
L. De Pasquale, Dr. D'Amico, M. Verardo, Dr. Petrini, and Dr. Bertini report no disclosures. Dr. De Benedetti reports that his institution has received liberal contribution to support research activities from Abbott, Bristol-Myers Squibb, Novartis, Novimmune, Pfizer, and Roche. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J, Grange RW. Dysregulated intracellular signaling and inflammatory gene expression during initial disease onset in Duchenne muscular dystrophy. Am J Phys Med Rehabil 2009; 88: 502– 522 [DOI] [PubMed] [Google Scholar]
- 2. Wagner KR. Approaching a new age in Duchenne muscular dystrophy treatment. Neurotherapeutics 2008; 5: 583– 591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pescatori M, Broccolini A, Minetti C, et al. Gene expression profiling in the early phases of DMD: a constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J 2007; 21: 1210– 1226 [DOI] [PubMed] [Google Scholar]
- 4. Griggs RC, Moxley RT, 3rd, Mendell JR, et al. Prednisone in Duchenne dystrophy. a randomized, controlled trial defining the time course and dose response: Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol 1991; 48: 383– 388 [DOI] [PubMed] [Google Scholar]
- 5. Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev 2008; 1: CD003725 [DOI] [PubMed] [Google Scholar]
- 6. Angelini C, Pegoraro E, Turella E, Intino MT, Pini A, Costa C. Deflazacort in Duchenne dystrophy: study of long-term effect. Muscle Nerve 1994; 17: 386– 391 [DOI] [PubMed] [Google Scholar]
- 7. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol; 10: 479– 489 [DOI] [PubMed] [Google Scholar]
- 8. Torchinsky MB, Blander JM. T helper 17 cells: discovery, function, and physiological trigger. Cell Mol Life Sci; 67: 1407– 1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology; 129: 311– 321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007; 204: 1849– 1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003; 52: 65– 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 2002; 8: 500– 508 [DOI] [PubMed] [Google Scholar]
- 13. Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008; 172: 146– 155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao L, Qiu de K, Ma X. Th17 cells: the emerging reciprocal partner of regulatory T cells in the liver. J Dig Dis; 11: 126– 133 [DOI] [PubMed] [Google Scholar]
- 15. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441: 235– 238 [DOI] [PubMed] [Google Scholar]
- 16. Buckner JH. Mechanisms of impaired regulation by CD4+CD25+Foxp3+ regulatory T cells in human autoimmune diseases. Nat Rev Immunol 2010; 10: 849– 859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. Foxp3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10: 490– 500 [DOI] [PubMed] [Google Scholar]
- 18. Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 2006; 212: 8– 27 [DOI] [PubMed] [Google Scholar]
- 19. Mazzone E, Martinelli D, Berardinelli A, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord 2010; 20: 712– 716 [DOI] [PubMed] [Google Scholar]
- 20. Khanna S, Reed AM. Immunopathogenesis of juvenile dermatomyositis. Muscle Nerve 2010; 41: 581– 592 [DOI] [PubMed] [Google Scholar]
- 21. Chevrel G, Page G, Granet C, Streichenberger N, Varennes A, Miossec P. Interleukin-17 increases the effects of IL-1β on muscle cells: arguments for the role of T cells in the pathogenesis of myositis. J Neuroimmunol 2003; 137: 125– 133 [DOI] [PubMed] [Google Scholar]
- 22. Tournadre A, Porcherot M, Cherin P, Marie I, Hachulla E, Miossec P. Th1 and Th17 balance in inflammatory myopathies: interaction with dendritic cells and possible link with response to high-dose immunoglobulins. Cytokine 2009; 46: 297– 301 [DOI] [PubMed] [Google Scholar]
- 23. Bilgic H, Ytterberg S, McNallan K, et al. IL-17 and IFN-regulated genes and chemokines as biomarkers of disease activity in inflammatory myopathies. Arthritis Rheum 2008; 58: 1 [Google Scholar]
- 24. Okiyama N, Sugihara T, Iwakura Y, Yokozeki H, Miyasaka N, Kohsaka H. Therapeutic effects of interleukin-6 blockade in a murine model of polymyositis that does not require interleukin-17A. Arthritis Rheum 2009; 60: 2505– 2512 [DOI] [PubMed] [Google Scholar]
- 25. Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum 2002; 46: 802– 805 [DOI] [PubMed] [Google Scholar]
- 26. Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol 2005; 237: 123– 130 [DOI] [PubMed] [Google Scholar]
- 27. Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006; 177: 566– 573 [DOI] [PubMed] [Google Scholar]
- 28. Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum 2004; 50: 650– 659 [DOI] [PubMed] [Google Scholar]
- 29. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 2003; 171: 6173– 6177 [DOI] [PubMed] [Google Scholar]
- 30. Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 2006; 203: 2673– 2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonderegger I, Rohn TA, Kurrer MO, et al. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol 2006; 36: 2849– 2856 [DOI] [PubMed] [Google Scholar]
- 32. Yoshimura T, Sonoda KH, Miyazaki Y, et al. Differential roles for IFN-γ and IL-17 in experimental autoimmune uveoretinitis. Int Immunol 2008; 20: 209– 214 [DOI] [PubMed] [Google Scholar]
- 33. Finnerty CC, Przkora R, Herndon DN, Jeschke MG. Cytokine expression profile over time in burned mice. Cytokine 2009; 45: 20– 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vetrone SA, Montecino-Rodriguez E, Kudryashova E, et al. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-β. J Clin Invest 2009; 119: 1583– 1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pegoraro E, Hoffman EP, Piva L, et al. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology 2011; 76: 219– 226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol 2009; 9: 137– 141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol 2008; 181: 7480– 7488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet 2009; 18: 482– 496 [DOI] [PMC free article] [PubMed] [Google Scholar]