Abstract
The significance of social situations is commonly context-embedded. Although the role of context has been extensively studied in basic sensory processing or simple stimulus-response settings, its relevance for social cognition is unknown. We propose the social context network model (SCNM), a fronto-insular-temporal network responsible for processing social contextual effects. The SCNM may 1) update the context and use it to make predictions, 2) coordinate internal and external milieus, and 3) consolidate context-target associative learning. We suggest the behavioral variant of frontotemporal dementia (bvFTD) as a specific disorder in which the reported deficits in social cognition (e.g., facial recognition, empathy, decision-making, figurative language, theory of mind) can be described as context impairments due to deficits in the SCNM. Disruption of orbitofrontal-amygdala circuit, as well as the frontal, temporal, and insular atrophy in bVFTD, suggests a relationship between context-sensitive social cognition and SCNM. In considering context as an intrinsic part of social cognition, we highlight the need for a situated cognition approach in social cognition research as opposed to an abstract, universal, and decontextualized approach. The assessment of context-dependent social cognition paradigms, the SCNM, and their possible application to neuropsychiatric disorders may provide new insight into bvFTD and other related frontal disorders.
Context-dependence effects are pervasive in everyday cognition. When we perceive objects and colors, we always perceive these among other objects and colors. We listen and speak within other word streams, and every atom of meaning emerges from a background of meanings. We perceive facial emotion together with body language, the prosody, and cues from the situation, all of them merged to understand the precise emotional significance. Acting appropriately in social interactions requires the interpretation of explicit and implicit contextual clues that orient our responses toward being polite, to make a joke or point out an irony, to say or not say something. Cognitive science and neuroscience research have evidenced context-dependence effects in similar domains of visual perception,1–3 emotion,4–7 language,8–14 and social cognition15,16 in both normal and neuropsychiatric conditions.
But what is context? Simply put, a contextual factor (X) is something that has an effect on a cognitive event and can be determined by observing how that event is affected when X is changed.17 However, this basic definition seems to miss the essence of contextual effects, which is best illustrated with a simple optical illusion. The Ebbinghaus illusion (figure 1A) depicts 2 identical central circles, surrounded by rings of circles. Despite the fact that they are the same size, one circle is perceived as small and the other as big. The contextual information available (the surrounding circles) creates the perception that the center circles are different sizes. Context seems to be more than a variable that affects a particular cognitive process. Rather, contextual cues seem to be an intrinsic part of the cognitive processes that enable the understanding of the specific significance of an object. This is illustrated by figure 1B. Like the size of the circles in the Ebbinghaus illusion, the meaning of the sentence “Mike, you are such a good dancer!” is dependent on the contextual information.
Figure 1. Context-dependent effects from visual perception to social cognition.
(A) The Ebbinghaus illusion. (B) The role of social context in semantic and situational meaning. Two different social contexts (in this case, indexed by the emotional expression, the dancing situation, and the other's actions) dramatically alter the meaning of the same sentence: “Mike, you are such a good dancer!” This figure makes a comparison between visual perception and social cognition by pointing out that they can both generate instances where the same stimulus is differentially experienced as a function of context. But there is also a difference. The perceptual illusion is obligatory (i.e., intrinsic to the hard-wiring of the visual system) whereas the utterance and interpretation of the comments in B are conditional. The femme fatale has the choice not to utter the comment and the young man has the option of interpreting the comment as wistful/friendly or sarcastic/cruel. The critical input of our brain (mainly prefrontal cortex) is probably based on its ability to coordinate conditional behaviors and interpretations in social situations. Illustration by Carlos Becerra (B).
