Abstract
Two hundred and three Plasmodium falciparum isolates from Jazan area, southwest Saudi Arabia, were typed for Pfcrt, Pfmdr1, dhps, and dhfr mutations associated with resistance to chloroquine, mefloquine, halofantrine, artemisinin, sulfadoxine-pyrimethamine, and the neutral polymorphic gene Pfg377. A large proportion (33%) of isolates harbored double mutant dhfr genotype (51I,59C,108N). However, only one isolate contained mutation dhps-437G. For Pfcrt, almost all examined isolates (163; 99%) harbored the mutant genotype (72C,73V,74I,75E,76T), whereas only 49 (31%) contained the mutant Pfmdr1 genotype (86Y,184F,1034S,1042N), 109 (66%) harbored the single mutant genotype (86N,184F,1034S,1042N), and no mutations were seen in codons 1034, 1042, and 1246. Nonetheless, three new single-nucleotide polymorphisms were detected at codons 182, 192, and 102. No differences were seen in distribution of drug resistance genes among Saudis and expatriates. There was a limited multiplicity (5%), mean number of clones (1.05), and two dominant multilocus genotypes among infected individuals in Jazan. A pattern consistent with limited cross-mating and recombination among local parasite was apparent.
Introduction
The Arabian Peninsula lies at the fringes of malaria endemicity, where successful control efforts have brought local transmission to a halt in many parts of this region. At the same time, limited foci in Yemen and southern Saudi Arabia remain malarious, with a high prevalence of drug-resistant Plasmodium falciparum parasites.1–6
In Saudi Arabia, malaria transmission is confined to southwestern regions (the Aseer and Jazan provinces), where P. falciparum is the prevailing species and Anopheles arabiensis is the main vector.7–11 However, malaria cases are still reported in different parts of the country, mostly brought by travelers from endemic sites in the south or expatriates from outside the country.1,12
Chloroquine was the drug of choice for the treatment of uncomplicated malaria cases for many years. However, the emergence and spread of chloroquine resistance (CQR)13–15 has lead to the introduction of sulfadoxine-pyrimethamine (SP) therapy, which is effective. Nonetheless, recently health authority in Saudi Arabia has adopted artemisinin combination therapy (ACT), consisting of artemisinin plus SP. A recent study has revealed high prevalence of Pfcrt76T and Pfmdr186Y mutations among P. falciparum parasites in Jizan.9 In addition, mutation dhfr59R was suspected12; however, no data were presented on codons 51 and 108 or codons 436, 437, 540, and 581 on dhps, which are critical markers for pyrimethamine and sulfadoxine resistance and subsequent SP failure.
Here, we have extended the above findings and examined 16 single-nucleotide polymorphisms (SNPs) on dhfr, dhps, Pfcrt, and Pfmdr1 genes implicated in resistance to an array of antimalarial drugs, including SP, chloroquine, amodiaquine, artemisinin, and lumefantrine. Some of these genes have recently been suggested to have an antagonistic selective role.16,17 Therefore, the pattern of response of P. falciparum to different combination antimalarial therapy may be influenced by mutations in these genes, and their role in predicting response to combination therapy can be of paramount importance.
Materials and Methods
Study subjects.
Jazan region, southwest Saudi Arabia, is endemic for malaria, where the vast majority of the cases are caused by P. falciparum and An. arabiensis is the main vector. Malaria transmission occurs after the rainy season from November to March and the appearance of Anopheles mosquitoes. P. falciparum in the region was at one time susceptible to chloroquine; however, resistance emerged in the late 1990s,14 and mutations associated with CQR have escalated in the region.9 This finding prompted the change of treatment policy to artemisinin-based combination therapies (ACTs).18
The present study examined 203 samples obtained, with informed consent, from microscopy-confirmed P. falciparum malaria patients from 11 hospitals and polyclinics in Jazan (Figure 1). The majority of patients (163; 80%) were Saudi; 145 (89%) were residents of Jazan, and 18 (11%) were visitors from other parts of the country. The remaining 40 (20%) were expatriates: 18 (45%) were residents, and 22 (55%) were visitors. Most of the patients (85%) were males. Their ages ranged from 2 months to 80 years, the largest group being between 20 and 29 years (16%).
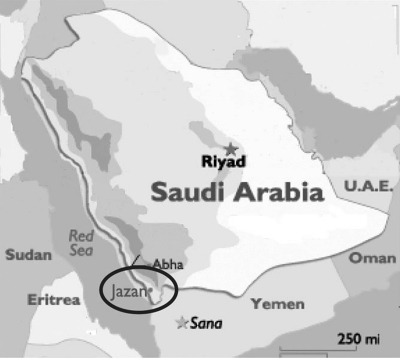
Figure 1.
Geographical location of Jazan, southwest Saudi Arabia. Samples analyzed in this study were collected from 203 patients in 11 hospitals and polyclinics in Jazan region.
Finger prick blood samples were spotted onto filter paper (Whatman 3M, Polybags Ltd., Greenford, Middlesex, UK) and individually sealed in plastic envelopes. All patients who participated in the study signed a consent form, and the study was approved by the Ethics Committee at King Khalid University, Abha, Saudi Arabia.
Dhfr, dhps, Pfcrt, and Pfmdr1 genes.
P. falciparum DNA was prepared from filter paper samples of blood as described previously.19 Alleles of the dhfr, dhps, Pfcrt, and pfdmdr1 genes were amplified using two rounds of polymerase chain reaction (PCR) as described previously.1,12,19–22 The amplified fragment of each gene encompasses mutations associated with pyrimethamine resistance (dhfr-51, -59, -108, and -164), sulfadoxine resistance (dhps-436, -437, -540, and -581), CQR (Pfcrt-72, -74, -75, and -76), and multidrug resistance gene-1 (Pfmdr1-86, -184, -1034, and -1042).
