At the beginning of the 21st century malaria remains one of the world's most widespread and intractable diseases, parasites of the genus Plasmodium, especially P. falciparum, being responsible for many millions of deaths and much human misery. The malaria parasite Plasmodium is genetically sophisticated, with complex survival strategies that have consistently outmaneuvered attempts to control it with antimalarial drugs. The completion of the P. falciparum genome project will greatly help the development of new methods of counterattack, but to understand the data the project produces, a detailed understanding of the parasite's biology is essential. The research by Salmon and colleagues reported in the previous issue of PNAS (1) tackles a fundamental unsolved problem of Plasmodium biology, highly relevant to the development of novel antimalarials.
In malaria infections, parasites injected by mosquitoes into the blood pass to the liver where they invade its main cell type, the hepatocyte. Within these, the parasites feed and grow, then multiply, each generating many hundreds of merozoites, minute, lemon-shaped infectious cells not much larger than a bacterium. These move into the bloodstream where each can invade a red blood cell (RBC), becoming entirely enclosed in this cell, and again feeding and multiplying, although this time to about 16 merozoites (in P. falciparum). These escape from the depleted RBC and invade new ones. After many cycles within RBCs, sexual forms (gametocytes) are produced, which when taken by a mosquito in a blood meal, break out of their enclosing RBCs as gametes, which fuse together and reinfect the insect.
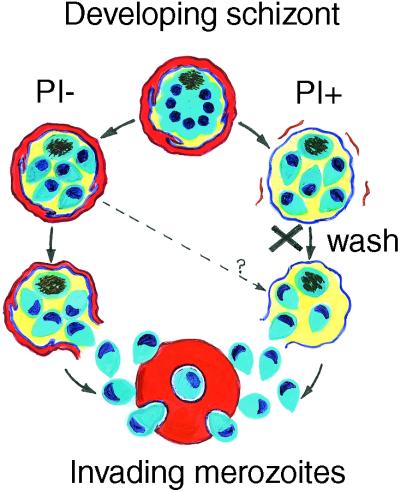
During RBC invasion, the merozoite induces a pit to form in the RBC surface and this closes over the parasite to form a minute bubble (the parasitophorous vacuole, PV) inside the RBC, where the parasite stays through successive stages—ring, trophozoite, and schizont—until its merozoite offspring mature and leave. The membrane lining the PV (the parasitophorous vacuole membrane, PVM) receives new parasite proteins and lipids, which enlarge it as the parasite grows. By the time that the schizont buds off its cluster of merozoites, most of the RBC hemoglobin has been consumed by the parasite. The merozoite cluster is now enclosed in a bag composed of two membranes—an inner PVM and an outer RBC membrane (Fig. 1). Normally, these then rupture to release the merozoites for another round of RBC invasion.
Figure 1.
Diagram comparing the release of merozoites from schizonts in the absence of protease inhibitors (Left, PI−) and in their presence (Right, PI+). The X indicates that the protease inhibitors have been removed by washing, allowing the escape of merozoites and reinvasion. On the left the red cell membrane (red-brown) and the PV (blue) remains intact until final merozoite release, whereas on the right the red cell membrane is lost, exposing the PVM, as reported by Salmon et al. (1).
Great efforts currently are being made to develop a malaria vaccine, but the history of malaria teaches that the parasite is extremely resourceful, and new antimalarial drugs are sure to continue playing a vital role in malaria treatment and control. The paper by Salmon and colleagues (1) reports new information relevant to drug development, concerning the release of merozoites from RBCs, a phase in the malaria parasite's life cycle that has been oddly neglected in the past, considering its potential as a drug target as well as being an engaging biological puzzle.
A defining feature of merozoite release is its susceptibility to protease inhibitors, discovered in 1983 when Hadley et al. (2) showed that when the protease inhibitors leupeptin and chymostatin were added to cultures of P. knowlesi (a simian malaria parasite), merozoites matured normally but were unable to escape from their RBC-schizont confines (Fig. 1). When the inhibitors were washed away, merozoites exited in the normal fashion and could invade RBCs successfully. This technique has been used to prepare large numbers of viable P. knowlesi merozoites for red cell invasion studies (2, 3). A few years later, Lyon and Haynes (4) discovered a similar effect in P. falciparum and added the protease inhibitors pepstatin and antipain to the list of blocking agents. Such parasites appeared under the light microscope as spherical clusters of merozoites (Fig. 1), each group enclosed in a delicate membranous covering identified as the RBC membrane by its reaction to anti-RBC antibodies, although permeable to antibodies against the enclosed merozoites.
