Abstract
Little information is available on transplacental transmission of Leishmania spp. We determined the frequency and impact of congenital infection caused by Leishmania panamensis or L. donovani in experimentally infected hamsters. A polymerase chain reaction showed that congenital transmission occurred in 25.8% (24 of 93) of offspring born to L. panamensis-infected hamsters and 14.6% (11 of 75) offspring born to L. donovani-infected hamsters. Mortality during lactation was higher in offspring born to L. panamensis-infected hamsters and offspring born to L. donovani-infected hamsters than controls, and lymphoproliferation to Leishmania was more frequent in offspring born to L. panamensis-infected hamsters (17.4%, 11 of 63) than in offspring born to L. donovani-infected hamsters (8.5%, 3 of 35). After weaning, only offspring born to L. donovani-infected hamsters had lower weight gain (P < 0.001) and hematocrit levels (P = 0.0045) than controls. Challenge of offspring born to L. panamensis-infected hamsters with L. panamensis showed no differences in lesion evolution, and offspring born to L. donovani-infected hamsters were more susceptible to L. donovani challenge than controls. Consequently, prenatal exposure of hamsters to L. donovani significantly increased the mortality risk and susceptibility to secondary homologous infection.
Introduction
The impact of congenital transmission of Leishmania has not been studied in persons in disease-endemic regions and consequently epidemiologic data are lacking. Although transplacental infection with other species of the family Trypanosomatidae such as Trypanosoma cruzi, the etiologic agent of Chagas' disease, resulted in 1,136 cases during 1994–2001,1,2 in the particular case of Leishmania donovani, only 11 cases of congenital transmission have been documented.3 In these cases, the possibility of vector transmission to the newborn was ruled out because the mothers were living in non-endemic regions free of phlebotomine sand flies.4,5 The difficulty in diagnosing congenital leishmaniasis is attributed to the lack of evident pathologic changes in newborns or length of the pre-patent period of visceral leishmaniasis (VL), which could be similar to that reported for primary infections in disease-endemic areas.6,7 Cutaneous leishmaniasis (CL) is also a systemic disease that seems to disseminate mainly through the lymphatic system, but the possibility of hematogenous circulation also has been documented in animals and humans.8–11 However, no attempts have been made to evaluate the feasibility of congenital transmission upon infections with Leishmania of the Viannia subgenus.
Experimental infections focused on congenital transmission of VL are scarce, and culture techniques used in earlier studies either failed to show transplacental transmission to offspring from infected hamsters or found markedly low transmission rates.12,13 Nevertheless, more recent studies in BALB/c mice and dogs by using polymerase chain reaction (PCR) to detect parasite DNA indicated that congenital transmission of VL could be more frequent than previously suspected.14,15
Although congenital infections with VL have been reported in humans and dogs, the frequency of its occurrence is still unclear.16–18 The purpose of this study was to explore the feasibility of transplacental transmission of New World CL and determine the frequency of congenital VL in a hamster model. For this purpose, we used the Syrian golden hamster, which is susceptible to most Leishmania species, including L. donovani and L. panamensis.19–21 Also, we determined the impact of in utero infection or exposure to leishmanial antigens on the newborn health and its susceptibility to a subsequent Leishmania challenge.
Materials and Methods
All experimental protocols involving hamsters followed the international guidelines for animal experimentation and were approved by the Institutional Animal Care and Use Committee of Centro Internacional de Entrenamiento e Investigaciones Medicas according to the Guiding Principles for Biomedical Research Involving Animals (Council for International Organizations of Medical Sciences) and the Colombian Law 84 of 1989, resolution #0084300 of 1993.
Offspring born to infected female hamsters.
Offspring born to hamsters with chronic CL or VL (CHR-offspring), were obtained from 3–4-month-old female hamsters infected with L. (Viannia) panamensis or L. (Leishmania) donovani one month before mating. To obtain offspring born to female hamsters pregnant during the acute phase of infection (AC-offspring), females were mated during a one-week period and then infected with the corresponding Leishmania species.22 This infection protocol assumed that female infection occurred between the second and sixth day of gestation. Infected female hamsters that did not become pregnant were excluded from the study, and groups of pregnant, non-infected hamsters were used as sources of control offspring (CTR-offspring). Females remained alone in the cage during gestation (the normal length of pregnancy is 16–18 days) and subsequently with the litter until weaning at day 21 of birth.
Female hamsters were infected intradermally in the snout with 1×104 cultured promastigotes of L. panamensis (MHOM/COL/84/1099) harvested at the stationary phase of growth (sixth day of culture) as described.8 Cultures were initiated from recent isolates obtained from infected hamsters to ensure strain pathogenicity. Cultured promastigotes of L. donovani (MHOM/IN/DD8/1968) also were harvested from the stationary phase of culture, and anethestetized female hamsters were infected with 1×106 parasites through the intraperitoneal route. Acute or chronic phases of infection represented distinct time points that could potentially lead to different congenital transmission rates and offspring immune responses.
Clinical evaluation of offspring born to infected mothers.
Offspring were maintained with the infected or uninfected mothers until weaned at 21 days of birth, which is the standard lactation period. The growth rate of the offspring based on change in body weight in grams and mortality was recorded every 15 days from the seventh to 45th day of age. Mortality was recorded in all the experimental groups, which were distributed as follows: 183 offspring born to females infected with L. panamensis (CHR-offspring, n = 79; AC-offspring, n = 104); 156 offspring born to females infected with L. donovani (CHR-offspring, n = 47; AC-offspring, n = 109); and 116 age-matched controls born to uninfected females (CTR-offspring). Hematocrits were evaluated at 45 days of age in L. donovani offspring (n = 92) and control offspring (CTR-offspring) (n = 20) by using heparinized capillary tubes. At the end of this clinical observation, animals were distributed into different experimental groups as shown in Figure 1.