PCR fragments were then sequenced, and mutations at the above genes were identified. The nested PCR products were first purified using ExoSAP-IT (USB) as described by the manufacturer. Sequencing was carried out using BigDye Terminator v3.1 cycle (ABI, United Kingdom) and run on a thermocycler (Gene Amp PCR system 9700; ABI, United Kingdom). Initial sequence comparison with the PlasmoDB database of P. falciparum genomic sequences was carried out with basic local alignment search tool N (BLASTN) BioEdit and Lasergene Software.
Pfg377 gene.
The Pfg377 gene contains four regions, the most polymorphic being region 3, which encodes seven degenerate amino acid repeats; alleles of this gene, thus, vary by multiples of 21 base pairs.23 Thus, Pfg377 was proven to be highly polymorphic in natural P. falciparum and a useful marker for analysis of parasite diversity.24–26 PCR primers and conditions for amplification of region 3 were as described previously,27 with minor modification where the nested primer 377R3D1 was labeled. The fluorescent PCR products were analyzed in an ABI 3100 sequencer, and alleles were visualized and sized on Genescan (Applied Biosystems).
Statistical analysis.
The prevalence of an allele was calculated as its percentage of all the alleles detected at a given locus among the isolates examined. The multiplicity of infection was defined as the proportion of people who carry more than one allele (genotype) for any of the examined genes, and the minimum number of clones per infection was estimated as the largest number of alleles at any of the examined loci.28 SPSS program (16.0.0) has been used to calculate odd ratio (OR), χ2 test, and P value.
Results
dhfr and dhps genotypes.
Tables 1 and 2 show distribution of dhfr and dhps alleles and corresponding genotypes in Jazan among the 176 and 179 P. falciparum isolates examined for dhfr and dhps, respectively. For dhfr, mutations N51I and 108N were frequent, occurring at prevalences of 33% and 34%, respectively. However, other mutations, at codons 59R and 164L, associated with high-level SP resistance were not seen. Similarly, dhps mutations associated with SP resistance at codons 436, 437, 540, and 581 were not seen, with the exception of mutation 437G, which was detected in one isolate (Tables 1 and 2).
Table 1.
Prevalence of alleles of dhfr, dhps, Pfcrt, and Pfmdr and the polymorphic control gene Pfg377 among 165 P. falciparum isolates from Jazan, southwest Saudi Arabia
| dhfr | dhps | Pfcrt | Pfmdr1 | Pfg377 | |||||
|---|---|---|---|---|---|---|---|---|---|
| dhfr alleles | N (%) | dhps alleles | N (%) | Pfcrt alleles | N (%) | Pfmdr1 alleles | N (%) | Pfg377 alleles (bp) | N (%) |
| 108N | 60 (34) | 437G | 1 (0.56) | 74M | 2 (1) | 86Y | 51 (31) | 269 | 2 (1.2) |
| 108S | 117 (66) | 437A | 178 (99.4) | 74I | 162 (99) | 86N | 118 (69) | 289 | 1 (0.6) |
| 51I | 58 (33) | 75N | 2 (1) | 184F | 159 (96) | 310 | 49 (28) | ||
| 51N | 117 (67) | 75E | 162 (99) | 184Y | 5 (3) | 331 | 82 (48) | ||
| 76K | 2 (1) | 352 | 38 (22) | ||||||
| 76T | 162 (99) | ||||||||
Table 2.
Prevalence of dhfr, dhps, pfcrt and pfmdr genotype among 165 P. falciparum isolates in Jazan, southwest Saudi Arabia
| dhfr genotypes | dhps genotypes | Pfcrt genotypes | Pfmdr1 genotypes | ||||
|---|---|---|---|---|---|---|---|
| Wild type (NCSI) | 116 (65.7) | Sensitive (SAKA) | 177 (99.4) | Wild type (CVMNK) | 2 (1) | Wild type (NYSN) | 5 (3) |
| Single mutant (NCNI) | 2 (1.14) | Single mutant (SGKA) | 1 (0.56) | Triple mutant (CVIET) | 162 (99) | Single mutant (NFSN) | 109 (66) |
| Double mutants (ICNI) | 58 (33.9) | Double (YFSN) | 49 (31) | ||||
Wild-type dhfr (N51 C59 S108) and dhps (A437 K540) genotypes were predominant, existing at prevalences of 65.90% and 99.44%, respectively. However, single- (N108) and double-mutant (I51N108) dhfr genotypes were less prevalent at 1.14% and 32.95%, respectively, whereas the triple mutant (I51 R59 N108) associated with high-level pyrimethamine resistance29,30 was not seen in the region (Tables 1 and 2).
Pfcrt genotypes.
CQR-associated mutations in Pfcrt were extremely high in Jazan area; 162 (99%) of 164 isolates successfully examined harbored the genotype (C72 V73 I74 E75 T76), and only 2 (1%) isolates carried the wild type (C72 V73 M74 N75 K76) (Tables 1–3).
Table 3.