Salmon and colleagues (1) have now reexamined this issue, using a combination of a cysteine protease inhibitor (designated E64), electron microscopy, and improved, specific antibody labels for the RBC membrane and PVM. From this work they conclude there is only one membrane, the PVM around the merozoite cluster, the RBC membrane having been lost during cluster maturation. This unexpected finding shows that the RBC membrane can be lost independently of the PVM in the presence of certain protease inhibitors, so that there must be two separable types of membrane lysis. These findings prompt several questions, for example, which proteases are responsible for PVM lysis? Which protease, if any, is needed for RBC membrane lysis? How do proteases reach their targets? And what determines the timing of merozoite release?
Blood stages of Plasmodium have several sets of proteases, a number active in feeding on RBC hemoglobin and not relevant to merozoite release (5), but others present in schizonts and possible agents of merozoite release. These include two (or more) serine proteinases, plasmepsin II, and a cysteine proteinase (5–7). All of these are found in late schizonts, and except for the last, also have been localized by immunofluorescence antibody staining to the PV. All can degrade red cell skeleton or membrane proteins, a requirement for red cell lysis (see e.g., refs. 8 and 9). Proteases involved in merozoite release could be liberated by the schizont before the merozoites have formed, to be activated at the time of release, or could be secreted by the merozoites themselves. Such enzymes would have to cross the PVM to gain access to the RBC membrane, and as the PVM is apparently permeable to antibodies (4), it is likely to be leaky to enzymes. However, to identify which of these (or other) enzymes fits the case, specific inhibitors are needed to prevent lysis of the RBC membrane as well as the PVM.
Protease inhibitors may, of course, directly block proteases from lysing membranes, but they are also likely to interfere with the maturation of other molecules that would be the actual agents of lysis, as most merozoite (and many schizont) proteins are cleaved proteolytically before they can be active.
Merozoites contain a number of proteases that may be involved in both escape and invasion (see ref. 6). Electron microscopy also shows that mature merozoites inside schizonts secrete material that appears to disrupt the PVM and perhaps the RBC membrane, too, a process blocked by protease inhibitors (3). Significantly, merozoite invasion into RBCs also is prevented by a number of protease inhibitors (2, 4, 10), suggesting that merozoite release and RBC invasion may be two sides of the same coin. There would be significant advantage for the parasite if merozoites were involved in their own release, as they would by this time be mature enough to invade an RBC, thus minimizing exposure to the host's defenses.
These ruminations on the effects of protease inhibitors do not address exactly what happens during normal merozoite release. The details of this event are not yet entirely clear. Winograd and colleagues (11) recently have analyzed this event in culture, using video microscopy and concluded that an aperture is made through both the PVM and red cell membrane to allow merozoites to exit in an orderly fashion. Moreover, rather unexpectedly, the red cell membrane and some hemoglobin persisted until after the merozoites had escaped. It would seem from this therefore that although there may be two parts to the release mechanism, they usually operate together to ensure a simultaneous breakdown of the two barriers.