Contextual effects are present at every level, from basic perception to social interaction. This means that we do not perceive objects or process cognitive events in an abstract and universal way. The specific significance of an object, emotion, word, or social situation depends on the contextual effects. During normal cognition, our brains do not process targets and contexts separately; rather, targets are in context. The brain dynamically constrains and shapes cognitive processing, parallel to external context.18 If this is true, cognition is a highly situated process that is dependent on the internal and external events that occur during a particular cognitive process. Contextual cues evoke previous experiences, allowing coordination between internal (previous experiences) and external (specific situations) processes for all cognition. Context-based predictions streamline cognitive processing, and ambiguous objects can be correctly processed by contextual cues.1 Considering abstract or decontextualized targets, which are rarely encountered in everyday cognition, would increase the ambiguity of cognitive processing. This is not a trivial point and implies that cognitive process models based on universal and acontextual approaches are blind to an intrinsic property of cognition: its contextual and situated nature. Depending on contextual processing, perception of the same targets (objects, language, emotion, or social cues) would be very different, and neural processes may also differ.
CONTEXT AND SOCIAL COGNITION
Comprehension of social cognition, from basic emotional facial processing to complex social decision-making, are mostly embedded in a social context. The essence of social phenomena occurs in a situational frame or background in which the specific social meaning emerges. These context-sensitive aspects include face processing, empathy, emotional inference, theory of mind, figurative language, decision-making, social norms, and attitudes. The structural processing and emotional recognition of a face usually occur within a background that includes emotional body language and other convergent information (e.g., prosody, situational clues). The empathy for pain involves the capacity to share and recognize feelings that are being experienced by another in pain. When we see a person suffering from physical pain (e.g., during an assault), we can experience empathy, but depending on the contextual situation, we might feel at risk and decide to escape or attack. The theory of mind consists of the ability to attribute cognitive and affective mental states to oneself and others. Usually, the emotional and cognitive mental states of others are inferred using contextual information. Beliefs, intentions, and other mental states are more reliably inferred when the frames and contextual shortcuts those states are embedded in can be accessed. Multiple figurative aspects of language (e.g., the way of saying something other than the literal meaning of the words) are used during everyday social speech. The intrinsic “figurative” content of the speech (e.g., rhetorical, ironic, or metaphorical) is easily and correctly recognized when the audience has access to paralinguistic cues, emotional body language, and the specific situational circumstance of the speech. We make hundreds of small decisions every day. In some situations, we decide based on ambiguous clues without predicting the outcome, but in other situations we cannot make a decision without full knowledge of the risks and potential consequences. Social norms guide our behavior; it is appropriate to be serious and quiet at a funeral, but friendly, humorous, and sociable at a party. Finally, how we express feelings about other races or ethnic or social groups are modulated by different social situations, specifically the presence or absence of members of these groups. In brief, social cognition processes (e.g., facial processing, emotional inference, empathy, theory of mind, decision-making, social norms, and social attitudes) seem to be embedded in specific contextual circumstances that help build intrinsic social meaning.
Neuroscience research has assessed the role of context in lower domains (e.g., visual perception) with relatively exhaustive anatomic and explanatory models,1,19 but its role in higher domains (e.g., social cognition) is not well understood. Although contextual effects have been proposed as an intrinsic part of social phenomena,20 and it is well known that brain region activation is modulated by social context, no current model has been suggested. We propose that the contextual influence on social cognitive processing depends on a fronto-insular-temporal network that 1) updates contextual cues and uses them to make predictions, 2) coordinates the internal and external milieus, and 3) consolidates context-target associative learning. Moreover, we propose that a specific neuropsychiatric disorder can be described as having strong contextual social cognition impairments.