Multi-locus haplotypes of 93 P. falciparum isolates with single-clone infection in Jazan area, western Saudi Arabia
| Haplotype | dhfr | dhps | Pfg377 | Pfcrt | Pfmdr1 | Frequency | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 51I | 59R | 108N | 164L | 436F | 437G | 450E | 613S | Size | 72S | 74I | 75E | 76T | 86Y | 184F | 034C | 1042D | ||
| 1 | N | C | S | I | S | A | K | A | 310 | C | I | E | T | N | Y | S | N | 1 |
| 2 | N | C | S | I | S | A | K | A | 331 | C | I | E | T | N | Y | S | N | 1 |
| 3 | N | C | S | I | S | A | K | A | 310 | C | I | E | T | N | F | S | N | 7 |
| 4 | N | C | S | I | S | A | K | A | 331 | C | I | E | T | N | F | S | N | 20 |
| 5 | N | C | S | I | S | A | K | A | 352 | C | I | E | T | N | F | S | N | 18 |
| 6 | N | C | S | I | S | A | K | A | 310 | C | I | E | T | Y | F | S | N | 13 |
| 7 | N | C | S | I | S | A | K | A | 331 | C | I | E | T | Y | F | S | N | 6 |
| 8 | N | C | S | I | S | A | K | A | 352 | C | I | E | T | Y | F | S | N | 2 |
| 9 | I | C | N | I | S | A | K | A | 331 | C | I | E | T | N | Y | S | N | 1 |
| 10 | I | C | N | I | S | A | K | A | 310 | C | I | E | T | N | F | S | N | 7 |
| 11 | I | C | N | I | S | A | K | A | 331 | C | I | E | T | N | F | S | N | 10 |
| 12 | I | C | N | I | S | A | K | A | 352 | C | I | E | T | N | F | S | N | 3 |
| 13 | I | C | N | I | S | A | K | A | 331 | C | I | E | T | Y | F | S | N | 1 |
| I | C | N | I | S | A | K | A | 310 | C | I | E | T | Y | F | S | N | 4 | |
| Total | 93 | |||||||||||||||||
Pfmdr1 alleles and genotypes
Genotyping of Pfmdr1 was completed for 165 P. falciparum isolates. We found mutations only in codons 86 and 184 and no mutations at codons 1034 and 1042. At codon 86, 118 (69%), 51 (30%), and 2 (1%) isolates harbored the wild-type allele (86N), the resistance allele (86Y), and mixed alleles (86N/86Y), respectively. For codon Y184F, 5 (3%), 159 (96%), and 1 (1%) carried the wild-type allele (184Y), the resistance allele (84F), and mixed alleles (184Y/184F), respectively (Tables 1 and 2). In addition, 0 of 46 isolates examined for codon 1246 were found to harbor a mutant allele, despite the fact that 13 and 38 of these isolates had mutation at codons N86Y and Y184F, respectively.
Furthermore, three new SNPs were detected: T306C in codon 102, T546G in codon 182, and C575G in codon 192. The first two SNPs are synonymous, and the last one is non-synonymous. Mutation T546G was more common, seen among five isolates, compared with the other two mutations, which were each found in one isolate. This mutation has recently been reported in some P. falciparum isolates in India.31
Among the 163 isolates with complete sequence data, five (3%) isolates carried the wild genotype (N86 Y184 W184 S1034 N1042). However, 108 (65%) and 49 (30%) harbored single- (N86 L87 F184 W186 S1034 N1042) and double-mutant genotypes (Y86 L87 F184 W186 S1034 N1042), respectively (Tables 1–3).
Distribution of resistance genotypes among Saudis and expatriates.
The majority of isolates (163; 80%) were obtained from Saudis: 145 (89%) were residents of Jazan, and 18 (11%) were Saudi visitors. In addition, 40 (20%) samples were collected from expatriates: 18 (45%) were residents, and 22 (55%) were visitors.
The risk of harboring a resistance strain of P. falciparum among Saudi and non-Saudi was equal (OR = 1), with 95% confidence interval (CI) at 0.458–2.182. No significant association was seen between harboring resistance strain and gender (χ2 = 0.79), living status (χ2 = 0.865), or nationality (χ2 = 1 at P < 0.005). However, the only mutant allele in dhps gene was harbored by an expatriate. Similarly, the wild-type Pfcrt allele was only carried by two expatriated visitors (Table 4).
Table 4.
Distribution of mutant alleles of dhfr, dhps, Pfcrt, and Pfmdr among P. falciparum isolates from Jazan among Saudi and non-Saudi patients
| dhfr alleles | dhps alleles | Pfcrt alleles | Pfmdr1 alleles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 51N | 51I | 108S | 108N | 437A | 437G | 74M/75N/76K | 74I/75E/76T | 86N | 86Y | 184Y | 184F | |
| Saudi | ||||||||||||
| Resident | 89 | 46 | 88 | 47 | 135 | 0 | 0 | 123 | 89 | 39 | 3 | 123 |
| Visitors | 6 | 0 | 5 | 1 | 7 | 0 | 0 | 7 | 6 | 0 | 1 | 6 |
| Total | 95 | 46 | 93 | 48 | 143 | 0 | 0 | 130 | 95 | 39 | 4 | 129 |
| Expatriates | ||||||||||||
| Resident | 5 | 2 | 5 | 2 | 8 | 1 | 0 | 9 | 7 | 2 | 0 | 8 |
| Visitors | 18 | 10 | 18 | 10 | 28 | 0 | 2 | 23 | 16 | 10 | 1 | 22 |
| Total | 23 | 12 | 23 | 12 | 36 | 1 | 2 | 32 | 23 | 12 | 1 | 30 |
| Total | 118 | 58 | 116 | 60 | 178 | 1 | 2 | 162 | 118 | 53 | 5 | 160 |
| Total | 176 | 176 | 179 | 164 | 171 | 165 | ||||||
| χ2* | 0.001 | 0.012 | NC† | NC | 0.839 | 1.254 | ||||||
| P value‡ | 0.978 | 0.914 | NC | NC | 0.36 | 0.534 | ||||||
| OR§ | 0.989 | 1.043 | NC | NC | 0.693 | 1.032 | ||||||
Degree of freedom (df) for all χ2 = 1 except for Pfmdr1/184 allele (df = 2).
