Abstract
In areas where visceral leishmaniasis is anthroponotic, asymptomatically infected patients may play a role in transmission. Additionally, the number of asymptomatic patients in a disease-endemic area will also provide information on transmission dynamics. Libo Kemkem and Fogera districts (Amhara State, Ethiopia) are now considered newly established areas to which visceral leishmaniasis is endemic. In selected villages in these districts, we conducted a study to assess the usefulness of different approaches to estimate the asymptomatic infection rate. Of 605 participants, the rK39 immunochromatographic test was able to detect asymptomatic infection in 1.5% (9 of 605), direct agglutination test in 5.3% (32 of 605), and leishmanin skin test in 5.6% (33 of 589); the combined use of serologic methods and leishmanin skin test enabled detecting asymptomatic infection in 10.1% (61 of 605). We conclude that the best option to detect asymptomatic infection in this new visceral leishmaniasis–endemic focus is the combined use of the direct agglutination test and the leishmanin skin test.
Introduction
Visceral leishmaniasis (VL) is a vector-borne disease caused by members of the Leishmania (Leishmania) donovani complex. It is endemic to 65 countries and, among them, Bangladesh, India, Nepal, Brazil, Sudan, and Ethiopia account for approximately 90% of the cases. The estimated annual incidence is 500,000 clinical cases with 59,000 associated deaths.1,2 Poor populations are particularly affected by VL, which is considered as one of the most neglected diseases.3 In addition, VL is currently spreading and re-emerging in different areas of the world with increasing public health concern.4
Visceral leishmaniasis is fatal if left untreated, and even with treatment the case fatality rate ranges from 4% to 10%.5,6 In stable disease-endemic areas, clinical disease appears only in a fraction of those infected, and another fraction will not develop the disease and remain asymptomatic.7 The prevalence of asymptomatic infection is different between and within different disease-endemic countries, and the number of asymptomatic infections usually exceeds the number of symptomatic infections, although this ratio can vary from 0.4:1 to 50:1.2
Visualization of parasite amastigotes by microscopic examination of bone marrow, spleen, or lymph node aspirates has been the gold standard method of VL diagnosis for many years. However, because this procedure is based on invasive sampling, its use for asymptomatic infection surveillance is not justified. In addition, in poor, remote, disease-endemic areas, the expertise and facilities required for these procedures may not be present. Thus, procedures based on less invasive sampling, such as serologic analysis or leishmanin skin test (LST) seem to be more suitable for this purpose.
Although detection of antibodies against Leishmania does not discriminate between current or past infection, serologic methods have been used to assess asymptomatic infection in different VL-endemic areas. These methods have been based on the direct agglutination test (DAT), rK39-immunochromatographic test (rK39-ICT), enzyme-linked immunosorbent assay, indirect immunofluorescent antibody test, or Western blot.8–12
The LST is a useful method for detecting cell-mediated immunity against Leishmania. Results for this test become positive after subclinical infection and, in this case, persist much longer than antibodies against Leishmania. The LST results also becomes positive within weeks-months after successful therapy against VL, indicating a healing or protective response.13,14 This makes the LST a valuable tool in detecting exposure to Leishmania parasites in epidemiologic surveys, and its usefulness to detect asymptomatic infection has been shown by different authors in different disease-endemic areas.11,15,16 In general, the LST would detect a higher proportion of asymptomatic infection than serologic analysis. However, given that serologic analysis and LST are based on different types of immune responses, in the absence of a gold standard, the combination of these two approaches would give a more realistic picture of the asymptomatic infection rate in a given disease-endemic area.17,18
In areas where VL transmission is anthroponotic, asymptomatic persons might play a role as reservoirs, and even in areas where VL is zoonotic it is speculated that these persons could also contribute to transmission.7,19 Thus, assessment of the prevalence and distribution of asymptomatic cases would contribute to a better understanding of VL transmission and help in developing control efforts.
In Ethiopia, VL is an endemic disease of increasing public health concern. It is estimated that 30% of VL patients in Ethiopia are malnourished and co-infection with human immunodeficiency virus (HIV) affects 40% in the northwestern region of this country, and these conditions are known to facilitate the spread of VL.20,21 In addition to the classical foci in the northwestern region along the border with Sudan (Humera and Metema) and those in the southern region (Lake Abaya region, Omo River, and Aba Roba plains), the disease has recently spread to previously non-endemic areas, such as Libo Kemkem and Fogera (highland districts in Amhara State) where a VL outbreak occurred in 2004–2005.15 Thus, VL is currently a priority in the public health agenda of the Amhara State Health Bureau, and there is a need to generate epidemiologic data on VL in Amhara State.