FRONTOTEMPORAL DEMENTIA AND SOCIAL CONTEXT
The behavioral variant of frontotemporal dementia (bvFTD) is characterized by insidiously progressive changes in personality and social interaction that typically precede other cognitive deficits. On the whole, perception, episodic memory, visuospatial abilities, and praxias are intact or relatively well preserved. Conversely, deficits in social interaction, lack of empathy for others,21 disinhibition, and impulsiveness22 are evident. The recently revised criteria require possible bvFTD cases to present with 3 out of 6 clinically discriminating features: disinhibition, apathy/inertia, loss of sympathy/empathy, perseverative/compulsive behaviors, hyperorality, and dysexecutive neuropsychological profile. Probable bvFTD is further characterized by functional disability and characteristic neuroimaging, and bvFTD with definite frontotemporal lobar degeneration requires histopathologic confirmation or pathogenic mutation identification.22 Patients may present with compulsiveness, perseverations, or stereotyped repetitive acts,23 loss of self-consciousness,24 diminished interest for activities or hobbies, or withdrawal and apathy.25 Increased appetite with a tendency for sweet foods is common, and hypersexuality and hyperorality may develop, especially in the advanced stages of the disease. Early diagnosis is difficult because behavioral problems dominate the clinical picture while cognitive functions are still relatively intact. People with bvFTD often score normally on mental status screens, and conventional structural brain imaging (CT and MRI) may not be sensitive to the early changes associated with bvFTD.21 Therefore, early diagnosis relies on clinical interviews and caregiver reports; it can be considerably difficult to distinguish bvFTD from primary psychiatric syndromes.
Patients with bvFTD have consistent deficits in several domains of social cognition such as recognizing emotions in facial expressions,26 empathy processing,27 decision-making,28,29 figurative language,15 theory of mind,30,31 and interpersonal norms.32 We propose that this specific pattern of social cognition impairment can be understood as a general impairment of social context processing information that is the result of an abnormal fronto-insular-temporal network.
A NEUROANATOMIC PATHWAY: THE SOCIAL CONTEXT NETWORK MODEL
Context-based predictions make social cognition more efficient. Prototypical situations in the environment are represented in “context frames” that integrate information about the meanings of social targets (e.g., an emotional face, a speech) that are likely to appear in a specific scene with information about their relationships.
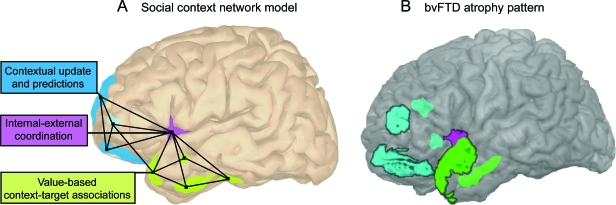
We propose that there exists a cortical network that mediates the processing of such contextual associations. This social context network involves regions of the frontal, insular, and temporal cortices. As detailed in figure 2, we postulate that frontal areas (e.g., orbitofrontal cortex, lateral prefrontal cortex, superior orbital sulcus) update and associate ongoing contextual information in relation to episodic memory and target-context associations. The temporal regions (amygdala, hippocampus, perirhinal and parahippocampal cortices) index the value learning of target-context associations. Finally, the insular cortex coordinates internal and external milieus in an internal motivational state. In this way, the insula would provide information integration from internal states and social contexts to produce a global feeling state.
Figure 2. The social context network model (SCNM) and behavioral variant of frontotemporal dementia (bvFTD).
(A) The SCNM. Lateral view of the left hemisphere showing the proposed fronto-insular-temporal network (light blue, violet, and green regions of interest, respectively). In this context network, prefrontal areas (PFC) such as frontopolar and dorsolateral-prefrontal cortices would be involved in the generation of focused predictions via the update of associative activation of representations in the specific context. The insular cortex would provide the convergence point for emotional and cognitive states related to the coordination between external and internal milieus, facilitating the fronto-temporal interaction in social context processing. Finally, target-context associations stored in the temporal regions would be integrated with feature-based information processed in frontal regions. Connected nodes represent the fronto-insular-temporal interactions. (B) bvFTD atrophy pattern. Lateral view of earliest regions thought to be damaged in bvFTD, in the frontal, insular, and temporal areas (light blue, violet, and green regions of interest, respectively). Note the partial overlap with the nodes of the SCNM. Highlighted regions correspond to the following Brodmann areas: frontal (BA46, B10, BA11, BA24); temporal (BA20, BA21, BA38); and insula (BA48).
Updating and predicting: A crucial role for the frontal lobe in contextual integration.