Not calculated (NC), because the sample was less than two in either resistant (dhps) or sensitive (Pfcrt) alleles.
P values in calculated alleles were > 0.05; thus, all are non-significant, and all hypotheses are accepted.
OR calculated at 95% confidence interval.
Pfg377 alleles and parasite diversity.
At least five Pfg377 alleles, varying in size between 269 and 352 base pairs, were detected. The difference between each of the two closest alleles is approximately 21 base pairs, which reflects variation in the number of the amino acids repeat of region 3.23 The common alleles (331, 310, and 352 base pairs) existed at a prevalence of 48%, 28%, and 22%, respectively. However, alleles (269 and 289 base pairs) existed at a very low prevalence of 1% each (Tables 1 and 2).
To evaluate the genetic background of P. falciparum isolates in Jazan, we examined the rate of parasite multiplicity of infection and mean number of clones per infection. The vast majority of isolates (95%) carried a single clonal infection with a single allele at each of the examined genes. The mean number of clones per infection (1.05) was very close to one. In addition, two major haplotypes (multilocus genotypes with identical alleles for all loci), haplotypes 4 and 5, existed at prevalences of 20% and 18%, respectively, among the 93 P. falciparum isolates with single-clone infection) (Table 3).
Discussion
The southwestern region of Saudi Arabia (Jazan) is a major malaria-endemic site,32 where chloroquine was previously the drug of choice for the treatment of malaria cases. In this area, P. falciparum remained sensitive to chloroquine up until the late 1990s,15 unlike neighboring malaria-endemic sites, such as Yemen and the closest east African countries,3,33,34 where CQR was common. The health authority in Saudi Arabia has lately changed the first line for treatment of malaria to ACT: SP plus artesunate.
Limited studies have examined dug resistance genes in the Jazan area and shown the presence of mutant alleles of some drug resistance genes: Pfcrt-76T, Pfmdr1-86Y, and dhfr-59R.9,12 The present study has extended the above findings and analyzed 16 SNPs in four genes involved in resistance to commonly used antimalaria drugs. dhfr and dhps mutations associated with SP resistance existed at low prevalence in Jazan area; however, mutations in Pfcrt and Pfmdr1 genes linked with chloroquine, amodiaquine, mefloquine, and possibly artemisinin35 are common, which is in agreement with a recent report from the area.35
The low prevalence of dhfr and dhps implies that the current use of SP in Jazan may be imposing only weak selection on these genes or that there has been insufficient time for selection to leave a molecular signature. Evidence of selection is generally found in dhfr earlier than dhps36,37; thus, it is likely that we may be seeing the initial phase of SP resistance in Jazan. An additional point that supports this hypothesis is that almost all the resistance dhfr genotypes consist of double mutants (I51N108) rather than the highly resistance triple mutant (I51 R59, N108) found in Africa and Southeast Asia where SP resistance is well-established.38,39 Thus, it is possible that highly resistance genotypes have not spread in this region. A limited study carried out in 2005 in the Jazan area has previously reported the presence of dhfr-59R allele.12 However, this allele was not detected in the current study. This discrepancy is unexpected, because the dhfr-59R allele is associated with high-level resistance to pyrimethamine.22 SP has been used in Jazan for sometime as a second line to chloroquine, and the above allele is expected to be under favorable selection in the face of drug pressure. Therefore, the small sample size (N = 19) analyzed by Al-Harthi12 may not have been representative of the Jazan parasite population, and the two isolates with 59R alleles may had been obtained from expatriates with asymptomatic chronic infection who acquired it outside Jazan; it is known to be capable of lasting for some months.40 Saudi Arabia is at great risk of imported malaria because of the large number of expatriate workers and the millions of annual visitors from malaria-endemic countries, for Hajj and Omrah.7 Additional analysis of microsatellite loci flanking the dhfr and dhps genes can indicate whether the mutant dhfr genotype in Jizan has evolved locally or shows close similarity to the common high-level resistance lineage seen in southeast Asia and Africa.38,39 This finding is of particular importance, because the only isolate that carried the mutation dhps 437G, which is associated with SP failure, was seen in an expatriate (Table 4).
Nonetheless, effective management of malaria outbreaks at health centers and clinics in Jazan using artesunate plus SP (ACT) can currently still control imported malaria and restrain escalation of drug resistance. However, the presence of a single mutant lineage carrying mutation 437 is alarming, because this mutation has been linked to high rate of SP failure in many sites.22 Continued molecular surveillance and in vivo drug efficacy assessments are recommended to guide appropriate policy revisions before widespread therapeutic failure.
High prevalence of the Pfcrt-resistance genotype (99%) seen among P. falciparum parasites in Jazan reflects the high level of CQ selection. The only mutant Pfcrt genotype seen (C72 I74 E75 T76) is similar to the common genotype in Africa CVIET, which originated in Southeast Asia.41 This finding is not surprising in view of the high rate of expatriates and pilgrims from Asia and Africa. Analysis of microsatellites flanking the Pfcrt gene in Jazan will shed light on its genetic background and whether it has evolved locally or not.39
The change from chloroquine to artemisinin + SP as the first-line antimalarial in Jazan is expected to decrease the CQR genotype and restore the chloroquine wild-type allele within the parasite population. This occurrence has been documented consistently after withdrawal of chloroquine in many African countries, such as Malawi,42 Tanzania,43 and Kenya.44 Therefore, it was unexpected to find Pfcrt-K76T at fixation. The persistence of the Pfcrt-K76T allele suggests an ongoing use of chloroquine or related drugs such as amodiaquine45 because of the recent shift to ACT,12 which can lead to selection of the mutant genotype. In addition, chloroquine, although not used for treatment of falciparum malaria, can be used for infections that are thought to be P. vivax but are actually unrecognized mixed infections or misdiagnosed P. falciparum infections.46 A large number of expatriates from the Indian subcontinent may import P. vivax infection.