To support VL control, facilities for treatment, mobile teams for surveillance, community mobilization, and active case detection strategies have been established. To contribute to this initiative the UBS-Optimus Foundation supported the project entitled Visceral Leishmaniasis and Malnutrition in Amhara State, Ethiopia, which among its specific objectives aimed to characterize nutritional, immunologic, and parasitologic aspects in the child population from this area, and it is within the framework of this project that we have explored the usefulness of rK39-ICT, DAT, and LST to detect asymptomatic Leishmania infection in children from different sub-districts of Libo Kemkem and Fogera. Furthermore, detection of asymptomatic infection in children will provide information about the status of VL transmission in this highland focus. In addition, this information will contribute to assessment of the magnitude of asymptomatic infection, which can help in early detection and treatment, thus contributing to decreased transmission, morbidity, and mortality. The information obtained can also be useful in a scenario in which HIV spreads to Leishmania-endemic areas, and help to foresee new VL cases among asymptomatic persons as a consequence of a reactivation of previous Leishmania infections after acquired HIV.22
Materials and Methods
Study site.
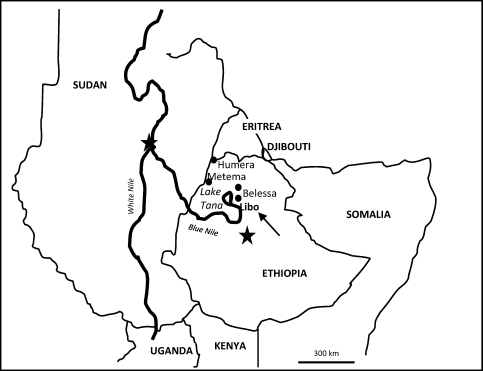
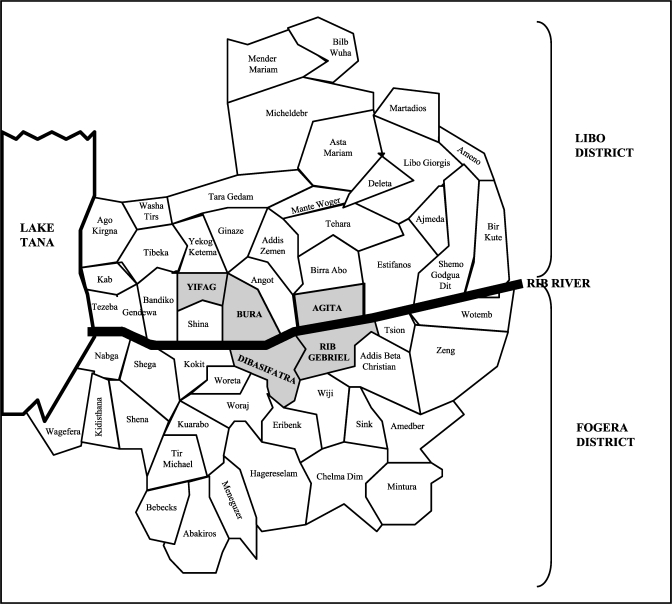
The study was conducted during May–July 2009 in the districts (weredas) of Libo Kemkem and Fogera, Amhara State, Ethiopia (Figures 1 and 2). These are adjacent districts most affected by the outbreak of VL that occurred in 2004–2005.15 According to the 2009 census, the population was 198,374 (males = 100,951 and females = 198,374) and 226,595 (males = 115,693 and females = 110,902) for Libo Kemkem and Fogera, respectively. The districts are located in a black cotton clay soil flat plain (1800–2000 meters above sea level). Human activities related to intensive cultivation of teff, maize, beans, oilseeds, rice, and cotton have reduced the natural vegetation to scattered clumps of acacia trees. Most of the area is flooded during the rainy season (July–September) and dried up during the dry season (November–May), resulting in deep cracks in the soil surface, which could become breeding sites for the putative vector Phlebotomus orientalis.23,24
Figure 1.
Location of the study area in Ethiopia.
Figure 2.
Location of the sub-districts in which the study was conducted (gray background) in Ethiopia.
Study population.
Population sampling was carried out by using a multi-staged cluster survey. Primary sampling units were sub-districts (kebeles) with high incidence of VL according to the 2008 register of the Addis Zemen VL Treatment Center: Agita, Bura, and Yifag from Libo Kemkem district and Dibasifatra and Rib Gebriel from Fogera district. Secondary sampling units were randomly selected villages (gotts) in each of the selected sub-districts. Tertiary sampling units were randomly selected households in each of the villages. All children with a reported age between 4 and 15 years living in the household at the time of the survey were eligible for the study, as long as they were asymptomatic and had no history of VL.
