Abstract
A spectrum of enteropathies, characterized by small intestinal inflammation, reduced absorptive capacity, and increased intestinal permeability, commonly affect people in developing countries. This subclinical intestinal pathology facilitates microbial translocation across the compromised intestinal barrier, leading to chronic systemic inflammation that may adversely impact health. Environmental enteropathy (EE), ubiquitous among people living in unhygienic conditions, likely mediates two interlinked public health problems of childhood, stunting and anemia, and underlies poor oral vaccine efficacy in developing countries. Human immunodeficiency virus (HIV) enteropathy, which frequently overlaps with EE, may contribute to immune activation and modulate HIV disease progression. The interacting effects of infection and enteropathy drive a vicious cycle that can propagate severe acute malnutrition, which underlies almost half of under-5-y deaths. Enteropathies are therefore highly prevalent, interacting causes of morbidity and mortality in developing countries. Interventions to prevent or ameliorate enteropathies have potential to improve the health of millions of people in developing countries.
Almost half a century ago it was recognized that most people living in developing countries had an abnormality of the small intestine (Figure 1).1,2 Histologically, this lesion was characterized by flattened villi and an inflammatory infiltrate, much like a milder form of celiac disease, but the vast majority of people appeared healthy. The disorder was labeled “tropical enteropathy” (TE) and was hypothesized to arise from unhygienic environmental conditions, because among migrants relocating to developed nations the condition slowly resolved.3 Some 30 years after these original observations, a series of studies in different populations around the world showed that TE was present across the tropics, but was absent in some tropical populations of high socio-economic status such as Qatar and Singapore.4 These studies used thin-layer chromatography to measure fractional excretion of four non-metabolizable sugars (xylose, rhamnose, lactulose, and 3-O-methyl D-glucose) as an assessment of intestinal permeability, although several groups assessed permeability using only lactulose and mannitol, which can be measured at higher throughput. Using this simpler approach, TE was revisited in studies from the Gambia that set out to explore the relationship between enteropathy and poor growth in infancy.5,6 From as early as 3–6 months of age, infants had increased intestinal permeability (raised L/M ratio), which correlated inversely with weight and length growth.6 This work refocused interest on TE, from being an incidental observation among people living in conditions of poverty, to being a disorder potentially underlying poor growth, which is so frequently observed among children in developing countries.7,8 However, direct demonstration of causality was not possible, nor was it established by what mechanism increased intestinal permeability might lead to growth failure.
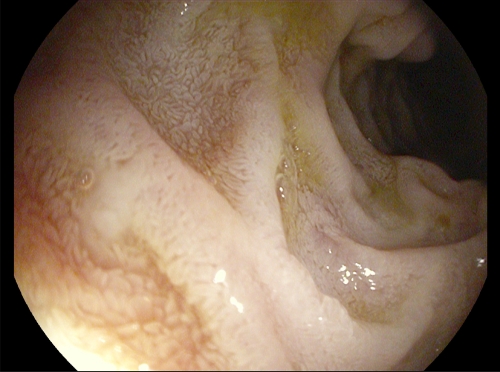
Figure 1.
Endoscopic appearance of environmental enteropathy. Endoscopic view of second part of the duodenum showing villi with characteristic changes of environmental enteropathy: fusion of villi so that instead of a finger-like appearance they take on a leaf-shaped appearance. Sometimes villous fusion goes further and takes on a cerebriform (sulcus and gyrus) appearance. Endoscopic image from Endoscopy Unit, University Teaching Hospital, Lusaka (P. Kelly).
The Importance Of The Intestinal Barrier
The intestinal mucosa has to maintain a barrier between the body's largest microbial ecosystem (containing ∼100 trillion organisms) and the sterile bloodstream. Intestinal barrier function comprises several distinct components: first, a mechanical barrier formed by the single layer of epithelial cells, which possess a continuous brush border and are joined by adherens junctions and tight junctions; second, an antimicrobial barrier, composed of defensins, immunoglobulins, and mucins secreted by the epithelial cells; third, an immunological barrier due to innate pattern recognition receptors in the mucosa together with immune cells in the subepithelial layer; and fourth, an ecological barrier created by the gut microbiota that deters pathogens. Disruption of this barrier increases intestinal permeability, enabling luminal contents, including bacteria and microbial-associated products, to leak into the systemic circulation, a process termed microbial translocation (MT). Probes used as markers of abnormal permeation, such as lactulose, have an intermediate molecular size (molecular weight 150–400 Da) and clearly do not reflect the pore size required for translocation of lipopolysaccharide (LPS) (> 10,000 Da) or a whole bacterium (typical size 0.5 × 2 μM), but it is believed that when lactulose permeation is increased, a “leak pathway” is established that enables macromolecules to cross the intestinal barrier.
In the Gambia, researchers went on to show that infants had evidence of endotoxin (LPS) in the blood, together with anti-endotoxin core antibodies (EndoCAb), which correlated with the degree of intestinal permeability.5 Furthermore, there was other evidence of chronic, low level immunostimulation, including raised lymphocyte and platelet counts, elevated C-reactive protein, and high plasma immunoglobulins, which correlated inversely with growth.5 Thus, a biologic model emerged in which infants living in conditions of poor sanitation and hygiene develop a chronic inflammatory enteropathy soon after birth, leading to reduced intestinal barrier function and passage of microbial-associated products across a “leaky gut” into the systemic circulation, where they chronically activate the immune system, leading to impaired growth.