Polymorphisms in the Pfmdr1 alleles (N86Y, Y184F, S1034C, N1042D, and D1246Y) may alter parasite responses to many antimalarial drugs, including chloroquine, quinine, mefloquine, and artemisinin.35,47 Mutations at codon 86 have been associated with CQR,35,47 whereas mutations at codons 184, 1034, 1042, and 1246 have been implicated to varying degrees in resistance to mefloquine and artesunate.35,47–50 An increased association between artesunate-mefloquine failure and a mutation at codon 184 has been seen in P. falciparum parasites in Cambodia.51 However, it is unlikely that the high frequency of mutations at codon 184 in the Jazan area is linked to mefloquine resistance, because this drug has not been in common use. A possible explanation is that the 184F allele has been driven by the high rate of CQR in the area.
A correlation between CQR and the Pfmdr1-N86Y mutation is well-established, because the Pfmdr1-N86Y mutation in conjunction with the Pfcrt-K76T mutation yields enhanced levels of resistance to chloroquine.21,34 However, we found fewer parasites with Pfmdr186Y than expected as Pfcrt76T reached fixation. Similarly, findings have been reported from other sites, and it has been postulated that Pfmdr1 N86Y mutation may confer a compensatory advantage for coping with chloroquine pressure, which may vary in different parasite populations.31,52
It has recently been suggested that some antimalarial drugs exert opposite directional selection on parasite genotypes.16,53 For example, artemisinin-lumefantrine (AL) and amodiaquine (AQ) exert opposite within-host selective effects on the Pfmdr1 genes of P. falciparum.16 Similarly, the Pfcrt-K76T mutation may enhance P. falciparum susceptibility to lumefantrine17 and thus, increase the benefits of using AL in areas affected by CQR P. falciparum malaria, such as Jazan. Therefore, the current use of ACT in Jazan may be exerting a novel pressure on the local parasites to select for alleles Pfmdr1 86N 184F 1246D and Pfcrt-74M 75N 76K,53 some of which are currently rare in Saudi Arabia; however, the wild-type pfmdr1-1246D is at fixation, and the mutant form was not seen in neighboring countries such as Iran.54,55 Whether the prevalence of the wild-type Pfmdr1 mutations will change under increasing artemisnin pressure will be of considerable interest in the future. However, currently, the apparent effectiveness of SP should protect artemisinin and reduce effective selective pressure.
The observed low genetic diversity of the P. falciparum in Jazan area probably reflects the impact of sustained control efforts, where limited transmission can restrict the gene pool. This finding is revealed in the low complexity (concurrent genotypes) of infection, where almost 95% of infected patients carried a single clone and only 5% are infected with multiple clones. Here, we have examined one polymorphic gene (pfg377); certainly, the addition of more polymorphic markers, such as msp-2, would have increased the rate of multiplicity. However, this increase is expected to be significantly higher because of the low transmission level and limited outcrossing in Jazan, which is evident by the occurrence of linkage disequilibrium. The level of multiplicity seen in Jazan is lower than the level in an area of low and seasonal transmission such as eastern Sudan, where the multiplicity of infection is approximately 20–40% and the mean number of clones per infection is 1.3.56
Thus, the markedly low level of diversity seen in Jazan is rare and seen typically in limited endemic sites such as island populations in Papua New Guinea and the Solomon Islands.57 The low within-host multiplicity can have profound effect of evolution of drug resistance. It affects both the strength of advantageous selection of resistance genotype in the presence of drug and the disadvantageous selection (fitness cost) in the absence of resistance.58,59 In addition, the low genetic diversity can result in limited opportunities for crossing and recombination to build up or break down existing multilocus drug resistance genotypes.60 In such areas, mutations associated with antimalarial drug resistance tend to reach fixation.61 Thus, it may take longer for the dominant Pfcrt haplotype in Kingdom of Saudi Arabia to lose its grip in the absence of chloroquine pressure compared with areas with high transmission such as Africa. The absence of mutations in Pfdhps in Jazan, which are already present in east Africa and Yemen, is a possible further illustration of such a low gene flow.
In summary, the present data provide baseline information on the prevalence of P. falciparum drug resistance-associated SNPs and highlight the low level of genetic diversity in the P. falciparum population in the Jazan area of western Saudi Arabia. Surveillance of molecular markers of drug resistance should be an integral part of the planned malaria eradication programs; therefore, the resistance dynamics can be assessed, and the most effective treatment can be selected.
ACKNOWLEDGMENTS
The authors thank the participants in this study and the staff of the Ministry of Health authorities in Jazan for their cooperation in this study. This study was supported by Grant LR-4-13 from King Abdulaziz City for Science and Technology (KACST), Saudi Arabia, and Sultan Qaboos University, Oman, Project IG/MED/BIOC/09/03.
Footnotes
Authors' addresses: Saad M. Bin Dajem, College of Science, King Khalid University, Abha, Kingdom of Saudi Arabia, E-mail: saad1426@gmail.com. Hissa M. Al-Farsi, Zainab S. Al-Hashami, and Hamza A. Babiker, Department of Biochemistry, Faculty of Medicine, Sultan Qaboos University, Muscat, Oman, E-mails: noornoor440@gmail.com, zainab44salam@gmail.com, and H.babiker@ed.ac.uk. Adel Ali H. Al-Sheikh, National Center for Training and Research, MOH, Jazan, Saudi Arabia, E-mail: adelalsheikh@gmail.com. Ahmed Al-Qahtani, Department of Biological and Medical Research, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia, E-mail: aqahtani@kfshrc.edu.sa.