Data collection.
Information on age, sex, residence, and clinical assessment was obtained for all participants by trained medical personnel (nurses and health officers) by using pretested questionnaires and protocols.
Asymptomatic case definition.
Asymptomatic persons were those who had a positive result in the rK39-ICT, DAT, or LST, and the absence of VL signs and symptoms (fever for > 2 weeks, in combination with either enlargement of spleen and/or liver, or weight loss).
Sample collection and storage.
Peripheral blood was collected in tubes containing Na2-EDTA (Sigma, St. Louis, MO) and immediately one drop was used for the rK39-ICT and two drops were spotted onto Whatman 3MM filter paper (Whatman International Ltd., Maidstone, United Kingdom). Filter papers were left to air dry and placed individually in sealed plastic bags. The plastic bags containing filter papers were kept in a chilled icebox and sent on the same day to the Amhara State Regional Laboratory, where they were stored at 4°C for further DAT analysis.
Ethical considerations.
The study was approved by the ethical review boards of Instituto de Salud Carlos III, the Armauer Hansen Research Institute, and the Ethiopian National Ethical Review Committee. Support letters were obtained from the Amhara State and health bureaus in different districts. Parents/guardians gave written informed consent before enrollment of their children in the study. For children > 11 years of age, verbal assents were also obtained in addition to the consent of their parents/guardians.
Detection of antibodies against Leishmania.
The rK39-ICT (Kalazar Detect® Rapid Test; InBios International Inc., Seattle, WA) was performed by using 1 drop of blood and 3 drops of chasing buffer according to the manufacturer's instructions. The DAT with freeze-dried antigen (ITMA-DAT/VL; Prince Leopold Institute of Tropical Medicine, Antwerp, Belgium) was performed by using the screening method according to the manufacturer's protocol. Blood samples with a titer ≥ 1:3,200 were considered positive.
Leishmanin skin test.
The LST was performed by using L. major antigen (Leishmanin batch 123-2; Pasteur Institute, Tehran, Iran). One hundred microliters of the antigen were intradermally inoculated on the volar surface of the forearm with a 1-mL sterile syringe and disposable needle. The test was read 48 hours later by using the ballpoint pen method. An induration with an average of two perpendiculars ≥ 5 mm was considered positive.
Data analysis.
Infection prevalence was calculated by using rK39-ICT, DAT, and LST results. The differences in infection prevalence between age group, sex, and location were compared by using Fisher's exact and chi-square tests. A P value < 0.05 was considered statistically significant. Data analysis was performed by using SPSS version 16.0 (SPSS Inc., Chicago, IL) and STATA version 10 (StataCorp LP, College Station, TX).
Results
A total of 639 children were screened; one of them was an active case of malaria, three had an active VL episode, and 28 reported having VL in the past. For two other children, their previous VL history was unknown. These findings resulted in 605 asymptomatic children without previous VL eligible for analyses. The active malaria and VL cases were referred to the Addis Zemen Health Center for further clinical examination and treatment.
Of the 605 participants, 309 were boys (51.1%) and 296 were girls (48.9%). The boy:girl ratio was approximately 1:1 in the two districts studied. The mean ± SD age of the participants was 8.8 ± 0.13 years (there was no difference between the sexes). The children were grouped according to their age into three groups: group 1 (< 5 years of age) consisted of 77 of 605 (12.7%) children, group 2 (5–9 years of age) consisted of 295 (48.8%) of 605 children, and group 3 (10–15 years of age) consisted of 233 (38.5%) of 605 children.
Antibodies against Leishmania were detected in 9 (1.5%) of 605 children by the rK39-ICT and in 32 (5.3%) of 605 by the DAT. Sixteen (2.6%) of the 605 children initially tested by LST were lost for analysis. This test showed a positive result for 33 (5.6%) of 589 children.
Globally, 38 (6.3%) of 605 children were seropositive (rK39-ICT and/or DAT positive), and 61 (10.1%) of 605 children were considered infected (positive by rK39-ICT and/or DAT and/or LST). For the group of 589 children tested by using the three methods, we observed that 27 of 37 seropositive children had a negative LST result, although 23 of 33 LST-positive children were seronegative. A detailed description of the performance of these three methods in the group of 589 children is shown in Table 1.
Table 1.