It is worth noting that escape from host cells is a widespread phenomenon in the group of intracellular parasites (apicomplexans), which includes P. falciparum. Indeed in Plasmodium species, we see a similar event in the escape of gametes from RBCs and hepatic merozoites from hepatocytes. Similar mechanisms also may operate in Toxoplasma gondii, a relative of Plasmodium, which invades many cells of the body including neurons in the brain, to cause toxoplasmosis, a disease prevalent in immunocompromised patients such as those with HIV-AIDS. Toxoplasma's invasive forms resemble malaria merozoites, and similarly, in laboratory cultures their invasion into tissue cells can be blocked by protease inhibitors (12); their escape from their host cell does not seem to have been studied. Cryptosporidium is another relative that causes debilitating gut infections in humans, and several other related species with similar invasive habits are notable pathogens of humans or domestic animals. Intriguingly, protease inhibitors are being used with some success in AIDS treatment to block the maturation of the HIV virus, and it would be interesting to know whether such medication has an effect on concurrent toxoplasmosis or malaria infections. Their actions on Plasmodium are at present conceived as mainly inhibiting enzymes involved in parasite feeding, but they may have a wider application in blocking merozoite release, too (9, 13, 14). Of course, many activities of the human body depend on proteolytic processes, so any new protease inhibitors will have to be tightly targeted and tested to avoid unwanted side effects. The prospects for designing a new breed of antimalarial and antitoxoplasmosis drugs based on protease inhibition are becoming more attractive as we know more about their biological targets and understand better the parasites' exits as well as their entrances.
Acknowledgments
Thanks are expressed to Dr. M. J. Blackman and Dr. C. Braun-Breton for their helpful advice. This work was supported by the Wellcome Trust (Grant 048244) to whom I also express my gratitude.
Footnotes
See companion article on page 271 in issue 1 of volume 98.
References
- 1.Salmon B L, Oksman A, Goldberg D E. Proc Natl Acad Sci USA. 2001;98:271–276. doi: 10.1073/pnas.011413198. . (First Published December 12, 2000; 10.1073/pnas.011413198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadley T J, Aikawa M, Miller L H. Exp Parasitol. 1983;55:306–311. doi: 10.1016/0014-4894(83)90027-9. [DOI] [PubMed] [Google Scholar]
- 3.Bannister L H, Mitchell G H. J Protozool. 1989;36:362–367. doi: 10.1111/j.1550-7408.1989.tb05527.x. [DOI] [PubMed] [Google Scholar]
- 4.Lyon J A, Haynes J D. J Immunol. 1986;136:2245–2251. [PubMed] [Google Scholar]
- 5.McKerrow J H, Sun E, Rosenthal P J, Bouvier J. Annu Rev Microbiol. 1993;47:821–853. doi: 10.1146/annurev.mi.47.100193.004133. [DOI] [PubMed] [Google Scholar]
- 6.Roggwiller E, Bétoulle M E M, Blisnick T, Braun-Breton C. Mol Biochem Parasitol. 1996;82:13–24. doi: 10.1016/0166-6851(96)02714-4. [DOI] [PubMed] [Google Scholar]
- 7.Raphael P, Takakuwa Y, Manno S, Liu S-C, Chishti A H, Hanspal M. Mol Biochem Parasitol. 2000;110:259–272. doi: 10.1016/s0166-6851(00)00283-8. [DOI] [PubMed] [Google Scholar]
- 8.Le Bonniec S L, Fournier C, Deregnaucourt C, Grellier P, Dhermy D, Lecomte M C, Schrével J. C R Acad Sci Serie III. 1996;319:1011–1017. [PubMed] [Google Scholar]
- 9.Le Bonniec S L, Deregnaucourt C, Redeker V, Banerjee R, Grellier P, Goldberg D E, Schrével J. J Biol Chem. 1999;274:14218–14223. doi: 10.1074/jbc.274.20.14218. [DOI] [PubMed] [Google Scholar]
- 10.Bernard F, Schrével J. Mol Biochem Parasitol. 1987;26:167–174. doi: 10.1016/0166-6851(87)90140-x. [DOI] [PubMed] [Google Scholar]
- 11.Winograd E, Clavijo C A, Bustamante L Y, Jaramillo M. Parasitol Res. 1999;85:621–624. doi: 10.1007/s004360050606. [DOI] [PubMed] [Google Scholar]
- 12.Conseil V, Soête M, Dubremetz J-F. Antimicrob Agents Chemother. 1999;43:1358–1361. doi: 10.1128/aac.43.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKerrow J H, Engel J C, Caffrey C R. Bioorg Med Chem. 1999;7:639–644. doi: 10.1016/s0968-0896(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal P J. Emerg Infect Dis. 1998;4:49–57. doi: 10.3201/eid0401.980107. [DOI] [PMC free article] [PubMed] [Google Scholar]