Several frontal areas (orbitofrontal cortex, lateral prefrontal cortex, superior orbital sulcus) seem to be involved in predictions, using the context to update the encoding and retrieval of episodic learning.33–36 Animal studies provide direct evidence for this hypothesis. Prefrontal neurons show rapid frontal adaptation to context-dependent behavioral significance in short-term context paradigms.37 These cells seem to update the same targets in different contexts.38 In the orbitofrontal cortex (OFC), neurons encode motivational context information.35 Primate lateral prefrontal cortex neurons are context-dependent, irrespective of differences in the cue's physical properties.35 In animal and human studies, the superior orbital sulcus (SOS) has also been directly related to the contextual update of visual targets.1 This frontal site would play an important role in the generation of focused predictions via the update of associative activation of representations in the specific context.19
Patients with frontal lobe lesions fail to recognize how context alters the meaning of stimuli.39 Thinking becomes concrete, and a patient's behavior is guided by superficial cues from the environment; some patients ignore the incongruity of context.39 Conversely, although these patients exhibit some characteristics that affect their behavior,40 they behave impeccably in the office. This is consistent with the idea that these patients are most impaired under circumstances with minimal external control of behavior.41
Interplay between internal and external milieus in the insular cortex.
The insula seems to be a convergence area that integrates internal and external milieus.42–45 This cortex uses previous experiences to connect intentions and motivations for a specific cognitive task. The insula integrates modality-specific feeling states and uncertainty with individual preferences and contextual information to produce a global feeling state.45 Thus, in our model, the insula would provide the convergence point for emotional and cognitive processes related to the coordination between external and internal states, facilitating the fronto-temporal interaction in social context processing.
Value-based learning of contextual associations in the temporal lobe.
Target-context associations can be considered to be the building blocks of contextual learning.34,46,47 Basic associative processes such as extinction48 and environment representation49 engage the hippocampus, amygdala, and related temporal sites (e.g., perirhinal cortex). Contextual markers are initially stored in the medial temporal lobes, where they cohere with featural information processed in frontal regions.50
Neuroimaging studies in humans suggest that the parahippocampal cortex receives polysensory and somatosensory information required for mediating global contextual associations.1 The parahippocampal cortex has also been associated with some aspects of episodic memory. Structures in the medial temporal lobe, including the hippocampus, and perirhinal and entorhinal cortices are thought to be important in associative processing. Unfortunately, there is not enough evidence to make a clear functional distinction between the subdivisions of the medial temporal lobe.1
Contextual effects in social cognition have not been assessed yet in a systematic and exhaustive approach. Nevertheless, recent reports using context manipulations in representative social cognition fields (table) offer links to specific brain regions engaged in the social context network model (SCNM).
Table.
Representative examples of contextual effects in social cognition
Abbreviations: ASD = autism spectrum disorder; FMR = fronto-mesolimbic regions; FTD = frontotemporal dementia; IFG = inferior frontal gyrus; MFC = medial frontal cortex; MFG = medial frontal gyrus; MPC = medial prefrontal cortex; OFC = orbitofrontal cortex; ONC = other neurodegenerative conditions; PFC = prefrontal cortex; PHC = parahippocampal cortex; SCZ = schizophrenia; TL = temporal lobe; ToM = theory of mind; VBM = voxel-based morphometry; VLPFC = ventrolateral prefrontal cortex.
References e1–e9 are available on the Neurology® Web site at www.neurology.org.
SCNM ANDbvFTD
We propose that the SCNM would provide an adequate model to understand bvFTD-related social impairments. Disruption of the orbitofrontal-amygdala circuit is thought to be responsible for the triad of bvFTD symptoms that includes disinhibition, stereotyped behaviors, and gluttony.25 The mesial and orbital frontal regions atrophy first, followed by the temporal pole, hippocampal formation, dorsolateral frontal cortex, and the basal ganglia. This pattern of atrophy progression has been shown to correlate with the volume of cortical and subcortical regions and with underlying neuronal loss.
