Agrobacterium tumefaciens is a soil-born pathogen with the unique ability to genetically transform plants. Agrobacterium infects plant wound sites, causing crown gall disease. This agriculturally significant disease results from the transfer of a single-stranded (ss) segment (the T-strand) of the bacterium's tumor-inducing plasmid to the host cell (reviewed in refs. 1–3; Fig. 1). On integration into the host genome, genes encoded by the T-strand direct the synthesis of plant growth hormones, resulting in tumorous proliferation of plant cells. T-strand genes also cause the plant to produce opines, compounds that represent a major carbon and nitrogen source uniquely metabolized by the bacterium. Thus, Agrobacterium has evolved a mechanism to genetically engineer host cells to create a favorable niche for itself. This natural ability has been coopted by researchers who use Agrobacterium to engineer plants for agricultural and research purposes.
Figure 1.
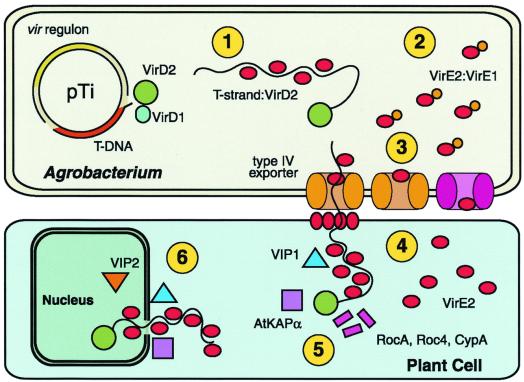
The six functions of VirE2. The vir regulon, encoding the major loci virA-E and virG-H, is expressed on detection of plant wound signals. VirD2 and VirD1 liberate the T-strand and VirD2 remains covalently bound to the 5′ end. 1: VirE2 coats the T-strand, protects it from degradation, and maintains it in a transportable conformation. 2: VirE2 associates with VirE1, required for VirE2 export. 3: VirE2 exits Agrobacterium via the type IV exporter independently, or as part of the T-complex. Alternatively, VirE2 may exit by an alternate pathway (pink; ref. 4). 4: VirE2 forms a pore in the plant plasma membrane allowing passage of the T-complex and coats the T-strand in the plant cytoplasm. 5: VirD2 and VirE2 interact with plant cytoplasmic chaperones (RocA, Roc4, and CypA). Other factors (AtKAPα, VIP1) may target the T-complex to the nucleus. 6: VirE2 interacts with nuclear factors (VIP2) that mediate interaction with chromatin and facilitate integration of the T-strand.
Of great interest is the mechanism that transfers DNA across the inner and outer bacterial membranes and the plasma membrane of the host. Although the specific mechanics are unknown, it is generally assumed that a type IV exporter transfers the T-strand across the bacterial membranes in a process analogous to conjugation. Agrobacterium is the prototypical example of a pathogen using a type IV exporter. Homologs of the exporter proteins are involved in bacterial conjugation, and are virulence proteins in several important human pathogens including Bordetella pertussis (whooping cough), Brucella suis (brucellosis), Helicobacter pylori (gastric ulcers), Legionella pneumophila (Legionnaire's disease), and Rickettsia prowazekii (epidemic typhus) (5, 6).
The biophysical studies of Dumas et al. (7) reported in this issue are surprising because they suggest that a single protein, VirE2 forms a membrane channel that transfers the T-strand through the plant plasma membrane. This result has implications for our understanding of Agrobacterium mediated transformation and, potentially, for delivery of DNA in gene therapy.
VirE2 is one of the most abundant Vir proteins. Fig. 1 summarizes its functions. VirE2 binds the T-strand cooperatively, without sequence specificity, and protects it from degradation (8–10). VirE2 and VirD2 contain nuclear localization sequences (NLS) that promote nuclear uptake of the T-complex (11, 12). VirE2:ssDNA complexes microinjected into plant cells give rise to nuclear accumulation of the ssDNA that can be blocked by nuclear import inhibitors (13). VirE2 may also assist nuclear uptake of the T-complex by keeping the T-strand in an unfolded state (9).
There is little doubt that VirE2 associates with the T-strand in the plant cell; however, there is controversy over whether this also occurs in the bacterium. VirE2's strong cooperative T-strand binding and abundance suggest association in Agrobacterium, as does the observed coimmunoprecipitation of the T-strand with VirE2 from Agrobacterium extracts and immunogold labeling of VirE2 “strings” in situ (8, 14). There is evidence, however, that VirE2 can enter the plant cell independent of the T-strand. First, coinfection with two Agrobacterium strains, one lacking T-strand but containing VirE2, the other lacking VirE2 but containing T-strand DNA, lead to successful plant transformation (15). Neither strain alone is capable of transformation. Second, Agrobacterium lacking VirE2 transforms transgenic plants that express the VirE2 protein (11), demonstrating that VirE2 is not required for T-strand export. Finally, VirE2 export can be inhibited without affecting T-strand export (16, 17).