Performance of the rK39-ICT, DAT, and LST for detection of visceral leishmaniasis in 589 children tested, Ethiopia*
| LST | DAT, no. positive/no. tested (%) | ||||
|---|---|---|---|---|---|
| + | – | Total | |||
| rK39-ICT† | rK39-ICT† | ||||
| + | – | + | – | ||
| + | 2/589 (0.3) | 6/589 (1.0) | 2/589 (0.3) | 23/589 (3.9) | 33/589 (5.6) |
| – | 0/589 (0.0) | 23/589 (3.9) | 4/589 (0.7) | 529/589 (89.8) | 556/589 (94.4) |
| 31/589 (5.3) | 558/589 (5.3) | Total = 589 (100) | |||
rK39-ICT = rK39-immunochromatographic test; DAT = direct agglutination test; LST = leishmanin skin test.
Global rK39-ICT positive = 8 (1.4%).
The mean ± SD age of those with asymptomatic infection was 10.6 ± 3.4 years. Analysis by age group showed a positive association between asymptomatic infection and age, and children 10–15 years of age had a higher asymptomatic infection rate (16.3%; P < 0.0001). This finding was common for all tests used.
A strong association was also found with regard to sex, with infection in boys being higher than in girls (13.9% versus 6.1%; P = 0.001). Differences in asymptomatic infection rate by age and sex are shown in Table 2.
Table 2.
Rate of asymptomatic infection of visceral leishmaniasis by sex and age, Ethiopia
| Age group (years) | Rate of asymptomatic infection, no. infected/no. tested (%) | ||
|---|---|---|---|
| Boys | Girls | Both sexes | |
| < 5 | 1/34 (2.9) | 1/43 (2.3) | 2/77 (2.6) |
| 5–9 | 16/140 (11.4) | 5/155 (3.2) | 21/295 (7.1) |
| 10–15 | 26/135 (19.3) | 12/98 (12.2) | 38/233 (16.3) |
| Total | 43/309 (13.9) | 18/296 (6.1) | 61/605 (10.1) |
The highest prevalence of asymptomatic infection was found in the selected villages in Bura (25.0%) and the lowest prevalence was found in villages in Agita (1.5%).
A detailed description of the results obtained by the three methods used by sex, age, and location is shown in Table 3.
Table 3.
Asymptomatic infection rates for visceral leishmaniasis detected by three tests and their combination by sex, age, and location, Ethiopia*
| Variable | Tests | ||||
|---|---|---|---|---|---|
| rK39-ICT, no. (%) | DAT, no. (%) | LST,† no. (%) | Seropositive, no. (%) | Infected, no. (%) | |
| Whole population (n = 605) | 9 (1.5) | 32 (5.3) | 33 (5.6) | 38 (6.3) | 61 (10.1) |
| Sex | |||||
| Boys (n = 309) | 7 (2.3) | 22 (7.1) | 23 (7.6) | 28 (9.1) | 43 (13.9) |
| Girls (n = 296) | 2 (0.7) | 10 (3.4) | 10 (3.5) | 10 (3.4) | 18 (6.1) |
| Age group (years) | |||||
| < 5 (n = 77) | 1 (1.3) | 2 (2.6) | 1 (1.3) | 2 (2.6) | 2 (2.6) |
| 5–9 (n = 295) | 3 (1.0) | 13 (4.4) | 8 (2.8) | 15 (5.1) | 21 (7.1) |
| 10–15 (n = 233) | 5 (2.1) | 17 (7.3) | 24 (10.6) | 21 (9.0) | 38 (16.3) |
| Sub-district | |||||
| Agita (n = 133) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 2 (1.5) | 2 (1.5) |
| Bura (n = 140) | 3 (2.1) | 17 (12.1) | 23 (16.5) | 18 (12.9) | 35 (25.0) |
| Dibasifatra (n = 133) | 5 (3.8) | 3 (2.2) | 4 (3.1) | 7 (5.3) | 10 (7.5) |
| Rib Gebriel (n = 132) | 1 (0.7) | 7 (5.3) | 6 (4.6) | 8 (6.1) | 11 (8.3) |
| Yifag (n = 67) | 0 (0.0) | 3 (4.5) | 0 (0.0) | 3 (4.5) | 3 (4.5) |
rK39-ICT = rK39-immunochromatographic test; DAT = direct agglutination test; LST = leishmanin skin test.
For 589 children.
Discussion
Our study shows the presence of asymptomatic Leishmania infection among children 4–15 years of age in the districts of Libo Kemkem and Fogera, in the new VL focus of Amhara State in northwestern Ethiopia. The observed overall asymptomatic infection rate was 10.1% (61 of 605). This rate was determined by using a combination of serologic methods, which detected 38 (6.3%) of 605 seropositive persons and LST, which detected 33 (5.6%) of 589 seropositive persons. As proposed initially, the combination of serologic analysis and LST enabled wider detection of asymptomatic infections.