The initial symptoms of FTD reflect the involvement of orbitofrontal cortex as well as the disruption of the rostral limbic system including the insula, the anterior cingulate cortex, the striatum, the amygdala, and the medial frontal lobes.51,52 This system is involved in a number of processes such as the evaluation of the motivational or emotional content of internal and external stimuli, error detection, response selection and decision-making, and subsequent regulation of context-dependent behaviors.53 Recent neuroimaging studies suggest that patients with FTD show predominantly right frontal, anterior insular, and anterior cingulate deterioration, with pronounced orbitofrontal cortex atrophy.51 Additionally, some studies have reported correlations between behavioral symptoms and brain structures, suggesting that the right orbitofrontal cortex regulates behavior together with a predominantly right-side network involving the insula and striatum.51 In addition, voxel-based morphometry studies have shown that patients with bvFTD have significant gray matter loss in the anterior insula and in a variety of frontal areas.54
Thus, a new research agenda linking context-sensitive social cognition, SCNM, and bvFTD is proposed. Implementing such an agenda would have several implications.
A NEW RESEARCH AGENDA
Theoretical implications.
In considering context as an intrinsic part of social cognition, we highlight the need for a situated cognition approach in social cognition research as opposed to an abstract, universal, and decontextualized approach. The so-called theories of embodied cognition propose that human mind is largely determined by the ecological coupling of sensorimotor systems, emotions, and the current environment. Theoretical claims of embodied cognition and related fields such as embodied emotion,55 embodied theory of mind,56 and cultural practices57,58 would be integrated with clinical and experimental approaches to study the contextual effects of social phenomena.
Integrating previous research into a new agenda.
Although the contextual nature of social events is far from controversial, there is no unifying theoretical background for research in animal models, lesion studies, neuropsychiatric reports, and experimental neuroscience. The SCNM provides an empirically testable set of hypotheses regarding contextual update, contextual prediction, target-context association, and contextual coordination of internal cognitive processes and external frames in social cognition paradigms. Typical approaches to emotional processing, mental inference, empathy, figurative language, and decision-making can be assessed in relation to contextual manipulations.
New hypothesis and tests for bvFTD and other neuropsychiatric disorders.
We proposed that the wide range of social cognition impairments in bvFTD can be partially explained by a general deficit in integrating social context and behavior. Because neuropsychological tests designed to detect impairment in social cognition are important bvFTD diagnostic tools,21 our hypothesis of social context impairments in bvFTD can be empirically evaluated and can provide insights into clinical, diagnostic, and cognitive models of this disorder. Although the complexity of social context can be a problem in experimental settings, the use of frames, background information, or multimodal designs (as used in other domains of contextual studies7,59) adapted to social cognition tasks can provide simple experimental shortcuts.
At the same time, a further contextual and ecological evaluation of bvFTD would provide more sensitive tools for bvFTD research (see below). In fact, bvFTD is one of the disorders in which patients present with behavioral problems despite good neuropsychological test scores (the so-called “frontal lobe mystery”40). This paradox could be partly explained by the use of an extended abstract and decontextualized neuropsychological evaluation. The mismatch between everyday cognition and neuropsychological profile in bvFTD can be partially explained by the abstract processing of standard assessment, which would perform better than context-dependent tasks. That is to say, bvFTD detection might improve if clinical tests were context-dependent.
CONTEXT AND THE FRONTAL LOBE MYSTERY
Mesulam41 suggests that frontal lobe symptoms would not be detected with traditional office-based neuropsychological tests, yet patients are impaired in their everyday lives. Most of the traditional tests used to assess executive functions were not originally created for the purpose of investigating patient populations; in many cases, there have been few modifications to these procedures for new applications. Therefore, traditional executive tests (e.g., the Wisconsin Card Sorting Test, the Stroop Task, and verbal fluency tasks) are not good “models of the world”40 because they are abstract tasks that lack the context of everyday life situations. For example, working memory is sensitive to distractibility and interference, so in a context in which the environment has plentiful stimuli and distracters, the bvFTD patient's working memory is more likely to fail than in a quiet and simple environment. The magnitude of the deficits depends on the context of the testing and on the degree to which the test requires the suppression of interference.60
Moreover, standard cognitive tests are included in an abstract and acontextual neuropsychological assessment. The office setting may introduce sufficient external structure to suppress some behavioral tendencies.41 These observations emphasize the importance of developing more ecological measures that include contextual sensitivity evaluation. Consequently, Burgess et al.40 invented 2 multitasking tests (Multiple Errands Test and Six Element Test) that represent some real-life situations. Because these tests represent the context of real-life situations, they more clearly reflect the difficulties that patients with bvFTD face. In a similar way, Torralva et al.31 constructed a more organic evaluation for the early detection of executive and social cognitive impairments in bvFTD.