References
- 1.Abdel-Hameed AA. Antimalarial drug resistance in the eastern mediterranean region. East Mediterr Health J. 2003;9:492–508. [PubMed] [Google Scholar]
- 2.Abdo-Rabbo A, Bassili A, Atta H. The quality of antimalarials available in yemen. Malar J. 2005;4:28. doi: 10.1186/1475-2875-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mekhlafi AM, Mahdy MAK, Al-Mekhlafi HM, Azazy AA, Fong MY. High frequency of Plasmodium falciparum chloroquine resistance marker (pfcrt t76 mutation) in Yemen: an urgent need to re-examine malaria drug policy. Parasit Vectors. 2011;4:94. doi: 10.1186/1756-3305-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawoud HA, Ageely HM, El-Sheikh AH, Heiba AA. Molecular surveillance of Plasmodium falciparum chloroquine resistance transporter variant t76 in jazan area, kingdom of Saudi Arabia. J Egypt Soc Parasitol. 2009;39:503–510. [PubMed] [Google Scholar]
- 5.WHO/Regional Office for the Eastern Mediterranean . Strategic plan for malaria control and elimination in the WHO Eastern Mediterranean Region 2006--2010. Cairo: World Health Organization Regional Office for the Eastern Mediterranea; 2007. p. 41. [Google Scholar]
- 6.Zaher T, Ahmadi M, Ibrahim A, El-Bahnasawy M, Gouda H, Shahat SAR. Malaria in Egypt, Saudi Arabia and Yemen: a clinical pilot study. J Egypt Soc Parasitol. 2007;37:969–976. [PubMed] [Google Scholar]
- 7.Al-Tawfiq JA. Epidemiology of travel-related malaria in a non-malarious areas in Saudi Arabia. Saudi Med J. 2006;27:1781–1782. [PubMed] [Google Scholar]
- 8.Bashwari LA, Mandil AM, Bahnassy AA, Al-Shamsi MA, Bukhari HA. Epidemiological profile of malaria in a university hospital in the eastern region of Saudi Arabia. Saudi Med J. 2001;22:133–138. [PubMed] [Google Scholar]
- 9.Bin Dajem SM, Al-Qahtani A. Analysis of gene mutations involved in chloroquine resistance in Plasmodium falciparum parasites isolated from patients in the southwest of Saudi Arabia. Ann Saudi Med. 2010;30:187–192. doi: 10.4103/0256-4947.62826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik GM, Seidi O, El-Taher A, Mohammed AS. Clinical aspects of malaria in the Asir region, Saudi Arabia. Ann Saudi Med. 1998;18:15–17. doi: 10.5144/0256-4947.1998.15. [DOI] [PubMed] [Google Scholar]
- 11.Omar MS, Malik GM, Al-Amari OM, Abdalla SE, Moosa RA. The rapid manual parasight-f test for diagnosing Plasmodium falciparum malaria in saudi arabia. Ann Saudi Med. 1999;19:159–162. doi: 10.5144/0256-4947.1999.159. [DOI] [PubMed] [Google Scholar]
- 12.Al-Harthi SA. Detection of drug resistance markers for chloroquine and pyrimethamine-sulfadoxine in Jazan area, Saudi Arabia using PCR and restriction digestion. J Egypt Soc Parasitol. 2007;37:17–30. [PubMed] [Google Scholar]
- 13.al Arishi HM, el Awad Ahmed F, al Bishi LA. Chloroquine-resistant Plasmodium falciparum malaria among children seen in a regional hospital, tabuk, Saudi Arabia. Trans R Soc Trop Med Hyg. 2001;95:439–440. doi: 10.1016/s0035-9203(01)90209-3. [DOI] [PubMed] [Google Scholar]
- 14.Alrajhi AA, Rahim I, Akood M, Hazmi M. Chloroquine-resistant Plasmodium falciparum cerebral malaria in a chloroquine-susceptible area. J Infect Dis. 1999;180:1738–1741. doi: 10.1086/315083. [DOI] [PubMed] [Google Scholar]
- 15.Ghalib HW, Al-Ghamdi S, Akood M, Haridi AEA, Ageel AAM, Abdalla RE. Therapeutic efficacy of chloroquine against uncomplicated, Plasmodium falciparum malaria in southwestern Saudi Arabia. Ann Trop Med Parasitol. 2001;95:773–779. doi: 10.1080/0003498012011127. [DOI] [PubMed] [Google Scholar]
- 16.Humphreys GS, Merinopoulos I, Ahmed J, Whitty CJM, Mutabingwa TK, Sutherland CJ, Hallett RL. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob Agents Chemother. 2007;51:991–997. doi: 10.1128/AAC.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisowath C, Petersen I, Veiga MI, Mårtensson A, Premji Z, Björkman A, Fidock DA, Gil JP. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt k76 allele after treatment with artemether-lumefantrine in africa. J Infect Dis. 2009;199:750–757. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosman A, Mendis KN. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am J Trop Med Hyg. 2007;77:193–197. [PubMed] [Google Scholar]
- 19.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Muhsin AA, Mackinnon MJ, Awadalla P, Ali E, Suleiman S, Ahmed S, Walliker D, Babiker HA. Local differentiation in Plasmodium falciparum drug resistance genes in sudan. Parasitology. 2003;126:391–400. doi: 10.1017/s0031182003003020. [DOI] [PubMed] [Google Scholar]
- 21.Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV, Coulibaly D. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 22.Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WM, Winstanley PA, Estrada-Franco JG, Mollinedo RE, Avila JC, Cespedes JL, Carter D, Doumbo OK. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis. 1997;176:1590–1596. doi: 10.1086/514159. [DOI] [PubMed] [Google Scholar]