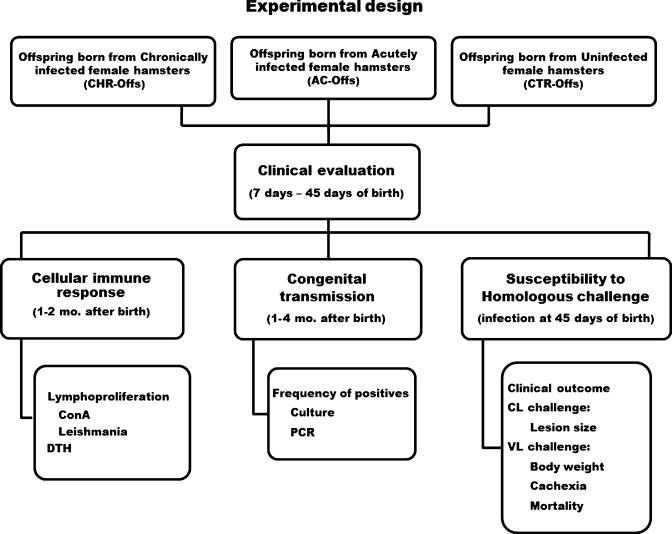
Figure 1.
Experimental design of the study. Offspring born to female hamsters pregnant during the acute phase of the infection (AC-offs) or chronic phase of the infection (CHR-offs) with Leishmania panamensis or L. donovani were subjected together with offspring born to uninfected female controls (CTR-offs) to clinical evaluations (weight and mortality) and distributed in subgroups to evaluate A, congenital transmission by means of culture (L. panamensis, AC-offs, n = 40, CHR offs, n = 33; L. donovani, AC-offs, n = 40, CHR-offs, n = 38) and polymerase chain reaction (PCR) (L. panamensis, AC-offs, n = 33, CHR offs, n = 60; L. donovani, AC-offs, n = 37, CHR-offs, n = 38), B, lymphoproliferative response to the homologous leishmanial antigen or delayed-type hypersensitivity (DTH) reaction (L. panamensis, AC-offs, n = 22, CHR offs, n = 14; L. donovani, AC-offs, n = 20, CHR-offs, n = 10, CTR-offs, n = 48), and C, acquired resistance to homologous challenge determined by clinical parameters in groups of male and female offspring (L. panamensis, male AC-offs, n = 14, female AC-offs, n = 12; male CHR-offs, n = 13, female CHR-offs, n = 12; male CTR-offs, n = 12; female CTR offs, n = 13; L. donovani, male AC-offs, n = 20, female AC-offs, n = 20, CHR-offs = not done because animals were not available, male CTR-offs, n = 10, female CTR-offs, n = 10). Evaluations of congenital transmission, DTH response, and resistance to challenge were conducted in different subsets of animals. Mo. = months; ConA = concanavalin A; CL = cutaneous leishmaniasis; VL = visceral leishmaniasis.
Identification of congenital transmission.
The frequency of congenital transmission was evaluated in offspring born to mothers with chronic infection (CHR-offspring) or born to mothers with acute infection (AC-offspring). Offspring between one and two months of age born to mothers infected either with L. panamensis (n = 140) or L. donovani (n = 78) were humanely killed, and samples of retropharyngeal lymph node, spleen, and liver were placed in Seneckjie's culture medium, incubated at 24°C, and inspected for parasites weekly for one month. For PCRs, tissue samples and serum were collected by using new sampling materials for each animal to avoid potential cross-contamination, and were subsequently stored at −20°C (n = 93, offspring born to mothers infected with L. panamensis; n = 75, offspring born to mothers infected with L. donovani).
Two PCR methods were used. For conventional PCR-hybridization, DNA from lymph node, spleen, and liver was isolated to detect amplified Leishmania kinetoplast DNA (kDNA) with a biotin-labeled probe as described.23 For real-time PCRs, DNA from lymph node, spleen, or serum was purified (NucleoSpin; Macherey-Nagel, Düren, Germany) and amplified with primers specific for a 120-basepair kDNA fragment of L. donovani (JW12, forward: 5′-GGGTAGGGGCGT TCTGCGAAA-3′; JW11, reverse: 5′-CCTATTTTACACC AACCCCCAGT-3′);24 or a 140-basepair kDNA minicircle region of L. panamensis (B4, forward: 5′-AATCGTACCACCCGACATGC-3′; 13B, reverse, 5′-ATATTACACCAACCCCTAATTGTGCA-3′). DNA was denatured (95°C for 10 seconds) and annealed (40 cycles at 60°C for 10 seconds) in a master mixture containing 20 μL of Fast Star DNA Master SYBR Green I (Hoffmann-La Roche Ltd., Basel, Switzerland), 750 nM of primers, and 100 ng or 500 ng of DNA (L. panamensis or L. donovani, respectively). Amplification curves and melting temperatures were obtained in the LightCycler 2.0 (Hoffmann-La Roche Ltd.). Specific products were identified by a melting temperature of 82.3°C for L. donovani and 83.5°C for L. panamensis. Standard curves prepared from tissue spiked with different parasite concentrations showed a sensitivity of 0.1 or 1 parasite of L. donovani or L. panamensis, respectively. The specificity of the amplification product was confirmed by electrophoresis and sequencing of the purified product (Wizard® SVG PCR Clean-Up; Promega, Madison, WI) in one congenital case of infection with L. donovani and one congenital case of infection with L. panamensis.
Cellular immune response.
Cellular immune response of the offspring was assessed by lymphoproliferation of retropharingeal lymph node lymphocytes and delayed-type hypersensitivity (DTH) reaction (Figure 1). Lymphocytes (5 × 105) of offspring born to mothers infected with L. panamensis (n = 63), offspring born to mothers infected with L. donovani (n = 35), and CTR offspring (n = 30) were stimulated in vitro over a two-day period with 2 μg/mL of concanavalin A or over a three-day period with 5 × 103 thawed-frozen L. panamensis or L. donovani promastigotes per well as described.25 The blastogenic response was expressed as the stimulation index (ratio of incorporation of tritiated thymidine over an eight-hour period of antigen-stimulated lymph node cells to that of non-stimulated cells of the same animal). A different group of offspring was used to evaluate sensitization to leishmanial antigens by DTH; these offspring were not subjected to PCR or lymphoproliferative studies because Montenegro antigen used in the test contains a large number (107/50 μL) of formalin-fixed promastigotes that could potentially give false-positive results in the PCR or lymphoproliferation assays. One and two months after weaning, Montenegro antigen was injected intradermally in the right foot of offspring born to mothers infected with L. panamensis (n = 36), offspring born to mothers infected with L. donovani (n = 20), and controls born to uninfected mothers (n = 48); the vehicle alone was injected in the left foot as a control. After 72 hours, induration in the foot was measured by using a digital caliper, and the DTH results were expressed as the difference between the diameter of the right and left foot.