What Causes Tropical Enteropathy?
Tropical enteropathy is a misnomer; although intestinal pathology varies geographically, TE is not confined to the tropics, and not all people in the tropics have enteropathy (see above). It is environmental conditions, rather than the location per se, that seem critical.4 TE is certainly seasonal, and its severity appears to be predicted by intestinal carriage of organisms with pathogenic potential, such as Citrobacter rodentium and hookworm.9 It is not found in newborns and fetuses in these settings,10 suggesting that unhygienic conditions during early childhood initiate a chronic intestinal pathology that only resolves once living standards are improved.11,12 Tropical enteropathy has therefore rightly been renamed “environmental enteropathy” (EE), although the precise etiology remains elusive. It is likely that repeated, often subclinical episodes of gastrointestinal infection with low doses of organisms possessing certain virulence factors, together with altered composition of the intestinal microbiota, lead to chronic enteric T cell-mediated inflammation.13 Counterintuitively, EE is associated with reduced expression of intestinal antimicrobial peptides.14
The Impact Of Enteropathies On Children In The Developing World
Stunting (low height-for-age [HAZ]) affects 32% of children living in developing countries and has a major impact on child health and development.7 Chronic undernutrition contributes to one-third of mortality in children < 5 years of age, leads to fewer years at school, reduced adult productivity, and an increased risk of stunting in subsequent generations.7 The first 2 years are a critical time for linear growth and, in particular, for brain growth and development of complex neural networks. Stunting develops between –9 months (fetal life) and +24 months (termed the critical first thousand days), and its effects are irreversible. Nutritional interventions have only had a marginal impact,15 leading researchers to consider other potential pathways leading to stunting.
Diarrhea is frequent among children living in conditions of poor sanitation and hygiene and has been shown to impact growth.16 –20 A pooled analysis of nine studies, conducted between 1978 and 1998 in Africa, Asia, and the Americas, showed a cumulative impact of diarrheal episodes on stunting by 2 years of age, with a similar effect across all studies.21 Interventions to improve hand washing, sanitation, and hygiene have been shown to reduce episodes of diarrhea.22 However, the estimated impact of improved sanitation and hygiene, achieved at 99% coverage, is only a 2.4% reduction in prevalence of stunting when modeled entirely through reductions in diarrhea.23 This modest impact may be because children show “catch up” growth between acute diarrheal episodes24,25; in contrast, children living in poor conditions do not recover from the chronic effects of EE, which may therefore be a more important pathway to stunting than is diarrhea.5
Anemia is a similar prevalent and intractable problem of young children in developing countries that often co-exists with stunting and whose etiology is frequently unexplained.26 The same chronic inflammatory pathway that is hypothesized to link enteropathy and stunting may plausibly lead to an anemia of chronic disease (Figure 2), although this potential mechanism has received little attention to date.
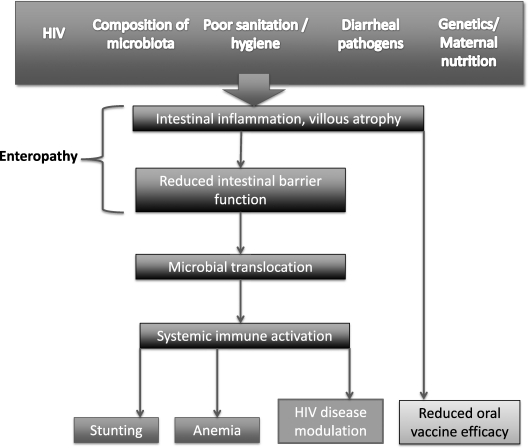
Figure 2.
Proposed causal pathway linking enteropathy with adverse health outcomes in developing countries. Several biologic, environmental, and possibly genetic factors promote the development of enteropathy, which is characterized by abnormal intestinal architecture and increased permeability. Enteropathy enables microbial translocation, which causes generalized activation of the innate and adaptive immune system. This chronic inflammatory pathway may mediate stunting and anemia in children living in developing countries; exacerbate immune activation and CD4 decline in human immunodeficiency virus (HIV)-infected individuals; and reduce oral vaccine efficacy.
Environmental enteropathy may also reduce the efficacy of oral vaccines, with major public health implications for children living in countries where efficacious vaccines are most needed.27 Although oral rotavirus vaccination is now recommended by the World Health Organization (WHO), its efficacy against severe rotavirus gastroenteritis in multi-country trials was far lower in sub-Saharan Africa (39.3%, 95% confidence interval [CI] = 19.1–54.7)28 and Asia (48.3%, 95% CI = 22.3–66.1)29 than in Europe/USA, where overall efficacy is 85–98%.30,31 The impact of EE on reducing vaccine efficacy may occur either through induction of regulatory T cells, which dampen the vaccine-specific immune response, or through destruction of the live attenuated vaccine by an over-vigorous local immune response.27 Alternatively, EE and reduced vaccine efficacy may both be effects of an extensive immunological disturbance. Data from a birth cohort in Dhaka, Bangladesh, in which 42% of children were stunted by 12 months of age, illustrate the link between undernutrition, enteropathy, and poor vaccine responses. Children who were stunted (HAZ < –2) by 6 months of age had significantly lower oral polio vaccine (OPV) responses at 1 year of age, compared with those who were not stunted by 6 months of age. Furthermore, there was a negative correlation between OPV serotype 3 responses and serum EndoCAb levels, suggesting that both enteropathy and undernutrition may be associated with OPV failure.32 The ongoing PROVIDE trial (www.clinicaltrials.gov identifier NCT01375647) is exploring the biologic basis for underperformance of enteric vaccines among children in Bangladesh.