The discordances observed between serologic analysis and LST were expected. Both LST and serologic analysis have been used to assess exposure to Leishmania, irrespective of disease presentation, and are frequently used in epidemiologic studies in Leishmania-endemic areas.9,25,26 Nevertheless, comparison of LST positivity and seroprevalence rates is complicated by different types of immune response detected by each test. The LST measures a delayed-type hypersensitivity reaction to Leishmania and relies on an in vivo cellular immune response to Leishmania antigens. Seropositivity is the result of a significant level of Leishmania-specific antibodies in the peripheral blood, which is based on a humoral immune response. The two differentiated groups of LST- positive/seronegative and LST-negative/seropositive children observed in our study are consistent with the lack of an association between LST and serologic results. The LST positivity appears later after infection and seems to be a sign of protective immunity against VL. Seropositivity is considered an indicator of more recent infection and has been related to disease progression.27,28 However, a recent seroepidemiologic study in Bihar, India, observed low disease conversion rate in asymptomatic DAT-positive persons.29
Different studies in VL-endemic areas have shown that LST detects a higher Leishmania infection rate in asymptomatic persons than serologic analyses.8,11,26,30–32 In contrast, our study shows that the infection rate obtained by serologic analysis is similar to that obtained with the LST, 6.3% versus 5.6% using DAT and rK39-ICT, and 5.3% versus 5.6% using DAT alone. Given that VL has recently been reported in our study area and that our study population is children, this finding can be associated with the longer time needed for development of a LST-positive response compared with seroconversion. If we consider that LST-positive conversion is the result of a repeated exposure to natural infection, this would also explain why LST positivity is higher in the older age group.
We observed a high discordance between DAT and rK39-ICT in our study (Table 1). Six persons with a positive rK39-ICT result were negative by DAT, 29 DAT-positive persons were negative by rK39-ICT result, and only three persons had a positive result by both serologic methods. Although Ritmeijer and others33 and Chappuis and others34 reported a lower sensitivity of rK39-ICT in Sudan, meta-analysis on the performance of DAT and rK39-ICT for active VL diagnosis concluded that both tests have a similar level of sensitivity. In addition, a recent study in Libo Kemkem that evaluated the performance of DAT and two rK39-ICT tests indicated that either approach is suitable for VL diagnosis in this area of Ethiopia.35 However, our study population was asymptomatic and higher positivity rates have been reported for DAT versus rK39-ICT when these methods are used to assess asymptomatic infection in other VL-endemic areas.7 Given that performance of a serologic test can depend on the stage of the disease/infection, the lower performance of rK39-ICT can be explained by its ability to detect antibody response against only a single antigen (rK39), whereas the DAT relies on detection of antibodies against a wide range of Leishmania antigens (whole freeze-dried promastigote).36 Another explanation is based on the nature of the tests. As proposed by Ter Horst and others, antibodies detected by rK39-ICT could be less able to react in a rapid reaction than in the overnight incubation used for DAT.37
Although the DAT has shown better performance in detecting asymptomatic infections in our study population, six persons with a positive result for the rK39-ICT result but a negative result for the DAT merits attention. A possible explanation for this finding might be the different volumes of blood tested with each method. Although in our study rK39-ICT was performed with one drop of blood, the DAT was performed with a 5-mm disk punched out from a spot of two drops of blood on a filter paper, which can be considered a lower amount of blood. In addition, Zijlstra and others reported that an rK39-based test (in an enzyme-linked immunosorbent assay format) detected asymptomatic infection earlier than the DAT.38
The increase of asymptomatic infection rate with age was consistent with an endemic focus of VL and a marked increase in persons > 5–9 years of age. In addition, asymptomatic infections in persons < 5 years of age is also consistent with active transmission, despite the low VL incidence observed after the outbreak.6
The present study indicates the appropriateness of combining serologic analysis (DAT) and LST to obtain a consistent picture of the asymptomatic infection rate in a VL-endemic area. This work also indicates that after the 2004–2005 VL outbreak, active transmission is still occurring in the villages studied and that L. donovani transmission can potentially be established in highlands (1,800–2,000 meters above sea level) in Ethiopia, which are commonly considered free of VL.
Our study has also enabled us to select appropriate tools to be used in different tasks during the UBS-Optimus Foundation project, such as estimation of infection prevalence, follow-up of those persons with asymptomatic infections, and further study of associations between malnutrition, Leishmania infection, and active disease. These tools have been locally evaluated and shown to be applicable under field conditions. They will enable investigators to conduct epidemiologic surveillance, thus contributing to ongoing efforts of the Amhara State Health Bureau to control spread of VL to adjacent areas.