Challenge of offspring born to infected and uninfected mothers.
Leishmania panamensis-infected offspring
Female and male offspring born to mothers infected with L. panamensis were challenged intradermally in the snout with 1 × 106 luciferase-transfected L. panamensis26 on the 45th day of birth (CHR-offspring, n = 25; AC-offspring, n = 26; CTR-offspring, n = 25). Development of cutaneous lesions in all groups was followed every 15 days until the end of the experiment on the 75th day post-challenge. At this point, the parasite burden was determined in the lesion and draining lymph node by using luminometry as described.22
Leishmania donovani-infected offspring
Forty-five days after birth, groups of female (n = 20) and male (n = 20) juvenile hamsters born to mothers infected during pregnancy with L. donovani were subjected to homologous intracardial challenge with 1 × 106 L. donovani promastigotes, and evolution of infection was compared with CTR-offspring born form uninfected mothers (n = 10 females, n = 10 males). Progression of VL was evaluated as follows: body weight, every 15 days up to 3 months post-challenge; hematocrit levels, monthly from the first to the third month post-challenge; cachexia, starting at three months post-challenge and up to the end of the experiment and at six months post-challenge. Cachexia was defined as clinically evident dehydratation (lack of skin elasticity of the back identified as failure to return to its normal position after pulling) and severe weight loss (> 10%). Offspring mortality was recorded every 15 days up to 6 months post-challenge, and the spleen weight as an indicator of splenomegaly was determined at the end of the experiment. Offspring born to mothers infected before pregnancy with L. donovani were not available for this set of experiments because of the small number of animals produced by these females.
Statistical analysis.
Statistical analysis was performed by using GraphPad InStat version 3.00 for Windows 95 (GraphPad Sotware, Inc., La Jolla, CA). The statistical test and number of animals used in each experiment is specified in the corresponding tables and figure legends.
Results
Clinical evaluation of offspring born to infected female hamsters.
The growth rate of offspring born to mothers infected with L. panamensis (n = 146) was equivalent to that of CTR-offspring (n = 162), as determined during the 21 days of lactation. Similarly, during the same period of lactation, no clinical manifestations of VL were observed in offspring from mothers infected with L. donovani (Figure 2A). However, the mortality rate up to the 45th day of age was significantly higher in offspring born to infected mothers than in offspring born to uninfected hamsters (Figure 2B). Analysis of the time at which mothers were infected showed that mortality was higher in offspring born to females with acute infection with both Leishmania species (L. panamensis or L. donovani) than in control offspring (CTR-offspring) (Figure 2B). The mortality rate in offspring born to chronically infected mothers was also significantly higher than in controls (Figure 2B). Despite higher mortality of offspring born to infected females, evaluations made on surviving offspring after weaning at 45 days of age indicated that the growth rate of offspring from L. panamensis-infected mothers was similar to that of controls. Conversely, CHR-offspring of mothers infected with L. donovani were clinically less fit, as demonstrated by lower weight gain and lower hematocrits than for CTR-offspring (Figure 2A and C) (P < 0.001 and P < 0.0001).
Figure 2.
Clinical evolution from the 7th to 45th day after birth of offspring born to hamster mothers infected at the chronic phase of the infection with Leishmania donovani (CHR-offs) or born to mothers infected during pregnancy (AC-offs) compared with offspring born to uninfected mothers (CTR-offs). A, Body weight at the end of lactation (21 days of birth) and after weaning at 45 days of age; CHR-offs and AC-offs had body weights similar to CTR-offs during lactation; after weaning, CHR-offs (n = 96) weighed significantly less than AC-offs (n = 68) or CTR-offs (n = 55) (**P < 0.001 each, by Tukey-Kramer multiple comparisons test). B, Mortality of AC-offs and CHR-offs infected with L. panamensis (L.p.) or L. donovani (L.d.) was higher than that of controls (CTR-offs) (L. panamensis AC-offs, n = 104, P = 0.001; L. panamensis CHR-offs, n = 79, P = 0.0014; L. donovani AC-offs, n = 109, P < 0.0001; L. donovani CHR-offs, n = 47, P = 0.019; CTR-offs, n = 162, by Fisher's exact test). C, Hematocrit at 45 days of age; L. donovani CHR-offspring (n = 52) had significantly lower hematocrit than L. donovani AC-offs (n = 40; ***P < 0.0001, by Mann-Whitney test) or CTR-offs (n = 20) (**P = 0.0045, by Mann-Whitney test).
Congenital transmission.
A low proportion of 140 (0.71%) offspring born to female hamsters infected with L. panamensis was positive when spleen samples were cultured at one month of age (Table 1), and none of the L. donovani cultures were positive (Table 2). However, molecular diagnosis by PCR dramatically increased detection of congenital transmission: 24 (25.8%) of 93 offspring born to mothers infected with L. panamensis and 11 (14.6%) of 75 offspring from mothers infected with L. donovani were positive (Tables 1 and 2). Occurrence of congenital transmission of CL was higher in offspring born to females during the acute phase of the infection (AC-offspring, 39.4%) than in offspring born to females during the chronic phase of infection (CHR-offspring, 18.3%) (P = 0.045) (Table 1). An opposite trend was observed in L. donovani (AC-offspring, 8%, CHR-offspring, 21%) (Table 2). Consequently, under the experimental conditions used in this set of experiments, congenital transmission of L. donovani tended to be lower than that of L. panamensis.
Table 1.
Frequency of congenital transmission to offspring from female hamsters infected intradermally with 104 promastigotes of Leishmania panamensis*
| Group | No. positive offspring/no. evaluated (%) | ||
|---|---|---|---|
| Culture | PCR† | P‡ | |
| CHR-offspring | 1/30 (3.3) | 11/60 (18.3)§ | 0.045 |
| AC-offspring¶ | 0/70 (0) | 13/33 (39.4) | |
| Total | 1/140 (0.7) | 24/93 (25.8) | |
Offspring were born to females in the chronic phase of the infection (CHR-offspring) or born to females infected during pregnancy (AC-offspring). PCR = polymerase chain reaction.
CHR-offspring were evaluated by PCR–hybridization or real-time PCR.