Enteropathy Is A Feature Of HIV Infection
By the 1980s, as the human immunodeficiency virus (HIV) pandemic emerged in sub-Saharan Africa, it was apparent that individuals progressing to acquired immunodeficiency syndrome (AIDS) frequently presented with diarrhea and weight loss (termed “slim disease”).33 Individuals with advanced disease developed a chronic, inflammatory enteropathy, frequently characterized by malabsorption and increased intestinal permeability.34,35 However, before late-stage AIDS most HIV-infected individuals had fairly normal gut structure and function.34,35 More recently, attention has refocused on the impact of HIV on the gut, following the observation that damage to the intestine may modulate HIV disease progression even before symptomatic gastrointestinal disease.36
HIV enteropathy is indistinguishable qualitatively from EE, and where these two enteropathies co-exist they differ only in degree. In a large quantitative analysis of 238 asymptomatic adults biopsied repeatedly over 3 years, crypt depth but not villous height was influenced by HIV; changes in absorption and permeability were only seen in late-stage HIV infection (CD4 count < 200 cells/μL).9 In patients with HIV-related diarrheal disease, changes in villous height were much more pronounced, irrespective of the infectious diagnosis; villous height correlated positively with nutritional status and negatively with markers of circulating TNF pathway activation.37 To our knowledge, eight published studies have reported morphometric (N = 6) and/or permeability (N = 4) data in both early and late HIV infection.9,34,35,37–41 Of the morphometric studies, four9,34,37,39 found no evidence of villous changes in early HIV infection but two38,40 did. Crypt changes were observed in early infection in two9,34 but not in others37–40; generally it was the larger studies that found the difference. Importantly, of the permeability studies, none found any evidence of enteropathy in early HIV.9,34,35,41 Taken together, although there are some enteropathic changes (crypt hyperplasia, cytokine dysregulation) in early HIV infection, the most important changes from the perspective of MT are predominantly seen in advanced disease.
It is well recognized that mucosal T cell depletion occurs in HIV infection.42 Recent studies have defined this immunological hit in more detail: the Th17 population of CD4 cells is most severely depleted and this occurs earlier in HIV infection than might have been suspected before refined techniques to define T cell subsets became available.43 Because Th17 cells play a major role in defense against bacterial, fungal, and parasitic infections, this may explain the spectrum of intestinal pathology seen in HIV infection. There is evidence that HIV itself can directly disrupt mucosal integrity of primary epithelial cells in vitro by down-regulating tight junction proteins, leading to increased intestinal permeability.44 Brenchley and others36 showed that mucosal damage enables MT to occur in HIV-infected individuals. In their study, the level of circulating microbial products correlated with the degree of immune activation, which is the hallmark of HIV infection and best indicator of disease progression.36 Intriguingly, sooty mangabeys, who develop non-pathogenic simian immunodeficiency virus infection, preserved Th17 cells in the gut and did not show signs of MT or immune activation.36 However, it remains debatable whether MT is a cause or consequence of advanced HIV disease.45 Several studies have confirmed the finding of higher levels of LPS in HIV-infected, compared with HIV-uninfected, individuals, and that LPS levels do not completely normalize despite effective antiretroviral therapy (ART)46–48; however, in these studies the level of MT did not correlate with HIV disease progression, whether assessed by viral load/CD4 count,46 proportion of activated T cells,48 or speed of clinical disease progression.47 In contrast, a recent case-control study of HIV-infected individuals in the SMART trial showed that those with the highest quartile of soluble CD14 levels, a marker of monocyte responsiveness to LPS, had a 6-fold higher risk of death compared with those in the lowest quartile.49 Taken together, although there is undoubtedly evidence of increased MT in the context of HIV infection, its contribution to disease progression remains uncertain. HIV enteropathy may be a modulator of disease progression, which is primarily driven by viral replication. Virologic suppression with ART is the most effective treatment to reduce the immune activation that underlies disease progression. However, residual MT and immune activation frequently persist, despite virologic control, and are associated with increased morbidity and a poor immunologic response to ART.36,49–52 Current trials (reviewed below) are exploring whether adjunctive interventions to improve enteropathy may modulate HIV disease outcome in the era of ART.
The Overlapping And Interacting Nature Of Enteropathies
The HIV epidemic affects populations already blighted by chronic stunting and food insecurity. HIV and malnutrition overlap, and the interacting effects of infection, enteropathy and immunodeficiency propagate morbidity and mortality. The spectrum of enteropathies caused by HIV, poor sanitation/hygiene, persistent diarrhea, inadequate diet, and severe acute malnutrition (SAM) compound each other (Figure 3). The overlapping and interacting nature of enteropathies was elegantly demonstrated in an early study from the Gambia,53 in which intestinal permeability was shown to rise progressively in a comparison of asymptomatic children (mean L/M ratio 0.42), well but undernourished children (L/M 0.52), malnourished children (L/M 1.3), and children with persistent diarrhea (L/M 2.85). Although the children who showed clinical recovery demonstrated improvement in intestinal integrity, many children do not recover from the persistent diarrhea-malnutrition cycle, particularly in the context of HIV infection where immunodeficiency and HIV enteropathy compound the situation further.