DISCUSSION
Behavior variant FTD is the prototypical disorder in which social behaviors are disrupted in certain contexts. However, there are other diseases in which the SCNM is disrupted, such as traumatic brain injury, structural lesions of the ventromedial prefrontal cortex, schizophrenia, autism, and dementing illness of any cause. Formulating the thought disorder of schizophrenia as one with deficits in the SCNM offers a parsimonious explanation of their difficulties that range from basic visual perceptual problems to their deficits in speech and social cognition.16
The assessment of context-dependent social cognition paradigms, the SCNM, and their possible application to neuropsychiatric disorders may provide new insight into bvFTD and other related disorders. The ideal experimental approach would be to have a battery of tasks that vary the degree of context for emotional/social stimuli, and that also verify whether there are any deficits in context processing for nonsocial stimuli. Further outstanding questions to be assessed with experimental paradigms are as follows: 1) How can contextual information be used for making predictions and acting in social situations? 2) How are social context frames integrated in the brain, and what elicits their activation? 3) Which paradigms are best suited to test the 3 components of the SCNM? 4) Which social cognition tests are more sensitive to contextual modulation in bvFTD and related disorders? 5) How can the different degenerating brain regions (frontal, insular, temporal) in bvFTD be tested in relation to the SCNM? The empirical answers to those questions may provide a strong framework for assessing a more sensitive contextual social cognition profile in frontal diseases.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Carlos Becerra and Blas Couto for their help in preparing figures 1B and 2. A.I. thanks Vladimir Lopez and Carlos Cornejo for their earlier influence on this work.
GLOSSARY
- bvFTD
behavioral variant of frontotemporal dementia
- OFC
orbitofrontal cortex
- SCNM
social context network model
- SOS
superior orbital sulcus
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Ibanez: principal investigator and corresponding author. F. Manes: corresponding author. Dr. Ibanez contributed to conceptualization, drafting, and revising of the manuscript. F. Manes contributed to conceptualization and revising the manuscript for content.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Bar M. Visual objects in context. Nat Rev Neurosci 2004; 5: 617– 629 [DOI] [PubMed] [Google Scholar]
- 2. Zhang NR, von der Heydt R. Analysis of the context integration mechanisms underlying figure-ground organization in the visual cortex. J Neurosci 2010; 30: 6482– 6496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz O, Hsu A, Dayan P. Space and time in visual context. Nat Rev Neurosci 2007; 8: 522– 535 [DOI] [PubMed] [Google Scholar]
- 4. Barrett LF, Bar M. See it with feeling: affective predictions during object perception. Philos Trans R Soc Lond B Biol Sci 2009; 364: 1325– 1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Gelder B. Towards the neurobiology of emotional body language. Nat Rev Neurosci 2006; 7: 242– 249 [DOI] [PubMed] [Google Scholar]
- 6. Meeren HK, van Heijnsbergen CC, de Gelder B. Rapid perceptual integration of facial expression and emotional body language. Proc Natl Acad Sci USA 2005; 102: 16518– 16523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barrett LF, Lindquist KA, Gendron M. Language as context for the perception of emotion. Trends Cogn Sci 2007; 11: 327– 332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aravena P, Hurtado E, Riveros R, Cardona JF, Manes F, Ibanez A. Applauding with closed hands: neural signature of action-sentence compatibility effects. PLoS One 2010; 5: e11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibanez A, Gleichgerrcht E, Hurtado E, Gonzalez R, Haye A, Manes FF. Early neural markers of implicit attitudes: N170 modulated by intergroup and evaluative contexts in IAT. Front Hum Neurosci 2010; 4: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ibanez A, Lopez V, Cornejo C. ERPs and contextual semantic discrimination: degrees of congruence in wakefulness and sleep. Brain Lang 2006; 98: 264– 275 [DOI] [PubMed] [Google Scholar]