P value of Fisher's exact test, congenital cases in CHR-offspring versus AC-offspring.
Seven (23.3%) of 30 were positive by conventional PCR–hybridization and 4 (13.3%) of 30 were positive by real- time PCR.
The gestational period of hamsters is 16–18 days; weaning took place 21 days after birth. Hamsters were evaluated between one and two months after birth by real-time PCR.
Table 2.
Frequency of congenital transmission to offspring from female hamsters infected intradermally with 106 promastigotes of Leishmania donovani*
| Group | No. positive offspring/no. evaluated (%) | ||
|---|---|---|---|
| Culture† | PCR‡ | P§ | |
| CHR-offspring | 0/38 (0) | 8/38 (21) | 0.19 |
| AC-offspring¶ | 0/40 (0) | 3/37 (8) | |
| Total | 0/78 (0) | 11/75 (15) | |
Offspring were born to females in the chronic phase of the infection (CHR-offspring) or to females infected during pregnancy (AC-offspring). PCR = polymerase chain reaction.
Samples from lymph node, spleen, and bone marrow were evaluated by culture.
Samples from serum, blood, or bone marrow were obtained between one and two months after birth and evaluated by real-time PCR.
P value of Fisher's exact test, CHR-offspring versus AC-offspring.
AC-offspring were born to female hamsters infected with L. donovani at 7–11 days of pregnancy.
We obtained samples from lymph nodes, whole blood, and serum from offspring born to mothers infected with L. panamensis. We found that lymph nodes were more frequently positive than other tissues: 20 (25%) of 79 for lymph nodes, 2 (2.9%) of 69 for serum, and 0% of 12 for whole blood. To evaluate congenital transmission of L. donovani, we obtained samples from bone marrow, whole blood, and serum. In these samples, parasite DNA was detected in 5 (13%) of 38 bone marrow samples, 1 (2.8%) of 35 whole blood samples, and 5 (8%) of 62 serum samples. Although we did not intend to compare results of PCR-hybridization with those of real-time PCR, we found that both methods yielded similar positive results for L. panamensis samples: 7 (23%) of 30 were positive by PCR-hybridization and 17 (25%) of 63 were positive by real-time PCR (this comparison was not made for L. donovani-infected samples).
Lymphoproliferation and DTH in offspring of infected and uninfected females.
To evaluate the immune response of offspring born to infected mothers, we test the proliferation of lymphocytes from retropharyngeal lymph nodes to the mitogen concanavalin A and Leishmania antigens. Eleven (17.4%) of 63 offspring from mothers infected with L. panamensis and 3 (8.5%) of 35 offspring from mothers infected with L. donovani showed positive blastogenic response to Leishmania antigens (Table 3). Although 1 of 30 offspring born to uninfected hamsters (3.3%, CTR-offspring) responded non-specifically to Leishmania antigens (stimulation index > 5), a higher proportion of offspring born to infected hamsters responded to leishmanial antigens (Table 3). Notably, the lack of blastogenic response to concanavalin A or Leishmania antigens in L. donovani CHR-offspring suggested that the cellular immune response of this group was severely impaired. The DTH test for a similar subgroup of hamsters showed that 5 (13.9%) of 36 offspring from mothers infected with L. panamensis and 2 (10%) of 20 offspring from mothers infected with L. donovani had positive reactions, and none of 48 CTR offspring showed a false-positive result (Table 3).
Table 3.
Cellular immune response of offspring born to hamsters infected with Leishmania panamensis or L. donovani*
| Offspring group | No. positive offspring/no. evaluated (%) | |||
|---|---|---|---|---|
| Lymphoproliferation† | DTH‡ | |||
| Con A | Leishmania | |||
| L.(Vianna) panamensis | CHR-offspring | 24/37 (64.9)§ | 7/37 (18.9) | 4/14 (28.6)¶ |
| AC-offspring | 11/26 (42.3)# | 4/26 (15.3) | 1/22 (4.5) | |
| L. (Leishmania) donovani | CHR-offspring | 0/18 (0)** | 0/20 | ND |
| AC-offspring | 13/15 (87) | 3/15 (20.0) | 2/20 (10)†† | |
| CTR-offspring | 22/30 (73.3) | 1/30 (3.3)‡‡ | 0/48 | |
T cell proliferation and delayed-type hypersensitivity (DTH) reaction was determined in offspring born to female hamsters in the chronic phase of the infection (CHR-offspring) or born to females infected during pregnancy (AC-offspring) compared with those born to uninfected female controls (CTR-offspring). ND = not done; low numbers of offspring BP were designed for parasitologic evaluations by polymerase chain reaction.
Lymphoproliferation of lymph node lymphocytes were obtained from offspring at 1–4 months of age, stimulated with concanavalin A (Con A) and specific Leishmania antigens (thaw-frozen parasites). Positive lymphoproliferative response was defined as a stimulation index > 5.0 (stimulation index = counts per minute stimulated cells/counts per minute nonstimulated cells).
Positive DTH was defined as a ≥ 0.3 mm difference in footpad diameter between the test (Leishmania antigens) and the control foot injected with vehicle (mean DTH + 3 SD of control offspring).
P = 0.05.
P = 0.0019.
P = 0.022.
P = 0.0001.
P = 0.08.
Statistical analysis of data indicated that the control offspring had a positive result could not be considered an outlier.
Analysis of L. donovani CHR-offs demonstrated that positive cellular immune responses to Leishmania antigens were less frequent than parasitologic positivity (0 of 20 positive lymphoproliferation results versus 8 of 38 positive PCR results) (P = 0.041). Non-significant differences between lymphopropliferation and parasite PCR positivity were found in other groups (Tables 2 and 3). We did not relate lymphoproliferation and parasite status in most animals, and no comparisons were made for L. donovani infections. However, data from L. panamensis CHR-offspring indicated that 3 of 10 PCR-positive animals also showed positive results in the lymphoproliferation assay. In AC-offspring, 2 of 10 animals also showed positive results by PCR and lymphoproliferation. Nevertheless, the small number of animals in these groups prevented us from drawing any conclusion.
Offspring susceptibility to a homologous challenge.