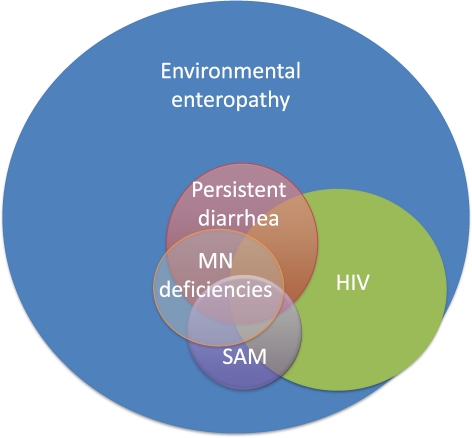
Figure 3.
Overlapping causes of enteropathy in the developing world. There are multiple underlying causes of enteropathy among people living in developing countries, which overlap on a background of environmental enteropathy. Environmental enteropathy is almost universal among people living in conditions of poverty. Overlapping causes of enteropathy shown here are based on known associations, but the size of each overlap is speculative and is not meant to represent prevalence. MN = micronutrient; SAM = severe acute malnutrition.
Malnutrition has therefore been viewed as an enteric infectious disease because of the vicious cycle in which diarrhea exacerbates malnutrition and malnutrition exacerbates diarrhea (reviewed in Reference 54). The impact of diarrheal episodes on weight and height has been clearly documented in multiple field studies. In a Guatemalan study undertaken in the 1960s, Mata55 elegantly demonstrated the progressive decline across growth centiles that occurred with each successive episode of diarrhea during early childhood. Most recently, the Global Enterics Multi-Center Study has provided new data on the profound sequelae of diarrheal episodes among children 0–59 months of age in seven African/Asian countries. Compared with age, gender, and location-matched controls, cases had a significant decrease in linear growth and a striking 7-fold (rate ratio 7.1; 95% CI 5.0, 10.1) increase in mortality 60 days after an episode of moderate-severe diarrhea.56 It is similarly well recognized that malnutrition impacts diarrhea.57–60 In an intensive prospective study of 61 children < 5 years of age in a Brazilian urban slum, children with malnutrition, measured either as low weight-for-age or low HAZ, had an increased incidence and duration of diarrhea in the subsequent 2 months, compared with better nourished children.60 The overlapping impact of enteric infection and malnutrition likely arises from a combination of impaired intestinal barrier function together with reduced capacity for mucosal repair caused by poor nutrient stores; reduced absorptive capacity, and nutrient loss, particularly in cases of persistent diarrhea; and reduced appetite in the face of increased catabolic demands.
The reciprocal interactions between malnutrition and enteropathy are well illustrated in a recent Dhaka birth cohort study.61 Enteropathy was shown to influence malnutrition, because both prolonged diarrhea during infancy and impaired intestinal barrier function (as measured by elevated serum EndoCAb at 6 months of age) were associated with stunting at 1 year of age; conversely, malnutrition was shown to influence enteropathy, because children who were born stunted were more likely to develop severe diarrhea, infection with Entamoeba histolytica, and diarrhea caused by Cryptosporidium and enterotoxigenic Escherichia coli during the first year of life. This study highlights the importance of preventing or treating enteropathy to reduce malnutrition, and the inter-generational impact of maternal undernutrition on infant enteropathy and malnutrition.
Other Enteropathies In The Developing World
Dietary factors also exacerbate enteropathy among children living in developing countries. Zinc deficiency, estimated to affect more than 20% of people worldwide,62 causes structural intestinal changes characterized by villous atrophy and reduced crypt cell production rate.63 Zinc deficient children experience increased diarrheal morbidity64; supplementation with zinc reduces the severity and duration of both acute and persistent diarrhea.65 EE may also exacerbate zinc deficiency by reducing resorption of endogenous zinc from the small intestine, which is critical for zinc homeostasis.66 Thus, zinc deficiency and EE are interacting factors that may propagate overt clinical disease in children with overlapping causes of enteropathy. Children with poor quality diets may also be exposed to aflatoxin, a fungal metabolite that contaminates inadequately stored crops such as maize and peanuts.67 Aflatoxin impairs intestinal integrity in animal models68 and is associated with stunting in children,67,69 potentially through the same pathway as EE.
It is also apparent that some emerging diseases of the developing world are associated with an underlying enteropathy. Obesity, diabetes, and the metabolic syndrome are all characterized by increased intestinal permeability.70 Mice receiving a high-fat diet have raised LPS levels, and the resulting immune activation causes insulin resistance through cytokine-mediated phosphorylation of insulin receptor 1.71 Given the rising prevalence of these “developed” diseases in areas of the developing world that are undergoing economic transition,72–74 enteropathies become an even more important pathological process in this setting that require further recognition and research.