- 11. Ibanez A, Riveros R, Aravena P, et al. When context is difficult to integrate: cortical measures of congruency in schizophrenics and healthy relatives from multiplex families. Schizophr Res 2011; 126: 303– 305 [DOI] [PubMed] [Google Scholar]
- 12. Ibanez A, Toro P, Cornejo C, et al. High contextual sensitivity of metaphorical expressions and gesture blending: a video event-related potential design. Psychiatry Res 2011; 191: 68– 75 [DOI] [PubMed] [Google Scholar]
- 13. Van Petten C, Luka BJ. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang 2006; 97: 279– 293 [DOI] [PubMed] [Google Scholar]
- 14. Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn Sci 2005; 9: 416– 423 [DOI] [PubMed] [Google Scholar]
- 15. Rankin KP, Salazar A, Gorno-Tempini ML, et al. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. Neuroimage 2009; 47: 2005– 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung YS, Mathews JR, Barch DM. The effect of context processing on different aspects of social cognition in schizophrenia. Schizophr Bull 2011; 37: 1048– 1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Jaegher H, Di Paolo E, Gallagher S. Can social interaction constitute social cognition? Trends Cogn Sci 2010; 14: 441– 447 [DOI] [PubMed] [Google Scholar]
- 18. Barrett LF, Lindquist KA, Gendron M. Language as context for the perception of emotion. Trends Cogn Sci 2007; 11: 327– 332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond B Biol Sci 2009; 364: 1235– 1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol 2009; 60: 693– 716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol 2011; 10: 162– 172 [DOI] [PubMed] [Google Scholar]
- 22. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456– 2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J Neurol Neurosurg Psychiatry 2000; 69: 178– 186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546– 1554 [DOI] [PubMed] [Google Scholar]
- 25. Hodges JR. Frontotemporal dementia (Pick's disease): clinical features and assessment. Neurology 2001; 56: S6– S10 [DOI] [PubMed] [Google Scholar]
- 26. Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia 2006; 44: 950– 958 [DOI] [PubMed] [Google Scholar]
- 27. Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain 2006; 129: 2945– 2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gleichgerrcht E, Ibanez A, Roca M, Torralva T, Manes F. Decision-making cognition in neurodegenerative diseases. Nat Rev Neurol 2010; 6: 611– 623 [DOI] [PubMed] [Google Scholar]
- 29. Manes F, Torralva T, Ibanez A, Roca M, Bekinschtein T, Gleichgerrcht E. Decision-making in frontotemporal dementia: clinical, theoretical and legal implications. Dement Geriatr Cogn Disord 2011; 32: 11– 17 [DOI] [PubMed] [Google Scholar]
- 30. Torralva T, Kipps CM, Hodges JR, et al. The relationship between affective decision-making and theory of mind in the frontal variant of frontotemporal dementia. Neuropsychologia 2007; 45: 342– 349 [DOI] [PubMed] [Google Scholar]
- 31. Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain 2009; 132: 1299– 1309 [DOI] [PubMed] [Google Scholar]
- 32. Rankin KP, Kramer JH, Mychack P, Miller BL. Double dissociation of social functioning in frontotemporal dementia. Neurology 2003; 60: 266– 271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol Psychiatry 2011; 69: 1133– 1139 [DOI] [PubMed] [Google Scholar]