We evaluated the outcome of a subsequent homologous challenge in offspring because this result could be a plausible scenario in humans inhabiting disease-endemic foci. We found no differences up to three months post-challenge regarding development of dermal lesions in offspring challenged intradermally in the snout with 1 × 106 L. panamensis promastigotes (mean evolution index of lesion size, observed value – initial baseline value/initial baseline value ± SD): AC-offspring, 1.25 ± 0.49, n = 26; CHR-offspring, 1.16 ± 0.39, n = 26; CTR-offspring, 1.24 ± 0.5, n = 25). No differences in parasite burden in the lesion or draining lymph node were found at the end of the experiment at three months post-challenge.
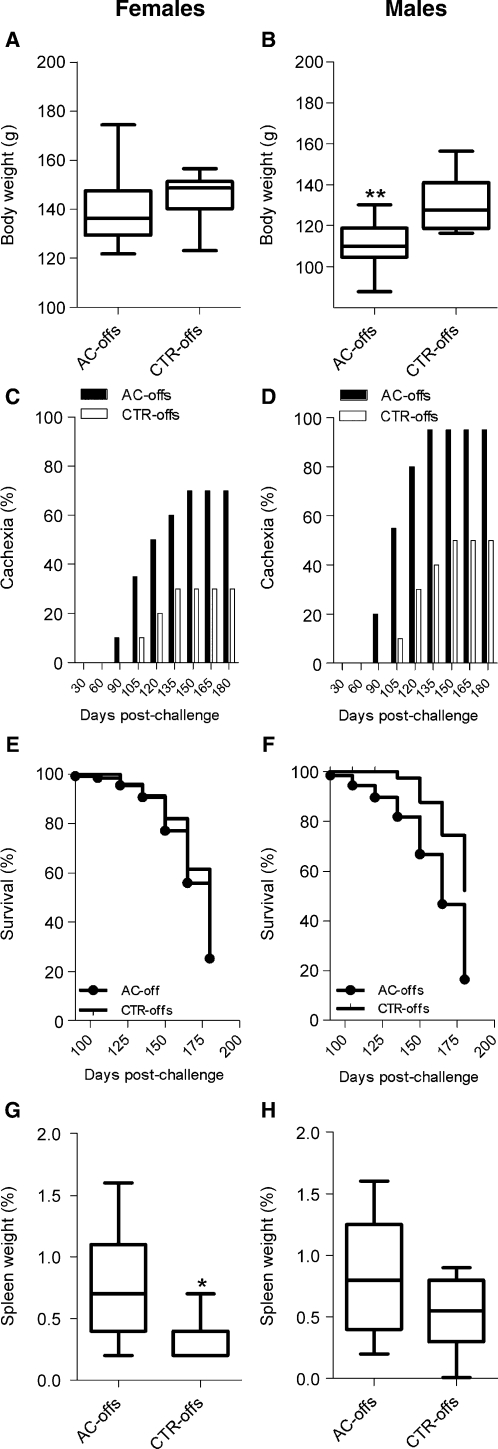
The influence of prenatal exposure to L. donovani upon a subsequent homologous challenge infection was evaluated only in offspring born to females during the acute phase of L. donovani infection (AC-offspring) because few offspring born to mothers during the chronic phase of VL were available and consequently were used to evaluate other parameters of the study (Table 3). We found that male and female AC-offspring responded differently to L. donovani challenge. Body weight at three months post-infection was similar in female AC-offspring and their matched female CTR-offspring (Figure 3A). However, male AC-offspring (n = 20) had significantly lower body weights after L. donovani challenge than their respective male CTR-offspring (n = 10) (Figure 3B) (P < 0.001). From the third to the sixth month post-challenge, both sexes of AC-offspring were more frequently cachectic than corresponding sex-matched, challenged CTR-offspring (P = 0.05, by Fisher's exact test, Figure 2C, and P = 0.008, by Fisher's exact test, (Figure 3D).
Figure 3.
Susceptibility of offspring born to Leishmania donovani-infected female hamsters to a homologous challenge than offspring born to uninfected females. Offspring born to mothers infected during pregnancy with L. donovani were challenged intraperitoneally with 1 × 106 stationary phase promastigotes at one month of age. A and B, Body weight at three months post-infection. No differences in body weight were found from 1–3 months after the challenge of female AC-offs (n = 20) compared with female CTR-offs (n = 10) (A); in contrast, the body weight of male AC-offs (n = 20) was significantly lower than male CTR-offs (n = 10) (**P < 0.001, by Tukey-Kramer test) (B). C and D, Cachexia. After three months post-challenge, overt disease was manifested and the percentage of female AC-offs (n = 20) (C) and male AC-offs (n = 20) (D) with cachexia (dehydration and severe weight loss) was significantly higher than in sex-matched CTR-offs (n = 10 females, n = 10 males) (males P = 0.0088 and females P = 0.0562, by Fisher's exact test). E and F, Kaplan-Meier survival curves. Female AC-offs (n = 20) had similar survival curves than female CTR-offs (n = 10) up to six months post-challenge (end of the experiment) (E); Survival of male AC-offspring (n = 20) was lower than that of male CTR-offspring (n = 10), P = 0.005, by log-rank Mantel-Cox test) (F). G and H, Splenomegaly. At the end of the experiment, surviving female AC-offs (n = 7) developed larger splenomegaly than female CTR-offs (n = 11, *P = 0.013, by t-test) (G) and the same trend was observed in male AC-offs (n = 8) compared with male CTR-offs (n = 9) (H). Splenomegaly is depicted as the percentage of spleen weight relative to body weight. AC-offs = mothers infected during pregnancy; CTR-offs = offspring born to uninfected mothers.
Survival of challenged female AC-offspring was similar to that of female CTR-offspring (Figure 3E) as opposed to that of male AC-offspring, which was significantly lower than their CTR-offspring (Figure 2F) (P = 0.009, by log-rank Mantel-Cox test). Despite the similar survival rate of female AC-offspring, they exhibited marked splenomegaly compared with female CTR-offspring (Figure 3G and Figure 4) (P = 0.013). The same trend was observed in male AC-offspring when compared with male CTR-offspring, but the difference was not statistically significant, probably because male AC-offspring with the largest spleens had already died, leaving only offspring with relatively smaller spleens available for evaluation (Figure 3H).
Figure 4.