- 23.Alano P, Read D, Bruce M, Aikawa M, Kaido T, Tegoshi T, Bhatti S, Smith DK, Luo C, Hansra S, Carter R, Elliott JF. Cos cell expression cloning of pfg377, a Plasmodium falciparum gametocyte antigen associated with osmiophilic bodies. Mol Biochem Parasitol. 1995;74:143–156. doi: 10.1016/0166-6851(95)02491-3. [DOI] [PubMed] [Google Scholar]
- 24.Nassir E, Abdel-Muhsin A-MA, Suliaman S, Kenyon F, Kheir A, Geha H, Ferguson HM, Walliker D, Babiker HA. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int J Parasitol. 2005;35:49–55. doi: 10.1016/j.ijpara.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Kheir A, Nwakanma D, Al-Gazali A, Akbarova Y, Al-Saai S, Swedberg G, Babiker HA. Transmission and cross-mating of high-level resistance Plasmodium falciparum dihydrofolate reductase haplotypes in the Gambia. Am J Trop Med Hyg. 2010;82:535–541. doi: 10.4269/ajtmh.2010.09-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nwakanma D, Kheir A, Sowa M, Dunyo S, Jawara M, Pinder M, Milligan P, Walliker D, Babiker HA. High gametocyte complexity and mosquito infectivity of Plasmodium falciparum in the Gambia. Int J Parasitol. 2008;38:219–227. doi: 10.1016/j.ijpara.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Menegon M, Severini C, Sannella A, Paglia MG, Sangaré D, Abdel-Wahab A, Abdel-Muhsin AA, Babiker H, Walliker D, Alano P. Genotyping of Plasmodium falciparum gametocytes by reverse transcriptase polymerase chain reaction. Mol Biochem Parasitol. 2000;111:153–161. doi: 10.1016/s0166-6851(00)00314-5. [DOI] [PubMed] [Google Scholar]
- 28.Hill WG, Babiker HA. Estimation of numbers of malaria clones in blood samples. Proc Biol Sci. 1995;262:249–257. doi: 10.1098/rspb.1995.0203. [DOI] [PubMed] [Google Scholar]
- 29.Cowman AF, Foote SJ. Chemotherapy and drug resistance in malaria. Int J Parasitol. 1990;20:503–513. doi: 10.1016/0020-7519(90)90198-v. [DOI] [PubMed] [Google Scholar]
- 30.Peterson DS, Milhous WK, Wellems TE. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mixson-Hayden T, Jain V, McCollum AM, Poe A, Nagpal AC, Dash AP, Stiles JK, Udhayakumar V, Singh N. Evidence of selective sweeps in genes conferring resistance to chloroquine and pyrimethamine in Plasmodium falciparum isolates in India. Antimicrob Agents Chemother. 2010;54:997–1006. doi: 10.1128/AAC.00846-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization World malaria report. 2008. p. P 42.http://books.google.com.om/books? Available at.
- 33.Alkadi HO, Al-Maktari MT, Nooman MA. Chloroquine-resistant Plasmodium falciparum local strain in taiz governorate, republic of Yemen. Chemotherapy. 2006;52:166–170. doi: 10.1159/000093592. [DOI] [PubMed] [Google Scholar]
- 34.Babiker HA, Pringle SJ, Abdel-Muhsin A, Mackinnon M, Hunt P, Walliker D. High-level chloroquine resistance in sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance gene pfmdr1. J Infect Dis. 2001;183:1535–1538. doi: 10.1086/320195. [DOI] [PubMed] [Google Scholar]
- 35.Saliba KJ, Caruana SR, Reed MB, Kirk K. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 36.Nzila AM, Mberu EK, Sulo J, Dayo H, Winstanley PA, Sibley CH, Watkins WM. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob Agents Chemother. 2000;44:991–996. doi: 10.1128/aac.44.4.991-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibley CH, Hyde JE, Sims PF, Plowe CV, Kublin JG, Mberu EK, Cowman AF, Winstanley PA, Watkins WM, Nzila AM. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 2001;17:582–588. doi: 10.1016/s1471-4922(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 38.Nair S, Williams JT, Brockman A, Paiphun L, Mayxay M, Newton PN, Guthmann J-P, Smithuis FM, Hien TT, White NJ, Nosten F, Anderson TJC. A selective sweep driven by pyrimethamine treatment in southeast Asian malaria parasites. Mol Biol Evol. 2003;20:1526–1536. doi: 10.1093/molbev/msg162. [DOI] [PubMed] [Google Scholar]
- 39.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 40.Babiker HA, Abdel-Muhsin ABA, Ranford-Cartwright LC, Satti G, Walliker D. Characteristics of Plasmodium falciparum parasites that survive the lengthy dry season in eastern sudan where malaria transmission is markedly seasonal. Am J Trop Med Hyg. 1998;59:582–590. doi: 10.4269/ajtmh.1998.59.582. [DOI] [PubMed] [Google Scholar]
- 41.Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, Magill AJ, Su XZ. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 42.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, Taylor TE, Plowe CV. Return of chloroquine antimalarial efficacy in malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 43.Alifrangis M, Lusingu JP, Mmbando B, Dalgaard MB, Vestergaard LS, Ishengoma D, Khalil IF, Theander TG, Lemnge MM, Bygbjerg IC. Short report: five-year surveillance of molecular markers of Plasmodium falciparum antimalarial drug resistance in Korogwe district, Tanzania: accumulation of the 581g mutation in the P. falciparum dihydropteroate synthase gene. Am J Trop Med Hyg. 2009;80:523–527. [PubMed] [Google Scholar]