Given the functions already attributed to VirE2, few would have predicted a direct role in T-strand transfer. VirE2 is hydrophilic with no predicted membrane spanning domains, yet fractionation experiments detect a small but significant portion of VirE2 in the bacterial outer membrane and periplasm (8). This observation prompted Dumas et al. (7) to investigate the significance of VirE2 membrane association. In vitro biophysical approaches demonstrated that VirE2 interacts with lipids and, interestingly, forms large, anion-selective, voltage-gated channels selective for transport of ssDNA.
Formation of pores large enough to allow passage of ssDNA may have deleterious effects in plant cells containing VirE2. Voltage gating in vitro suggests that the opening/closing of the channels may be regulated in vivo. Whereas gating may moderate the effects of pores formed in plant cells, it may be the role of the specific chaperone, VirE1, to prevent the formation of pores in Agrobacterium. VirE1 is required for export of VirE2 from Agrobacterium and may inhibit T-strand binding (18–20).
A pore-forming protein may also be exported by a L. pneumophila type IV exporter (21). Legionella multiplies in human macrophages inside a specialized phagosome that Legionella is able to manipulate and thus inhibit phagosome–lysosome fusion (22). Insertion of a pore-forming protein in the membrane of the phagosomal compartment may interfere with its targeting in the endocytic pathway. Legionella dotB mutants (dotB is homologous to Agrobacterium virB11) are defective in pore insertion (21). Whereas it is unlikely that the Legionella pore transfers DNA, type IV exporters in other bacterial pathogens may insert pore proteins in the membranes of their hosts.
The results of Dumas et al. (7) may assist development of gene therapy technologies. VirE2 mediated transformation may avoid problems inherent in the use of viral delivery systems. Once transported across the plasma membrane of the recipient cell, however, additional factors may be required for nuclear uptake and integration of the DNA. These factors may be lacking in non-plant hosts. For example, VirD2 contains an NLS that allows its nuclear import in animal and yeast cells (23–26). Although VirE2 localizes to plant nuclei (11, 13, 27), it does not exhibit nuclear import in intact animal cells (25, 26, 28). None of these experiments address integration of the DNA in the host genome. It is likely that specific host factors are required at this critical step.
Plant proteins have been identified in Arabidopsis thaliana that physically interact with VirD2 and VirE2 (28). AtKAPα, which belongs to a protein family known to mediate nuclear import (29), interacts with the VirD2 NLS, but not with VirE2 (30). Three isoforms of cyclophilins, RocA, Roc4, and CypA, also interact with VirD2 and may act as VirD2 chaperones in the plant cell (31). Two VirE2 interacting proteins, VIP1 and VIP2, have been identified (1). When expressed in yeast, VIP1, a bZIP protein, promotes nuclear import of VirE2. VIP2 is homologous to Drosophila Rga, a protein thought to mediate interaction between chromatin proteins and transcription complexes (28, 32). VIP2 may promote intranuclear transport or T-strand integration. Similar factors may be required for efficient transformation of non-plant organisms.
In summary, VirE2 performs an unusually large number of functions (their Fig. 1). Dumas et al. (7) report a sixth function: VirE2 forms channels in lipid bilayers. Thus, VirE2 may insert in the plant plasma membrane and facilitate passage of the T-strand (Fig. 1, function 4). Additional work will be required to confirm this proposed function in vivo, and it is critical to test whether these results can be repeated with larger molecules the size of the T-strand. Interesting questions arise from their results. How might VirE2, after membrane insertion, “uninsert” so as to participate in nuclear import? Does VirE1 also participate in the insertion process? The resourcefulness of the VirE2 protein is remarkable, performing multiple critical functions at several points in T-strand transfer. Will future research determine a seventh function? Perhaps, after evolving six functions, virE2 rested.
Acknowledgments
We thank V. Citovsky for critical comments and personal communications. The authors acknowledge the support of a National Science Foundation Postdoctoral Research Fellowship in Microbiology (to D.V.W.) and the Novartis Agriculture Discovery Institute (to the Department of Plant and Microbial Biology at the University of California, Berkeley).
Footnotes
See companion article on page 485.