Splenomegaly of offspring born to infected relative to uninfected hamster mothers upon challenge with Leishmania donovani. A marked splenomegaly was found in surviving female AC-offs (right) compared with CTR-offs (left) at six-months post-challenge. AC-offs = mothers infected during pregnancy; CTR-offs = offspring born to uninfected mothers.
Discussion
This study focused on the potentially negative effects that congenital infection could have on offspring. Our findings suggest that both Leishmania species tested could be transmitted in utero, and more importantly, that prenatal exposure to L. donovani modulates the immune response of the offspring and increases the risk for mortality or susceptibility to subsequent leishmanial infections.
As expected, PCRs were more sensitive than culture techniques in detecting congenital infections, demonstrating that approximately 25% of offspring born to infected mothers harbored L. panamensis. To our knowledge, this is the first time that congenital infection of a Leishmania species producing dermal leishmaniasis is reported. A small percentage of offspring showed DTH compared with PCR positivity, suggesting that the parasite or its antigens sensitized a low proportion of fetuses in utero.
No epidemiologic data for humans are available concerning congenital transmission of CL, but mucosal metastasis and isolation of parasites from lymph nodes and blood and diffuse CL in patients indicate that Leishmania species producing dermal pathologic changes are also disseminated systemically11,27 and may reach placental tissues. Moreover, PCR screening of dog populations suggested that Leishmania (Viannia) spp. circulate in the blood of asymptomatic dogs,10 and experimental infections in hamsters indicated that hematogenous dissemination is feasible in this animal model.9 Thus, the feasibility of congenital transmission of Leishmania (Viannia) spp. in humans requires further exploration.
We speculated that the phase of Leishmania infection of the mother (acute or chronic) could determine the frequency of congenital transmission. We found that congenital transmission of L. panamensis, as determined by PCR, was more frequent during an acute infection of the mother. Different factors could have contributed to this result. Hamsters infected with Leishmania (Viannia) sp. showed development of chronic but controlled infections in which the number of parasites tends to plateau or diminish with time post-infection.19 Leishmania (Viannia) sp. disseminates principally to draining lymph nodes, and circulates in low numbers in the blood of hamsters,9 decreasing the likelihood of transplacental transmission in females infected before pregnancy. Conversely, considerable numbers of promastigotes could have accessed the peripheral blood by spill over from intradermal infections of pregnant females (acutely infected females), thereby increasing hematogenous contact of L. panamensis with placental tissues. We expected to see a higher rate of congenital transmission in females chronically infected with L. donovani because of the progressive nature of the disease in the hamster model. However, these differences did not show statistical significance.
Previous studies using culture as diagnostic tool demonstrated either leishmanial antigen sensitization or low infection rates of offspring born to hamsters infected with L. donovani.12,13 In our study, we detected by PCR prenatal infection in 15% (n = 75) of the litters of female hamsters infected with L. donovani. In humans, there are few reports regarding transplacental infection of L. infantum or L. donovani from symptomatic or asymptomatic women.28,29 Nevertheless, in some of these cases, health of the newborns was severely affected.30 In Saudi Arabia31 and Pakistan,32 5–14% of cases occur in children before they are one year of age. In Brazil, 28% of VL cases are diagnosed in children less than four years of age,33 and in Colombia 85% of VL cases were found in children less than two years of age, including three-month-old babies.34 Although high biting rates of infected sand flies, parasite virulence, and childhood malnutrition play a significant role in the early appearance of overt disease, the contribution of vertical transmission still needs be defined.
Placentation in rodents is similar to that of humans (hemocorial), i.e., the chorionic cells are in direct contact with maternal blood, making the observations related to congenital infections amenable to extrapolation. However, in animals with other placentation types (endotheliochorial) in which the blood of the mother is not in intimate contact with chorionic cells, such as in carnivores, congenital infection is also possible, suggesting that Leishmania spp. have greater capacity to reach the fetus than commonly believed. Transplacental infection of L. infantum in dogs has been reported in experimental infections and, more importantly, in natural infections in which 32% of 52 fetuses showed PCR-positive results for different tissues.15 The epidemiologic implications of transplacental transmission in the principal domestic reservoir of VL are still unknown and should be clarified.
We found that prenatal exposure to both Leishmania species led to increased mortality during the early phase of lactation. Accordingly, studies in human populations suggested that even subclinical infections have a negative effect on the general health of humans, as shown by the decreased growth rate of asymptomatic, Montenegro skin test–positive children.35
Interestingly, we found sex-associated differences in hamster susceptibility to challenge infections after prenatal exposure to L. donovani. After homologous parasite challenge, males showed decreased weight gain and significantly higher mortality rates at any given time point than female or control male offspring. These results are consistent with those of previous studies with hamsters in which male susceptibility to L. panamensis or L. guyanensis was comparatively greater than that of females.20 Of potential epidemiologic relevance is the concept that in utero exposure to L. donovani resulted in increased susceptibility to a homologous parasite challenge, characterized by marked weight loss, splenomegaly, and mortality. From the standpoint of reservoir hosts and transmission dynamics, we speculate that puppies born to infected bitches also may have higher susceptibility to VL and become polysymptomatic earlier or more often, and therefore highly infective to sand flies.36,37
Offspring born to females during the chronic stage L. donovani infection had impaired cellular responses to Leishmania antigens and the mitogen concanavalin A, suggesting an in utero modulation of the immune response. A plausible explanation for these results is the development of specific T regulatory cell populations that induced tolerance and higher susceptibility during the first years of age, as reported for malaria38,39 and Wuchereria bancrofti infection.40 The low proportion of offspring born to females chronically infected with Leishmania that responded to leishmanial antigen contrast with the frequent T cell responses observed in dogs congenitally infected with L. infantum.18 Nevertheless, additional studies are necessary to define the parasitologic and host variables that conditioned the cellular immune response to L. donovani in the hamster model.
This, experimental study suggested that CL and VL could lead to congenital transmission resulting in symptomatic or asymptomatic infections. These experimental infections suggest that humans and reservoir hosts exposed in utero to L. donovani may have an increased mortality risk early in life or marked susceptibility to a secondary homologous infection. It could contribute to the maintenance of the transmission cycle and may influence future vaccination strategies. A closer look at vertically acquired infections aimed at defining their relative epidemiologic importance may help to adopt more knowledge-based prevention and control measures.
ACKNOWLEDGMENTS
We thank Osibar Jamauca for assistance with hamster husbandry.