Diagnosis, Prevention, And Treatment Of Enteropathies
Several large programs are currently addressing priority research and implementation questions related to intestinal infections and EE.75 The MAL-ED project (http://mal-ed.fnih.org/) is investigating the link between EE and malnutrition among children in eight countries. A panel of investigations conducted on longitudinal birth cohorts recruited at each site are assessing intestinal inflammation and barrier function, enteric infections, growth/development, and vaccine responses, to better inform rational design of intervention strategies to break the enteropathy-malnutrition cycle. One of the major challenges in young children, in the absence of intestinal biopsy material, is the paucity of well-validated, non-invasive biomarkers to assess enteropathy.75 The MAL-ED program is evaluating a range of enteropathy markers in urine, feces, and blood that may be associated with poor growth in early childhood.
Several large field programs currently underway are investigating strategies to reduce malnutrition and/or diarrheal disease among children in developing countries. Alive & Thrive (www.aliveandthrive.org) is a 5-year initiative underway in Ethiopia, Bangladesh, and Viet Nam, aimed at improving exclusive breastfeeding and complementary feeding practices in the first 2 years of life. These strategies have proven efficacy in reducing infant mortality, reducing frequency and severity of diarrhea, and improving long-term nutritional status,76 but are currently poorly adopted in many settings. Two trials due to start recruitment shortly in Zimbabwe (SHINE) and Kenya/Bangladesh (WASH Benefits) will test the hypothesis that improved sanitation/hygiene from birth will prevent or ameliorate EE and reduce childhood stunting. Another cluster-randomized trial underway in Orissa, India, (www.clinicaltrials.gov identifier NCT01214785) is investigating the impact of latrine construction and use on diarrheal disease, intestinal helminth infections, and nutritional status. A further trial in Malawi (NCT01440608) is investigating the effectiveness of high-dose zinc treatment or deworming with albendazole on intestinal permeability in rural 1–3 year old children at high risk of EE.
Previous trials have generally shown disappointing results with regards to treatment of underlying enteropathy in children with malnutrition, although interpretation of trial results is partly hindered by the absence of well-validated markers. Trehan and others77 conducted a double-blind, placebo-controlled trial of rifaximin, a non-absorbable antibiotic, in 3–5 year old rural Malawian children with EE. After 7 days of rifaximin or placebo, administered under direct observation of study staff, intestinal permeability as measured by the L/M ratio was not significantly different between groups, although no measures of MT were untaken in this study. The same research group undertook a trial of the probiotic Lactobacillus GG in rural Malawi, but again demonstrated no difference in intestinal integrity between groups after 30 days of probiotic or placebo.78 A probiotic and prebiotic functional food, given to Malawian children with SAM following stabilization, showed no improvement in nutritional cure compared with control.79 However, this does not necessarily imply that these approaches are futile. Choice of antibiotic/probiotic, treatment duration, age group, and primary outcome may all impact results, and some of these strategies should continue to be explored in future trials.
Several trials in HIV-infected individuals are investigating strategies to target enteropathy and reduce immune activation. One trial (NCT01090102) is using 5-aminosalicylic acid, an oral anti-inflammatory agent widely used in inflammatory bowel disease, to reduce enteric inflammation. Other trials are aiming to reduce MT, using either probiotics (NCT01184456), a prebiotic/probiotic mixture (NCT00688311), or the non-absorbed antibiotic rifaximin (ACTG trial 5286). It is hoped that results of these trials will clarify the role of enteropathy in HIV infection and may inform choice of adjunctive therapies, particularly for HIV-infected individuals with poor immunologic response to ART, in whom residual immune activation is known to be contributory.50
Next Steps
There is a fundamental need to better understand the basic science underlying enteropathies. The interaction between enterocytes, pathogens, and the mucosal immune system is likely to be critical in development and resolution of enteropathy. It is also becoming apparent that the intestinal microbiota has a profound impact on gut structure and function and nutritional status. Developments in high-throughput sequencing and gnotobiotic mouse models now enable characterization of the composition and function of gut microbial communities.80 Food type and nutrient load both impact the gut microbiota; conversely, changes in the gut microbiota impact nutrient use and energy balance.80 Transfer by gavage of the microbiota from a child with SAM into a gnotobiotic mouse causes malnutrition in the animal, and therapeutic feeding causes shifts in the composition and functional profile of the gut microbial community, demonstrating the bidirectional interaction between the microbiota and nutritional status.81
In summary, there is a strong rationale for developing interventions that prevent or ameliorate enteropathy, with the aim of reducing stunting, anemia, malnutrition, and improving HIV disease outcome. Such interventions have the potential to improve the health of millions of people in developing countries, especially among children < 5 years of age, who are most affected by the overlapping epidemics of stunting, malnutrition, and HIV.
Footnotes
Authors' addresses: Andrew Prendergast, Centre for Paediatrics, Blizard Institute, Queen Mary University of London, UK, E-mail: a.prendergast@qmul.ac.uk. Paul Kelly, Centre for Digestive Diseases, Blizard Institute, Queen Mary University of London, UK, E-mail: m.p.kelly@qmul.ac.uk.