- 34. Lang S, Kroll A, Lipinski SJ, et al. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci 2009; 29: 823– 832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watanabe M, Sakagami M. Integration of cognitive and motivational context information in the primate prefrontal cortex. Cereb Cortex 2007; 17 (suppl 1): i101– i109 [DOI] [PubMed] [Google Scholar]
- 36. Roca MA, Torralva T, Gleichgerrcht E, et al. The role of area 10 (BA10) in human multitasking and in social cognition: a lesion study. Neuropsychologia 2011; 49: 3525– 3531 [DOI] [PubMed] [Google Scholar]
- 37. Kusunoki M, Sigala N, Gaffan D, Duncan J. Detection of fixed and variable targets in the monkey prefrontal cortex. Cereb Cortex 2009; 19: 2522– 2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sigala N, Kusunoki M, Nimmo-Smith I, Gaffan D, Duncan J. Hierarchical coding for sequential task events in the monkey prefrontal cortex. Proc Natl Acad Sci USA 2008; 105: 11969– 11974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mesulam MM. The human frontal lobes: transcending the default mode through contingent encoding. Principles Frontal Lobe Function 2002; 1: 8– 31 [Google Scholar]
- 40. Burgess PW, Alderman N, Volle E, Benoit RG, Gilbert SJ. Mesulam's frontal lobe mystery re-examined. Restor Neurol Neurosci 2009; 27: 493– 506 [DOI] [PubMed] [Google Scholar]
- 41. Mesulam MM. Frontal cortex and behavior. Ann Neurol 1986; 19: 320– 325 [DOI] [PubMed] [Google Scholar]
- 42. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3: 655– 666 [DOI] [PubMed] [Google Scholar]
- 43. Ibanez A, Gleichgerrcht E, Manes F. Clinical effects of insular damage in humans. Brain Struct Funct 2010; 214: 397– 410 [DOI] [PubMed] [Google Scholar]
- 44. Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 2010; 214: 519– 534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 2009; 13: 334– 340 [DOI] [PubMed] [Google Scholar]
- 46. Greene AJ, Gross WL, Elsinger CL, Rao SM. An FMRI analysis of the human hippocampus: inference, context, and task awareness. J Cogn Neurosci 2006; 18: 1156– 1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus 2010; 20: 1139– 1153 [DOI] [PubMed] [Google Scholar]
- 48. Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry 2006; 60: 352– 360 [DOI] [PubMed] [Google Scholar]
- 49. Bilkey DK. Space and context in the temporal cortex. Hippocampus 2007; 17: 813– 825 [DOI] [PubMed] [Google Scholar]
- 50. Mayes AR, Roberts N. Theories of episodic memory. Philos Trans R Soc Lond B Biol Sci 2001; 356: 1395– 1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann NY Acad Sci 2007; 1121: 528– 545 [DOI] [PubMed] [Google Scholar]
- 52. Seeley WW. Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr Opin Neurol 2008; 21: 701– 707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boccardi M, Sabattoli F, Laakso MP, et al. Frontotemporal dementia as a neural system disease. Neurobiol Aging 2005; 26: 37– 44 [DOI] [PubMed] [Google Scholar]
- 54. Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage 2005; 24: 1042– 1051 [DOI] [PubMed] [Google Scholar]
- 55. Niedenthal PM. Embodying emotion. Science 2007; 316: 1002– 1005 [DOI] [PubMed] [Google Scholar]
- 56. Grafton ST. Embodied cognition and the simulation of action to understand others. Ann NY Acad Sci 2009; 1156: 97– 117 [DOI] [PubMed] [Google Scholar]
- 57. Hutchins E. The role of cultural practices in the emergence of modern human intelligence. Philos Trans R Soc Lond B Biol Sci 2008; 363: 2011– 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vogeley K, Roepstorff A. Contextualising culture and social cognition. Trends Cogn Sci 2009; 13: 511– 516 [DOI] [PubMed] [Google Scholar]
- 59. Bar M, Ullman S. Spatial context in recognition. Perception 1996; 25: 343– 352 [DOI] [PubMed] [Google Scholar]
- 60. Fuster JM. Overview of prefrontal functions: the temporal organization of action. In: The Prefrontal Cortex, 4th ed San Diego: Academic Press; 2008: 333–385 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.