ACKNOWLEDGMENTS
We thank the study participants for volunteering to participate in the study; the data collectors for performing field work; the Armauer Hansen Research Institute/All Africa Leprosy Rehabilitation and Training Center and the Fundación Española para la Cooperación Internacional, Salud y Política Social for providing logistic and technical support; the Amhara State Regional Laboratory for allowing us to use their laboratory facility and for creating a conducive environment during the field work; and Dr. Jorge Alvar (WHO/CDS/NTD/IDM) and the American Journal of Tropical Medicine and Hygiene for permission to adapt the figures published in the report by Alvar and others (Am J Trop Med Hyg 77: 2007, 275–282) and for obtaining Figures 1 and 2 in this report.
Footnotes
Financial support: This study was supported by the UBS-Optimus Foundation (Switzerland) through the project Visceral Leishmaniasis and Malnutrition in Amhara State, Ethiopia, the Spanish Ministry of Science and Innovation, and the Instituto de Salud Carlos III through the Network of Tropical Diseases Research (RICET RD06/0021/0009 and RD06/0021/0000).
Authors' addresses: Endalamaw Gadisa, Abraham Aseffa, Lawrence Yamuah, and Howard Engers, Armauer Hansen Research Institute, POB 1005, Jimma Road, All Africa Leprosy Rehabilitation and Training Center Compound, Addis Ababa, Ethiopia, E-mails: endalamawgadisa@yahoo.com, aseffaa@gmail.com, yamuahlk@ahrialert.org, and engersh@ahrialert.org. Estefanía Custodio, Centro Nacional de Medicina Tropical, Instituto de Salud Carlos III, Sinesio Delgado 6, 28029, Madrid, Spain, E-mail: ecustodio@isciii.es. Carmen Cañavate, Javier Nieto, Carmen Chicharro, Javier Moreno, and Israel Cruz, World Health Organization Collaborating Center for Leishmaniasis, Servicio de Parasitología, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Ctra. Majadahonda-Pozuelo Km2, 28220, Majadahonda, Madrid, Spain, E-mails: ccanave@isciii.es, fjnieto@isciii.es, cchichar@isciii.es, javier.moreno@isciii.es, and cruzi@isciii.es. Luis Sordo, Centro Nacional de Epidemiología, Instituto de Salud Carlos III, Monforte de Lemos 5, 28029, Consorcio de Investigación Biomédica de Epidemiología y Salud Pública, Madrid, Spain, E-mail: lsordo@isciii.es. Zelalem Abebe, Amhara Regional State Research Laboratory, POB 531, Bahir Dar, Ethiopia, E-mail: gebriyehailu@gmail.com.
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Bolaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 3.Yamey G, Torreele E. The world's most neglected diseases. BMJ. 2002;325:176–177. doi: 10.1136/bmj.325.7357.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dujardin JC. Risk factors in the spread of leishmaniases: towards integrated monitoring? Trends Parasitol. 2006;22:4–6. doi: 10.1016/j.pt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Berman JD. Human leishmaniasis: clinical, diagnostic and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 6.Herrero M, Orfanos G, Argaw D, Muguleta A, Aparicio P, Parreño F, Bernal O, Rubens D, Pedraza J, Lima MA, Flevaud L, Palma PP, Bashaye S, Alvar J, Bern C. Natural history of a visceral leishmaniasis outbreak in Highland Ethiopia. Am J Trop Med Hyg. 2009;81:373–377. [PubMed] [Google Scholar]
- 7.Topno RK, Das VN, Ranjan A, Pandey K, Singh D, Kumar N, Siddiqui NA, Singh VP, Kesari S, Kumar N, Bimal S, Kumar AJ, Meena C, Kumar R, Das P. Asymptomatic infection with visceral leishmaniasis in a diseases-endemic area in Bihar, India. Am J Trop Med Hyg. 2010;83:502–506. doi: 10.4269/ajtmh.2010.09-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa CH, Stewart JM, Gomes RB, Garcez LM, Ramos PK, Bozza M, Satoskar A, Dissanayake S, Santos RS, Silva MR, Shaw JJ, David JR, Maguire JH. Asymptomatic human carriers of Leishmania chagasi. Am J Trop Med Hyg. 2002;66:334–337. doi: 10.4269/ajtmh.2002.66.334. [DOI] [PubMed] [Google Scholar]