Footnotes
Financial support: This study was supported by the Departamento Administrativo de Ciencia, Tecnología e Innovación–Fondo Colombiano de Investigaciones Cientificas y Proyectos Especiales, Programa Nacional de Ciencia y Tecnología de la Salud (COLCIENCIAS), Colombia, project no. 22290414328, contract no. 440-2003.
Authors' addresses: Elvia Yaneth Osorio, Alex Peniche, Omar Saldarriaga, and Bruno Luis Travi, University of Texas Medical Branch, Galveston, TX, E-mails: ejosorio@utmb.edu, alpenich@utmb.edu, omsaldar@utmb.edu, and brltravi@utmb.edu. Luz D. Rodriguez, Laboratorio de Medicina Aviar, Instituto Colombiano Agropecuario–Instituto Colombiano Agropecuario, Bogotá DC, Colombia, E-mail: luz.rodriguezc@ica.gov.co. Diana Lucía Bonilla, Biology of Inflammation Center, Baylor College of Medicine, Houston, TX, E-mail: bonilla@bcm.edu. Hector Henao, Centro Internacional de Entrenamiento e Investigaciones Medicas, Cali, Colombia, E-mail: hhhenao@gmail.com.
References
- 1.Gürtler RE, Segura EL, Cohen JE. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerg Infect Dis. 2003;9:29–32. doi: 10.3201/eid0901.020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakur CP, Sinha GP, Sharma V, Barat D. The treatment of kala-azar during pregnancy. Natl Med J India. 1993;6:263–265. [PubMed] [Google Scholar]
- 3.Figueiró-Filho EA, Duarte G, El-Beitune P, Quintana SM, Maia TL. Visceral leishmaniasis (kala-azar) and pregnancy. Infect Dis Obstet Gynecol. 2004;12:31–40. doi: 10.1080/1064744042000210384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elamin A, Omer MI. Visceral leishmaniasis in a 6-week-old infant: possible congenital transmission. Trop Doct. 1992;22:133–135. doi: 10.1177/004947559202200326. [DOI] [PubMed] [Google Scholar]
- 5.Meinecke CK, Schottelius J, Oskam L, Fleischer B. Congenital transmission of visceral leishmaniasis (kala-azar) from an asymptomatic mother to her child. Pediatrics. 1999;104:e65. doi: 10.1542/peds.104.5.e65. [DOI] [PubMed] [Google Scholar]
- 6.Mattot M, Ninane J, Bigaignon G, Vermylen C, Cornu G. Visceral and cutaneous leishmaniasis in an European paediatric population. Acta Clin Belg. 1992;47:231–237. doi: 10.1080/17843286.1992.11718236. [DOI] [PubMed] [Google Scholar]
- 7.Al-Orainey IO, Gasim IY, Singh LM, Ibrahim B, Ukabam SO, Gonchikar D, Shekhawat BS. Visceral leishmaniasis in Gizan, Saudi Arabia. Ann Saudi Med. 1994;14:396–398. doi: 10.5144/0256-4947.1994.396. [DOI] [PubMed] [Google Scholar]
- 8.Travi B, Rey-Ladino J, Saravia NG. Behavior of Leishmania braziliensis s.l. in golden hamsters: evolution of the infection under different experimental conditions. J Parasitol. 1988;74:1059–1062. [PubMed] [Google Scholar]
- 9.Martinez JE, Travi BL, Valencia AZ, Saravia NG. Metastatic capability of Leishmania (Viannia) panamensis and Leishmania (Viannia) guyanensis in golden hamsters. J Parasitol. 1991;77:762–768. [PubMed] [Google Scholar]
- 10.Reithinger R, Lambson BE, Barker DC, Davies CR. Use of PCR to detect Leishmania (Viannia) spp. in dog blood and bone marrow. J Clin Microbiol. 2000;38:748–751. doi: 10.1128/jcm.38.2.748-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergel C, Palacios R, Cadena H, Posso CJ, Valderrama L, Perez M, Walker J, Travi BL, Saravia NG. Evidence for Leishmania (Viannia) parasites in the skin and blood of patients before and after treatment. J Infect Dis. 2006;194:503–511. doi: 10.1086/505583. [DOI] [PubMed] [Google Scholar]
- 12.Herman RN, Fahey JR. Sensitization of offspring of Leishmania donovani-infected hamsters to immunization and of offspring of immunized hamsters to challenge. Am J Trop Med Hyg. 1982;31:9. doi: 10.4269/ajtmh.1982.31.730. [DOI] [PubMed] [Google Scholar]
- 13.Escobar MA, Saravia NG. Transmisión Congenital Experimental de Leishmania donovani chagasi. Cali, Colombia: Biomédica: Revista del Instituto Nacional de Salud; 1991. [Google Scholar]
- 14.Rosypal AC, Lindsay DS. Non-sand fly transmission of a North American isolate of Leishmania infantum in experimentally infected BALB/c mice. J Parasitol. 2005;91:1113–1115. doi: 10.1645/GE-586R.1. [DOI] [PubMed] [Google Scholar]
- 15.Pangrazio KK, Costa EA, Amarilla SP, Cino AG, Silva TM, Paixão TA, Costa LF, Dengues EG, Diaz AA, Santos RL. Tissue distribution of Leishmania chagasi and lesions in transplacentally infected fetuses from symptomatic and asymptomatic naturally infected bitches. Vet Parasitol. 2009;165:327–331. doi: 10.1016/j.vetpar.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Rosypal AC, Troy GC, Zajac AM, Frank G, Lindsay DS. Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. J Parasitol. 2005;91:970–972. doi: 10.1645/GE-483R.1. [DOI] [PubMed] [Google Scholar]
- 17.da Silva SM, Ribeiro VM, Ribeiro RR, Tafuri WL, Melo MN, Michalick MS. First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet Parasitol. 2009;166:159–162. doi: 10.1016/j.vetpar.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Boggiatto PM, Gibson-Corley KN, Metz K, Gallup JM, Hostetter JM, Mullin K, Petersen CA. Transplacental transmission of Leishmania infantum as a means for continued disease incidence in North America. PLoS Negl Trop Dis. 2011;5:e1019. doi: 10.1371/journal.pntd.0001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hommel M, Jaffe CL, Travi B, Milon G. Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann Trop Med Parasitol. 1995;89((Suppl 1)):55–73. doi: 10.1080/00034983.1995.11813015. [DOI] [PubMed] [Google Scholar]