- 44.Mwai L, Ochong E, Abdirahman A, Kiara SM, Ward S, Kokwaro G, Sasi P, Marsh K, Borrmann S, Mackinnon M, Nzila A. Chloroquine resistance before and after its withdrawal in Kenya. Malar J. 2009;8:106. doi: 10.1186/1475-2875-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochong EO, Van den Broek IVF, Keus K, Nzila A. Short report: association between chloroquine and amodiaquine resistance and allelic variation in the Plasmodium falciparum multiple drug resistance 1 gene and the chloroquine resistance transporter gene in isolates from the upper nile in southern Sudan. Am J Trop Med Hyg. 2003;69:184–187. [PubMed] [Google Scholar]
- 46.Wang X, Mu J, Li G, Chen P, Guo X, Fu L, Chen L, Su X, Wellems TE. Decreased prevalence of the Plasmodium falciparum chloroquine resistance transporter 76t marker associated with cessation of chloroquine use against P. falciparum malaria in Hainan, People's `Republic of China. Am J Trop Med Hyg. 2005;72:410–414. [PubMed] [Google Scholar]
- 47.Roper C, Walliker D, Duraisingh MT, Warhurst DC. Increased sensitivity to the antimalarials mefloquine and artemisinin is conferred by mutations in the. Mol Microbiol. 2000;36:955–961. doi: 10.1046/j.1365-2958.2000.01914.x. [DOI] [PubMed] [Google Scholar]
- 48.Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, Zalewski C, Kawamoto F, Miller RS, Meshnick SR. Resistance to antimalarials in southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob Agents Chemother. 2003;47:2418–2423. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price RN, Cassar C, Brockman A, Duraisingh M, van Vugt M, White NJ, Nosten F, Krishna S. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob Agents Chemother. 1999;43:2943–2949. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sidhu ABS, Uhlemann A-C, Valderramos SG, Valderramos J-C, Krishna S, Fidock DA. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis. 2006;194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah NK, Alker AP, Sem R, Susanti AI, Muth S, Maguire JD, Duong S, Ariey F, Meshnick SR, Wongsrichanalai C. Molecular surveillance for multidrug-resistant Plasmodium falciparum, Cambodia. Emerg Infect Dis. 2008;14:1637–1640. doi: 10.3201/eid1410.080080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vathsala PG, Pramanik A, Dhanasekaran S, Devi CU, Pillai CR, Subbarao SK, Ghosh SK, Tiwari SN, Sathyanarayan TS, Deshpande PR, Mishra GC, Ranjit MR, Dash AP, Rangarajan PN, Padmanaban G. Widespread occurrence of the Plasmodium falciparum chloroquine resistance transporter (pfcrt) gene haplotype svmnt in P. falciparum malaria in India. Am J Trop Med Hyg. 2004;70:256–259. [PubMed] [Google Scholar]
- 53.Lekana-Douki JB, Dinzouna Boutamba SD, Zatra R, Zang Edou SE, Ekomy H, Bisvigou U, Toure-Ndouo FS. Increased prevalence of the Plasmodium falciparum pfmdr1 86n genotype among field isolates from Franceville, Gabon after replacement of chloroquine by artemether-lumefantrine and artesunate-mefloquine. Infect Genet Evol. 2011;11:512–517. doi: 10.1016/j.meegid.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Jalousian F, Dalimi A, Samiee SM, Ghaffarifar F, Soleymanloo F, Naghizadeh R. Mutation in pfmdr1 gene in chloroquine-resistant Plasmodium falciparum isolates, southeast Iran. Int J Infect Dis. 2008;12:630–634. doi: 10.1016/j.ijid.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Zakeri S, Afsharpad M, Kazemzadeh T, Mehdizadeh K, Shabani A, Djadid ND. Association of pfcrt but not pfmdr1 alleles with chloroquine resistance in iranian isolates of Plasmodium falciparum. Am J Trop Med Hyg. 2008;78:633–640. [PubMed] [Google Scholar]
- 56.Babiker HA, Walliker D. Current views on the population structure of Plasmodium falciparum: implications for control. Parasitol Today. 1997;13:262–267. doi: 10.1016/s0169-4758(97)01075-2. [DOI] [PubMed] [Google Scholar]
- 57.Ballif M, Hii J, Marfurt J, Crameri A, Fafale A, Felger I, Beck H-P, Genton B. Monitoring of malaria parasite resistance to chloroquine and sulphadoxine-pyrimethamine in the Solomon Islands by DNA microarray technology. Malar J. 2010;9:270. doi: 10.1186/1475-2875-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huijben S, Nelson WA, Wargo AR, Sim DG, Drew DR, Read AF. Chemotherapy, within-host ecology and the fitness of drug-resistant malaria parasites. Evolution. 2010;64:2952–2968. doi: 10.1111/j.1558-5646.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wargo AR, Huijben S, de Roode JC, Shepherd J, Read AF. Competitive release and facilitation of drug-resistant parasites after therapeutic chemotherapy in a rodent malaria model. Proc Natl Acad Sci USA. 2007;104:19914–19919. doi: 10.1073/pnas.0707766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackinnon MJ. Drug resistance models for malaria. Acta Trop. 2005;94:207–217. doi: 10.1016/j.actatropica.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Griffing S, Syphard L, Sridaran S, McCollum AM, Mixson-Hayden T, Vinayak S, Villegas L, Barnwell JW, Escalante AA, Udhayakumar V. Pfmdr1 amplification and fixation of pfcrt chloroquine resistance alleles in Plasmodium falciparum in Venezuela. Antimicrob Agents Chemother. 2010;54:1572–1579. doi: 10.1128/AAC.01243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]