- 9.Schenkel K, Rijal S, Koirala S, Koirala S, Vanlerberghe V, Van der Stuyft P, Gramiccia M, Boelaert M. Visceral leishmaniasis in southeastern Nepal: a cross-sectional survey on Leishmania donovani infection and its risk factors. Trop Med Int Health. 2006;11:1792–1799. doi: 10.1111/j.1365-3156.2006.01735.x. [DOI] [PubMed] [Google Scholar]
- 10.Sundar S, Maurya R, Singh RK, Bharti K, Chakravarty J, Parekh A, Rai M, Kumar K, Murray HW. Rapid, noninvasive diagnosis of visceral leishmaniasis in India: comparison of two immunochromatographic strip tests for detection of anti-K39 antibody. J Clin Microbiol. 2006;44:251–253. doi: 10.1128/JCM.44.1.251-253.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riera C, Fisa R, López-Chejade P, Serra T, Girona E, Jiménez MT, Muncunill J, Sedeño M, Mascaró M, Udina M, Gállego M, Carrió J, Forteza A, Portús M. Asymptomatic infection by Leishmania infantum in blood donors from the Balearic Islands (Spain) Transfusion. 2008;48:1383–1389. doi: 10.1111/j.1537-2995.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 12.Romero HD, Silva LA, Silva-Vergara ML, Rodrigues V, Costa RT, Fernandes Guimarães S, Alecrim W, Moraes-Souza H, Prata A. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am J Trop Med Hyg. 2009;81:27–33. [PubMed] [Google Scholar]
- 13.Zijlstra EE, El-Hassan AM, Ismael A, Ghalib HW. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1994;51:826–836. doi: 10.4269/ajtmh.1994.51.826. [DOI] [PubMed] [Google Scholar]
- 14.Khalil EA, Ayed NB, Musa AM, Ibrahim ME, Mukhtar MM, Zijlstra EE, Elhassan IM, Smith PG, Kieny PM, Ghalib HW, Zicker F, Modabber F, Elhassan AM. Dichotomy of protective cellular immune responses to human visceral leishmaniasis. Clin Exp Immunol. 2005;140:349–353. doi: 10.1111/j.1365-2249.2005.02768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvar J, Bashaye S, Argaw D, Cruz I, Aparicio P, Kassa A, Orfanos G, Parreño F, Babaniyi O, Gudeta N, Cañavate C, Bern C. Kala-azar outbreak in Libo Kemkem, Ethiopia: epidemiologic and parasitologic assessment. Am J Trop Med Hyg. 2007;77:275–282. [PubMed] [Google Scholar]
- 16.Gidwani K, Rai M, Chakravarty J, Bolaert M, Sundar S. Evaluation of leishmanin skin test in Indian visceral leishmaniasis. Am J Trop Med Hyg. 2009;80:566–567. [PubMed] [Google Scholar]
- 17.De Gouvêa Viana L, De Assis TS, Orsini M, da Silva AR, De Souza GF, Caligiorne R, Da Silva AC, Peruhype-Magalhaes V, Vieira Marciano AP, Martins-Filho OA, Rabello A. Combined diagnostic methods identify a remarkable proportion of asymptomatic Leishmania (Leishmania) chagasi carriers who present modulated cytokine profiles. Trans R Soc Trop Med Hyg. 2008;102:548–555. doi: 10.1016/j.trstmh.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Biglino A, Bolla C, Concialdi E, Trisciuoglio A, Romano A, Ferroglio E. Asymptomatic Leishmania infantum infection in an area of northwestern Italy (Piedmont Region) where such infections are traditionally nonendemic. J Clin Microbiol. 2010;48:131–136. doi: 10.1128/JCM.00416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barao SC, De Fonseca Camargo-Neves VL, Resende MR, Da Silva LJ. Human asymptomatic infection in visceral leishmaniasis: a seroprevalence study in an urban area of low endemicity. Preliminary results. Am J Trop Med Hyg. 2007;77:1051–1053. [PubMed] [Google Scholar]
- 20.Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burki T. East African countries struggle with visceral leishmaniasis. Lancet. 2009;374:371–372. doi: 10.1016/s0140-6736(09)61401-x. [DOI] [PubMed] [Google Scholar]
- 22.Alvar J, Cañavate C, Gutiérrez-Solar B, Jiménez M, Laguna F, López-Vélez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–312. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elnaiem DA, Connor SJ, Thomson MC, Hassan MM, Hassan HK, Aboud MA, Ashford RW. Environmental determinants of the distribution of Phlebotomus orientalis in Sudan. Ann Trop Med Parasitol. 1998;92:877–887. doi: 10.1080/00034989858925. [DOI] [PubMed] [Google Scholar]