- 20.Travi BL, Osorio Y, Melby PC, Chandrasekar B, Arteaga L, Saravia NG. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect Immun. 2002;70:2288–2296. doi: 10.1128/IAI.70.5.2288-2296.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melby PC, Chandrasekar B, Zhao W, Coe JE. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol. 2001;166:1912–1920. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- 22.Osorio Y, Bonilla DL, Peniche AG, Melby PC, Travi BL. Pregnancy enhances the innate immune response in experimental cutaneous leishmaniasis through hormone-modulated nitric oxide production. J Leukoc Biol. 2008;83:1413–1422. doi: 10.1189/jlb.0207130. [DOI] [PubMed] [Google Scholar]
- 23.Weigle KA, Labrada LA, Lozano C, Santrich C, Barker DC. PCR-based diagnosis of acute and chronic cutaneous leishmaniasis caused by Leishmania (Viannia) J Clin Microbiol. 2002;40:601–606. doi: 10.1128/JCM.40.2.601-606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolas L, Milon G, Prina E. Rapid differentiation of Old World Leishmania species by LightCycler polymerase chain reaction and melting curve analysis. J Microbiol Methods. 2002;51:295–299. doi: 10.1016/s0167-7012(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 25.Osorio Y, Melby PC, Pirmez C, Chandrasekar B, Guarín N, Travi BL. The site of cutaneous infection influences the immunological response and clinical outcome of hamsters infected with Leishmania panamensis. Parasite Immunol. 2003;25:139–148. doi: 10.1046/j.1365-3024.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 26.Roy G, Dumas C, Sereno D, Wu Y, Singh AK, Tremblay MJ, Ouellette M, Olivier M, Papadopoulou B. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol. 2000;110:195–206. doi: 10.1016/s0166-6851(00)00270-x. [DOI] [PubMed] [Google Scholar]
- 27.Romero I, Téllez J, Suárez Y, Cardona M, Figueroa R, Zelazny A, Gore Saravia N. Viability and burden of Leishmania in extralesional sites during human dermal leishmaniasis. PLoS Negl Trop Dis. 2010;4(pii):e819. doi: 10.1371/journal.pntd.0000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eltoum IA, Zijlstra EE, Ali MS, Ghalib HW, Satti MM, Eltoum B, el-Hassan AM. Congenital kala-azar and leishmaniasis in the placenta. Am J Trop Med Hyg. 1992;46:57–62. doi: 10.4269/ajtmh.1992.46.57. [DOI] [PubMed] [Google Scholar]
- 29.Bogdan C, Schönian G, Bañuls AL, Hide M, Pratlong F, Lorenz E, Röllinghoff M, Mertens R. Visceral leishmaniasis in a German child who had never entered a known endemic area: case report and review of the literature. Clin Infect Dis. 2001;32:302–306. doi: 10.1086/318476. [DOI] [PubMed] [Google Scholar]
- 30.Pagliano P, Carannante N, Rossi M, Gramiccia M, Gradoni L, Faella FS, Gaeta GB. Visceral leishmaniasis in pregnancy: a case series and a systematic review of the literature. J Antimicrob Chemother. 2005;55:229–233. doi: 10.1093/jac/dkh538. [DOI] [PubMed] [Google Scholar]
- 31.Al-Orainey IO, Gasim IY, Singh LM, Ibrahim B, Ukabam SO, Gonchikar D, Shekhawat BS. Visceral leishmaniasis is endemic in southern Saudi Arabia. Ann Saudi Med. 1994;14:396–398. doi: 10.5144/0256-4947.1994.396. [DOI] [PubMed] [Google Scholar]
- 32.Ayub S, Khalid M, Mujtaba G, Bhutta RA. Profile of patients with cutaneous leishmaniaisis from Multan. J Pak Ned Assoc. 2001;51:279–281. [PubMed] [Google Scholar]
- 33.Silva ES, Gontijo CM, Pacheco RS, Fiuza VO, Brazil RP. Visceral leishmaniasis in the Metropolitan Region of Belo Horizonte, State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2001;96:285–291. doi: 10.1590/s0074-02762001000300002. [DOI] [PubMed] [Google Scholar]
- 34.Salgado DPC, Rodríguez JA. Leishmaniasis Visceral en Niños: Afecta Principalmente a Menores de Dos Años. Revisión de 20 Años de Experiencia. Bogota: Revista Colombiana de Pediatría. Ed. Legis; 1998. pp. 160–165. [Google Scholar]
- 35.Cunha DF, Lara VC, Monteiro JP, Romero HD, Cunha SF. Growth retardation in children with positive intradermic reaction for leishmaniasis: preliminary results [in Portuguese] Rev Soc Bras Med Trop. 2001;34:25–27. doi: 10.1590/s0037-86822001000100004. [DOI] [PubMed] [Google Scholar]
- 36.Deane LM. Leishmaniasis. New York: Elsevier; 1985. (Leishmaniasis in Brazil. Human Parasitic Diseases). [Google Scholar]
- 37.Travi BL, Tabares CJ, Cadena H, Ferro C, Osorio Y. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitologic status and infectivity for sand flies. Am J Trop Med Hyg. 2001;64:119–124. doi: 10.4269/ajtmh.2001.64.119. [DOI] [PubMed] [Google Scholar]
- 38.Flanagan KL, Halliday A, Burl S, Landgraf K, Jagne YJ, Noho-Konteh F, Townend J, Miles DJ, van der Sande M, Whittle H, Rowland-Jones S. The effect of placental malaria infection on cord blood and maternal immunoregulatory responses at birth. Eur J Immunol. 2010;40:1062–1072. doi: 10.1002/eji.200939638. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra I, Dent A, Mungai P, Wamachi A, Ouma JH, Narum DL, Muchiri E, Tisch DJ, King CL. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med 6. 2009;e1000116 doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malhotra I, Mungai PL, Wamachi AN, Tisch D, Kioko JM, Ouma JH, Muchiri E, Kazura JW, King CL. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J Infect Dis. 2006;193:1005–1013. doi: 10.1086/500472. [DOI] [PubMed] [Google Scholar]