References
- 1.Desai HG, Borkar AV, Pathare SM, Dighe PK, Jeejeebhoy KN. ‘Flat’ jejunal mucosa in the tropics. Indian J Med Sci. 1969;23:1–5. [PubMed] [Google Scholar]
- 2.Schenk EA, Samloff IM, Klipstein FA. Morphology of small bowel biopsies. Am J Clin Nutr. 1968;21:944–961. doi: 10.1093/ajcn/21.9.944. [DOI] [PubMed] [Google Scholar]
- 3.Lindenbaum J, Harmon JW, Gerson CD. Subclinical malabsorption in developing countries. Am J Clin Nutr. 1972;25:1056–1061. doi: 10.1093/ajcn/25.10.1056. [DOI] [PubMed] [Google Scholar]
- 4.Menzies IS, Zuckerman MJ, Nukajam WS, Somasundaram SG, Murphy B, Jenkins AP, Crane RS, Gregory GG. Geography of intestinal permeability and absorption. Gut. 1999;44:483–489. doi: 10.1136/gut.44.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:1332–1338. doi: 10.1093/jn/133.5.1332. [DOI] [PubMed] [Google Scholar]
- 6.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–910. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]
- 7.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 8.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–e480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 9.Kelly P, Menzies I, Crane R, Zulu I, Nickols C, Feakins R, Mwamsa K, Mudenda V, Katubulushi M, Greenwald S, Farthing M. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70:412–419. [PubMed] [Google Scholar]
- 10.Baker SJ. Subclinical intestinal malabsorption in developing countries. Bull World Health Organ. 1976;54:485–494. [PMC free article] [PubMed] [Google Scholar]
- 11.Gerson CD, Kent TH, Saha JR, Siddiqi N, Lindenbaum J. Recovery of small-intestinal structure and function after residence in the tropics. II. Studies in Indians and Pakistanis living in New York City. Ann Intern Med. 1971;75:41–48. doi: 10.7326/0003-4819-75-1-41. [DOI] [PubMed] [Google Scholar]
- 12.Lindenbaum J, Gerson CD, Kent TH. Recovery of small-intestinal structure and function after residence in the tropics. I. Studies in Peace Corps volunteers. Ann Intern Med. 1971;74:218–222. doi: 10.7326/0003-4819-74-2-218. [DOI] [PubMed] [Google Scholar]
- 13.Veitch AM, Kelly P, Zulu IS, Segal I, Farthing MJ. Tropical enteropathy: a T-cell-mediated crypt hyperplastic enteropathy. Eur J Gastroenterol Hepatol. 2001;13:1175–1181. doi: 10.1097/00042737-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Dhaliwal W, Bajaj-Elliott M, Kelly P. Intestinal defensin gene expression in human populations. Mol Immunol. 2003;40:469–475. doi: 10.1016/s0161-5890(03)00156-1. [DOI] [PubMed] [Google Scholar]
- 15.Dewey KG, Adu-Afarwuah S. Maternal and Child Nutrition. Blackwell Publishing Ltd; Program in International and Community Nutrition, University of California, Davis, CA: 2008. (Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 17.Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157:166–175. doi: 10.1093/aje/kwf179. [DOI] [PubMed] [Google Scholar]
- 18.Rowland MG, Rowland SG, Cole TJ. Impact of infection on the growth of children from 0 to 2 years in an urban West African community. Am J Clin Nutr. 1988;47:134–138. doi: 10.1093/ajcn/47.1.134. [DOI] [PubMed] [Google Scholar]
- 19.Assis AM, Barreto ML, Santos LM, Fiaccone R, da Silva Gomes GS. Growth faltering in childhood related to diarrhea: a longitudinal community based study. Eur J Clin Nutr. 2005;59:1317–1323. doi: 10.1038/sj.ejcn.1602245. [DOI] [PubMed] [Google Scholar]
- 20.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30:1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 21.Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Mølbak K, Valentiner-Branth P, Lanata CF, Black RE, Childhood Malnutrition, Infection Network. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 23.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP, Shekar M. Maternal and Child Undernutrition Study Group What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 24.Briend A, Hasan KZ, Aziz KM, Hoque BA. Are diarrhoea control programmes likely to reduce childhood malnutrition? Observations from rural Bangladesh. Lancet. 1989;2:319–322. doi: 10.1016/s0140-6736(89)90498-4. [DOI] [PubMed] [Google Scholar]
- 25.Moy RJ, de C Marshall TF, Choto RG, McNeish AS, Booth IW. Diarrhoea and growth faltering in rural Zimbabwe. Eur J Clin Nutr. 1994;48:810–821. [PubMed] [Google Scholar]
- 26.DeMaeyer E, Adiels-Tegman M. The prevalence of anemia in the world. World Health Stat Q Rep. 1985;38:302–316. [PubMed] [Google Scholar]
- 27.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 2010;8:129. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, Levine MM, Lewis K, Attah-Poku M, Ojwando J, Rivers SB, Victor JC, Nyambane G, Hodgson A, Schödel F, Ciarlet M, Neuzil KM. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomized, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 29.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, Le HT, Coia ML, Lewis K, Rivers SB, Sack DA, Schödel F, Steele AD, Neuzil KM, Ciarlet M. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomized, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gzillare P, Innis BL, Gervantes Y, Linhares AC, López P, Macias-Parra M, Ortega-Barria E, Richardson V, Riveria-Medina DM, Rivera L, Salinas B, Pavia-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Torniepporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenoogle A, Clemens R, De Vos B, O'Ryan M. Human Rotavirius Vaccine Study Group Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 31.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Compens D, Karvonen A, Watt JP, O'Brian KL, DiNubile MJ, Boslego JW, Offit PA, Heaton PM. Rotavirus Efficacy and Safety Trial (REST) Study Group Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 32.Petri WA., Jr . Malnutrition, Gut-Microbial Interactions and Mucosal Immunity to Vaccines. New Delhi, India: 2011. (Genetic Basis of Malnutrition). November 7–11. [Google Scholar]