References
- 1.Tzfira T, Citovsky V. Mol Plant Pathol. 2000;1:201–212. doi: 10.1046/j.1364-3703.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 2.Zupan J, Muth T R, Draper O, Zambryski P. Plant J. 2000;23:11–28. doi: 10.1046/j.1365-313x.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Oger P M, Schrammeijer B, Hooykaas P J, Farrand S K, Winans S C. J Bacteriol. 2000;182:3885–3895. doi: 10.1128/jb.182.14.3885-3895.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Li C M, Nester E W. Proc Natl Acad Sci USA. 2000;97:7545–7550. doi: 10.1073/pnas.120156997. . (First Published June 13, 2000; 10.1073/pnas.120156997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie P J. Trends Microbiol. 1997;5:264–265. doi: 10.1016/S0966-842X(97)88833-6. [DOI] [PubMed] [Google Scholar]
- 6.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 7.Dumas F, Duckely M, Pelczar P, Van Gelder P, Hohn B. Proc Natl Acad Sci USA. 2001;98:485–490. doi: 10.1073/pnas.011477898. . (First Published January 9, 2001; 10.1073/pnas.011477898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christie P J, Ward J E, Winans S C, Nester E W. J Bacteriol. 1988;170:2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citovsky V, Wong M L, Zambryski P. Proc Natl Acad Sci USA. 1989;86:1193–1197. doi: 10.1073/pnas.86.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das A. Proc Natl Acad Sci USA. 1988;85:2909–2913. doi: 10.1073/pnas.85.9.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citovsky V, Zupan J, Warnick D, Zambryski P. Science. 1992;256:1802–1805. doi: 10.1126/science.1615325. [DOI] [PubMed] [Google Scholar]
- 12.Howard E A, Zupan J R, Citovsky V, Zambryski P C. Cell. 1992;68:109–118. doi: 10.1016/0092-8674(92)90210-4. [DOI] [PubMed] [Google Scholar]
- 13.Zupan J R, Citovsky V, Zambryski P. Proc Natl Acad Sci USA. 1996;93:2392–2397. doi: 10.1073/pnas.93.6.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorstenson Y R, Zambryski P C. J Bacteriol. 1994;176:1711–1717. doi: 10.1128/jb.176.6.1711-1717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otten L, De Greve H, Leemans J, Hain R, Hooykaas P, Schell J. Mol Gen Genet. 1984;195:159–163. [Google Scholar]
- 16.Binns A N, Beaupre C E, Dale E M. J Bacteriol. 1995;177:4890–4899. doi: 10.1128/jb.177.17.4890-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee L Y, Gelvin S B, Kado C I. J Bacteriol. 1999;181:186–196. doi: 10.1128/jb.181.1.186-196.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundberg C, Meek L, Carroll K, Das A, Ream W. J Bacteriol. 1996;178:1207–1212. doi: 10.1128/jb.178.4.1207-1212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X R, Christie P J. J Bacteriol. 1999;181:4342–4352. doi: 10.1128/jb.181.14.4342-4352.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng W, Chen L, Peng W T, Liang X, Sekiguchi S, Gordon M P, Comai L, Nester E W. Mol Microbiol. 1999;31:1795–1807. doi: 10.1046/j.1365-2958.1999.01316.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz M A. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Relic B, Andjelkovic M, Rossi L, Nagamine Y, Hohn B. Proc Natl Acad Sci USA. 1998;95:9105–9110. doi: 10.1073/pnas.95.16.9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziemienowicz A, Gorlich D, Lanka E, Hohn B, Rossi L. Proc Natl Acad Sci USA. 1999;96:3729–3733. doi: 10.1073/pnas.96.7.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guralnick B, Thomsen G, Citovsky V. Plant Cell. 1996;8:363–373. doi: 10.1105/tpc.8.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee Y, Gurel F, Gafni Y, Dingwall C, Citovsky V. Nat Biotechnol. 2000;18:433–437. doi: 10.1038/74500. [DOI] [PubMed] [Google Scholar]
- 27.Gelvin S B. J Bacteriol. 1998;180:4300–4302. doi: 10.1128/jb.180.16.4300-4302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzfira T, Rhee Y, Chen M H, Kunik T, Citovsky V. Annu Rev Microbiol. 2000;54:187–219. doi: 10.1146/annurev.micro.54.1.187. [DOI] [PubMed] [Google Scholar]
- 29.Powers M A, Forbes D J. Cell. 1994;79:931–934. doi: 10.1016/0092-8674(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 30.Ballas N, Citovsky V. Proc Natl Acad Sci USA. 1997;94:10723–10728. doi: 10.1073/pnas.94.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng W, Chen L, Wood D W, Metcalfe T, Liang X, Gordon M P, Comai L, Nester E W. Proc Natl Acad Sci USA. 1998;95:7040–7045. doi: 10.1073/pnas.95.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frolov M V, Benevolenskaya E V, Birchler J A. Genetics. 1998;148:317–329. doi: 10.1093/genetics/148.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]