- 24.Gebre-Michael T, Balkew M, Alamirew T, Gudeta N, Reta M. Preliminary entomological observations in a highland area of Amhara region, northern Ethiopia, with epidemic visceral leishmaniasis. Ann Trop Med Parasitol. 2007;101:367–370. doi: 10.1179/136485907X176382. [DOI] [PubMed] [Google Scholar]
- 25.El-Safi SH, Bucheton B, Kheir MM, Musa HA, El-Obaid M, Hammad A, Dessein A. Epidemiologyof visceral leishmaniasis in Atbara River area, eastern Sudan: the outbreak of Barbar el Fugara village (1996–1997) Microbes Infect. 2002;4:1439–1447. doi: 10.1016/s1286-4579(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 26.Hailu A, Gramiccia M, Kager PA. Visceral leishmaniasis in Aba Roba, south-western Ethiopia: prevalence and incidence of active and subclinical infections. Ann Trop Med Parasitol. 2009;103:659–670. doi: 10.1179/000349809X12554106963555. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoodi M, Khamesipour A, Dowlati Y, Rafati S, Momeni A, Emamjomeh M, Hejazi H, Modabber F. Immune response measured in human volunteers vaccinated with autoclaved Leishmania major vaccine mixed with low dose of BCG. Clin Exp Immunol. 2003;134:303–308. doi: 10.1046/j.1365-2249.2003.02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha PK, Bimal S, Pandey K, Singh SK, Ranjan A, Kumar N, Lal CS, Barman SB, Verma RB, Jeyakumar A, Das P, Bhattacharya M, Sur D, Bhattacharya SK. A community-based, comparative evaluation of direct agglutination and rK39 strip tests in the early detection of subclinical Leishmania donovani infection. Ann Trop Med Parasitol. 2008;102:119–125. doi: 10.1179/136485908X252278. [DOI] [PubMed] [Google Scholar]
- 29.Gidwani K, Kumar R, Rai M, Sundar S. Longitudinal seroepidemiologic study of visceral leishmaniasis in hyperendemic regions of Bihar, India. Am J Trop Med Hyg. 2009;80:345–346. [PubMed] [Google Scholar]
- 30.Manson-Bahr PE. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- 31.Evans TG, Teixeira MJ, McAuliffe IT, Vasconcelos I, Vasconcelos AW, Sousa AA, Lima JW, Pearson RD. Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis. 1992;166:1124–1132. doi: 10.1093/infdis/166.5.1124. [DOI] [PubMed] [Google Scholar]
- 32.Riera C, Fisa R, Udina M, Gállego M, Portús M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands, Spain) by different diagnostic methods. Trans R Soc Trop Med Hyg. 2004;98:102–110. doi: 10.1016/s0035-9203(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 33.Ritmeijer K, Melaku Y, Mueller M, Kipngetich S, O'Keeffe C, Davidson RN. Evaluation of a new recombinant K39 rapid diagnostic test for Sudanese visceral leishmaniasis. Am J Trop Med Hyg. 2006;74:76–80. [PubMed] [Google Scholar]
- 34.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. 2006;333:723. doi: 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cañavate C, Herrero M, Nieto J, Cruz I, Chicharro C, Aparicio P, Mulugeta A, Argaw D, Blackstock AJ, Alvar J, Bern C. Evaluation of two rK39 dipstick tests, direct agglutination test, and indirect fluorescent antibody test for diagnosis of visceral leishmaniasis in a new epidemic site in highland Ethiopia. Am J Trop Med Hyg. 2011;84:102–106. doi: 10.4269/ajtmh.2011.10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boelaert M, Rijal S, Regmi S, Singh R, Karki B, Jacquet D, Chappuis F, Campino L, Desjeux P, Le Ray D, Koirala S, Van der Stuyft P. A comparative study of the effectiveness of diagnostic tests for visceral leishmaniasis. Am J Trop Med Hyg. 2004;70:72–77. [PubMed] [Google Scholar]
- 37.Ter Horst R, Tefera T, Assefa G, Ebrahim AZ, Davidson RN, Ritmeijer K. Field evaluation of rK39 test and direct agglutination test for diagnosis of visceral leishmaniasis in a population with high prevalence of human immunodeficiency virus in Ethiopia. Am J Trop Med Hyg. 2009;80:929–934. [PubMed] [Google Scholar]
- 38.Zijlstra EE, Daifalla NS, Kager PA, Khalil EA, El-Hassan AM, Reed SG, Ghalib HW. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin Diagn Lab Immunol. 1998;5:717–720. doi: 10.1128/cdli.5.5.717-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]