- 33.Serwadda D, Mugerwa RD, Sewankambo NK, Lwegaba A, Carswell JW, Kirya GB, Bayley AC, Downing RG, Tedder RS, Clayden SA, Bayley AC, Downing RG, Tedder RS, Clayden SA. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet. 1985;2:849–852. doi: 10.1016/S0140-6736(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 34.Keating J, Bjarnason I, Somasundaram S, Macpherson A, Francis N, Price AB, Sharpstone D, Smithson J, Menzies IS, Gozzard BG. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995;37:623–629. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapembwa MS, Fleming SC, Sewankambo N, Serwadda D, Lucas S, Moody A, Griffin GE. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 1991;81:327–334. doi: 10.1042/cs0810327. [DOI] [PubMed] [Google Scholar]
- 36.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 37.Kelly P, Davies SE, Mandanda B, Veitch A, McPhail G, Zulu I, Drobniewski F, Fuchs D, Summerbell C, Luo NP, Pobee JO, Farthing JM. Enteropathy in Zambians with HIV related diarrhoea: regression modelling of potential determinants of mucosal damage. Gut. 1997;41:811–816. doi: 10.1136/gut.41.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batman PA, Miller AR, Forster SM, Harris JR, Pinching AJ, Griffin GE. Jejunal enteropathy associated with human immunodeficiency virus infection: quantitative histology. J Clin Pathol. 1989;42:275–281. doi: 10.1136/jcp.42.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummins AG, LaBrooy JT, Stanley DP, Rowland R, Shearman DJ. Quantitative histological study of enteropathy associated with HIV infection. Gut. 1990;31:317–321. doi: 10.1136/gut.31.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller AR, Griffin GE, Batman P, Farquar C, Forster SM, Pinching AJ, Harris JR. Jejunal mucosal architecture and fat absorption in male homosexuals infected with human immunodeficiency virus. Q J Med. 1988;69:1009–1019. [PubMed] [Google Scholar]
- 41.Bjarnason I, Sharpstone DR, Francis N, Marker A, Taylor C, Barrett M, Macpherson A, Baldwin C, Menzies IS, Crane RC, Smith T, Pozniak A, Gazzard BG. Intestinal inflammation, ileal structure and function in HIV. AIDS. 1996;10:1385–1391. doi: 10.1097/00002030-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 43.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenaulat AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redd AD, Gray RH, Quinn TC. Is microbial translocation a cause or consequence of HIV disease progression? J Infect Dis. 2011;203:744–745. doi: 10.1093/infdis/jiq107. author reply 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lester RT, Yao XD, Ball TB, McKinnon LR, Omange WR, Kaul R, Wachihi C, Jaoko W, Rosenthal KL, Plummer FA. HIV-1 RNA dysregulates the natural TLR response to subclinical endotoxemia in Kenyan female sex-workers. PLoS ONE. 2009;4:e5644. doi: 10.1371/journal.pone.0005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redd AD, Dabitao D, Bream JH, Charvat B, Laeyendecker O, Kiwanuka N, Lutalo T, Kigozi G, Tobian AA, Gamiel J, Neal JD, Oliver AE, Margolick JB, Sewankambo N, Reynolds SJ, Wawer MJ, Serwadda D, Gray RH, Quinn TC. Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc Natl Acad Sci USA. 2009;106:6718–6723. doi: 10.1073/pnas.0901983106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, Alfano M, Poli G, Rossouw T. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–733. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 49.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley M, Deeks SG, Sereti I, Douek DC. INSIGHT SMART Study Group Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt PW, Cao HL, Muzoora C, Ssewanyana I, Bennett J, Emenyonu N, Kembabazi A, Neilands TB, Bargsberg DR, Deeks SG, Martin JN. Impact of CD8+ T cell activation on CD4+ T cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25:2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, Morace G, Gori A, Monforte AD. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 53.Behrens RH, Lunn PG, Northrop CA, Hanlon PW, Neale G. Factors affecting the integrity of the intestinal mucosa of Gambian children. Am J Clin Nutr. 1987;45:1433–1441. doi: 10.1093/ajcn/45.6.1433. [DOI] [PubMed] [Google Scholar]
- 54.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mata LJ. The Children of Santa Maria Cauque: A Prospective Field Study of Health and Growth. Cambridge, MA: MIT Press; 1978. [Google Scholar]
- 56.Kotloff KL. “Top 5” attributable pathogens of moderate and severe diarrhea (by age, study site and clinical presentation) and mortality and linear growth consequences. 60th Annual Meeting of the American Society of Tropical Medicine and Hygiene; Philadelphia, PA: 2011. [Google Scholar]
- 57.Black RE, Brown KH, Becker S. Malnutrition is a determining factor in diarrheal duration, but not incidence, among young children in a longitudinal study in rural Bangladesh. Am J Clin Nutr. 1984;39:87–94. doi: 10.1093/ajcn/39.1.87. [DOI] [PubMed] [Google Scholar]
- 58.Palmer DL, Koster FT, Alam AK, Islam MR. Nutritional status: a determinant of severity of diarrhea in patients with cholera. J Infect Dis. 1976;134:8–14. doi: 10.1093/infdis/134.1.8. [DOI] [PubMed] [Google Scholar]
- 59.Tomkins A. Nutritional status and severity of diarrhoea among pre-school children in rural Nigeria. Lancet. 1981;1:860–862. doi: 10.1016/s0140-6736(81)92139-5. [DOI] [PubMed] [Google Scholar]
- 60.Schorling JB, McAuliffe JF, de Souza MA, Guerrant RL. Malnutrition is associated with increased diarrhoea incidence and duration among children in an urban Brazilian slum. Int J Epidemiol. 1990;19:728–735. doi: 10.1093/ije/19.3.728. [DOI] [PubMed] [Google Scholar]
- 61.Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA., Jr Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2011;54:185–192. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wuehler SE, Peerson JM, Brown KH. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Public Health Nutr. 2005;8:812–819. doi: 10.1079/phn2005724. [DOI] [PubMed] [Google Scholar]
- 63.Koo SI, Turk DE. Effect of zinc deficiency on the ultrastructure of the pancreatic acinar cell and intestinal epithelium in the rat. J Nutr. 1977;107:896–908. doi: 10.1093/jn/107.5.896. [DOI] [PubMed] [Google Scholar]
- 64.Bahl R, Bhandari N, Hambidge KM, Bhan MK. Plasma zinc as a predictor of diarrheal and respiratory morbidity in children in an urban slum setting. Am J Clin Nutr. 1998;68((Suppl 2)):414S–417S. doi: 10.1093/ajcn/68.2.414S. [DOI] [PubMed] [Google Scholar]
- 65.Lukacik M, Thomas RL, Aranda JV. A meta-analysis of the effects of oral zinc in the treatment of acute and persistent diarrhea. Pediatrics. 2008;121:326–336. doi: 10.1542/peds.2007-0921. [DOI] [PubMed] [Google Scholar]
- 66.Manary MJ, Abrams SA, Griffin IJ, Quimper MM, Shulman RJ, Hamzo MG, Chen Z, Maleta K, Manary MJ. Perturbed zinc homeostasis in rural 3–5-y-old Malawian children is associated with abnormalities in intestinal permeability attributed to tropical enteropathy. Pediatr Res. 2010;67:671–675. doi: 10.1203/PDR.0b013e3181da44dc. [DOI] [PubMed] [Google Scholar]
- 67.Gong YY, Egal S, Hounsa A, Turner PC, Hall AJ, Cardwell KF, Wild CP. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: the critical role of weaning. Int J Epidemiol. 2003;32:556–562. doi: 10.1093/ije/dyg109. [DOI] [PubMed] [Google Scholar]
- 68.Bouhet S, Oswald IP. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet Immunol Immunopathol. 2005;108:199–209. doi: 10.1016/j.vetimm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, Wild CP. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. BMJ. 2002;325:20–21. doi: 10.1136/bmj.325.7354.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev. 2011;12:272–281. doi: 10.1111/j.1467-789X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 71.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baalwa J, Byarugaba BB, Kabagambe KE, Otim AM. Prevalence of overweight and obesity in young adults in Uganda. Afr Health Sci. 2010;10:367–373. [PMC free article] [PubMed] [Google Scholar]
- 73.Shayo GA, Mugusi FM. Prevalence of obesity and associated risk factors among adults in Kinondoni municipal district, Dar es Salaam Tanzania. BMC Public Health. 2011;11:365. doi: 10.1186/1471-2458-11-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wee BS, Poh BK, Bulgiba A, Ismail MN, Ruzita AT, Hills AP. Risk of metabolic syndrome among children living in metropolitan Kuala Lumpur: a case control study. BMC Public Health. 2011;11:333. doi: 10.1186/1471-2458-11-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salazar-Lindo E, Allen S, Brewster DR, Elliott EJ, Fasano A, Phillips AD, Sanderson IR, Tarr PI. Latin American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Intestinal infections and environmental enteropathy: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39((Suppl 2)):S662–S669. doi: 10.1097/00005176-200406002-00013. [DOI] [PubMed] [Google Scholar]
- 76.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O'Hare D, Schanler RJ, Eidelman AI. American Academy of Pediatrics Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 77.Trehan I, Shulman RJ, Ou CN, Maleta K, Manary MJ. A randomized, double-blind, placebo-controlled trial of rifaximin, a nonabsorbable antibiotic, in the treatment of tropical enteropathy. Am J Gastroenterol. 2009;104:2326–2333. doi: 10.1038/ajg.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galpin L, Manary MJ, Fleming K, Ou CN, Ashorn P, Shulman RJ. Effect of Lactobacillus GG on intestinal integrity in Malawian children at risk of tropical enteropathy. Am J Clin Nutr. 2005;82:1040–1045. doi: 10.1093/ajcn/82.5.1040. [DOI] [PubMed] [Google Scholar]
- 79.Kerac M, Bunn J, Seal A, Thindwa M, Tomkins A, Sadler K, Bahwere P, Collins S. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet. 2009;374:136–144. doi: 10.1016/S0140-6736(09)60884-9. [DOI] [PubMed] [Google Scholar]
- 80.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith MI. Malnutrition, Gut-Microbial Interactions and Mucosal Immunity to Vaccines. New Delhi, India: 2011. (Metagenomic Studies of Humanized Gnotobiotic Mice Harboring the Gut Microbiomes of Malawian Twins Discordant for Kwashiorkor). November 7–11, 2011. [Google Scholar]