Abstract
We report the first establishment of in vitro cultivation and genotypic characterization of Leishmania siamensis isolated from an autochthonous disseminated dermal and visceral leishmaniasis in a Thai acquired immunodeficiency syndrome (AIDS) patient. The molecular identification has shown that the parasite was identical to L. siamensis, a recently described Leishmania species reported in the southern provinces of Thailand. The phylogenetic analysis has confirmed L. siamensis as closely related to the zoonotic Leishmania species L. enrietti.
Introduction
Recently, a few cases of autochthonous leishmaniasis have been reported from Thailand, especially from the southern provinces. However, these cases were diagnosed by detecting amastigotes in clinical specimens without confirmation of the species of Leishmania. In 2008, the first case of visceral leishmaniasis (VL) caused by a new species, L. siamensis, was described.1 This parasite was characterized by molecular methods. In 2010, a second case of L. siamensis infection was reported in a human immunodeficiency virus (HIV)-infected patient.2 Meanwhile, a Leishmania strain apparently identical to L. siamensis was detected as a causative agent of cutaneous leishmaniasis in cows and a horse in Germany and Switzerland.3,4 However, attempts to isolate the parasite from these human and animal infections by cultivation on axenic media were unsuccessful. This paper is the description of a disseminated dermal and visceral form in a Thai acquired immunodeficiency syndrome (AIDS) patient. For the first time, the parasite was isolated on culture medium and identified as L. siamensis (Trang strain) by molecular methods.
Case Report
A 32-year-old Thai female originally living in Trang, southern Thailand, who never traveled to leishmaniasis-endemic areas, was diagnosed with HIV infection in 2007. She was treated by stavudine (D4T)/lamivudine (3TC)/nevirapine (NVP) at a community hospital in Trang. In June of 2009, she developed skin rash and was referred to Trang Provincial Hospital. The treatment was changed to 3TC/D4T/efavirenz (EFV). She was diagnosed with cryptococcal meningitis two times when antifungal treatments, including amphotericin B and fluconazole, were given. During this period, her anemic condition was detected, and a blood transfusion was given. She refused to have bone marrow aspiration for investigating the cause of anemia. Antiretroviral therapy was changed to 3TC/TDF/liponaviar (LPV)/r and then LASTAVIR(D4T + 3TC)/LPV/r. She gained weight, and the skin rash was reduced. CD4 cell count increased from 32 to 107 cells/μL, and she had increased levels of Hb (10.1 g/dL) and Hct (30.7%). In March of 2010, she was diagnosed with cryptococcal meningitis and anemia. 3TC/D4T/LPV/r and fluconazole were given.
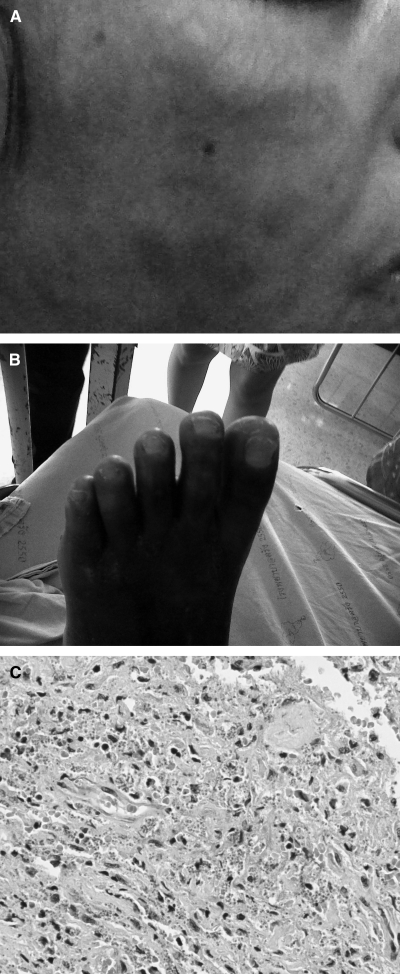
In July 2010, she complained of thick and hard skin nodules covering her body for 1 month. Dermatological examination revealed diffused irregular hard subcutaneous nodules varying in size, especially on her face, trunk, and extremities (Figure 1A and B). The nodules at lower extremities showed diffuse brownish scaly plaques. Skin biopsy was performed and sent to the Department of Pathology, Prince of Songkla Hospital, Songkhla. Pathological examination revealed diffuse dermal and subcutaneous infiltration of mixed inflammatory cells composed predominantly of macrophages with an admixture of lymphocytes and plasma cells. The macrophages contained numerous intracellular amastigotes of Leishmania (Figure 1C). Physical examination also revealed severe anemia and generalized hepatomegaly. Bone marrow aspiration and blood collection were performed and sent to the Department of Parasitology, Phramongkutklao College of Medicine, Bangkok. Leishmania infection was confirmed. In September 2010, the patient experienced seizure and died 2 weeks before amphotericin B was started.
Figure 1.
Disseminated dermal and visceral leishmaniasis: clinical and histopathological features. (A) Diffuse irregular hard surface in the subcutaneous tissue varying in size of large nodules on the face. (B) Diffuse brownish scaly plaques at lower extremities. (C) Histopathological examination of the skin biopsy revealing numerous intracellular amastigotes of Leishmania (hematoxylin–eosin staining).
From a bone marrow aspiration, we established an axenic culture of L. siamensis in both Scheneider's medium supplemented with 20% fetal bovine serum (FBS) and Novy-MacNeal-Nicolle (NNN) medium incubated at 25°C. Promastigote forms were observed on day 15 to have morphology similar to other Leishmania species, and the strain was then cryopreserved in liquid nitrogen. These promastigotes have been continuously maintained in both Scheneider's medium supplemented with 20% FBS and NNN medium. However, this parasite did not grow well in M199. Establishment of amastigotes in BALB/c mice has been ongoing research. A molecular identification was performed by polymerase chain reaction (PCR) amplification of the ssrRNA locus and the conserved part of the minicircle kinetoplastic DNA from both buffy coat and in vitro cultivated parasites.5,6 The PCR products amplified from the ssrRNA and the mini-circle kDNA were 540 bp (Genbank accession number JQ280883) and 117 bp, respectively. The ssrRNA sequence was 100% identical to the unpublished L. siamensis sequence (accession number JN885899).1 Lower identity (99%) of ssrRNA sequences was observed between a pair of Leishmania identified from this study and another 10 Leishmania species (L. amazonensis [GQ332354], L. naiffi [DQ182543], L. braziliensis [GQ332355], L. donovani [GQ332356], L. guyanensis [GQ332358], L. infantum [GQ332359], L. major [GQ332361], L. panamensis [GQ332362], L. mexicana [GQ332360], and L. tropica [GQ332363]) with different substitution positions.
There were 118 alignable sites of kDNA genes from eight species of studied Leishmania. The divergences of kDNA sequences of Leishmania isolated in this study were noticeably high. There was about a 74–78% match of kDNA sequences between Leishmania from the patient and selected Leishmania species, including L. amazonensis (EU370875), L. braziliensis (EU370882), L. donovani (EU370886), L. infantum (EU370899), L. major (EU370908), L. tropica (EU370912), and L. tarentolae (AF088235), and only a 63% consensus in the identity of kDNA sequences shown between Leishmania identified from the patient and L. lainsoni (K01770).
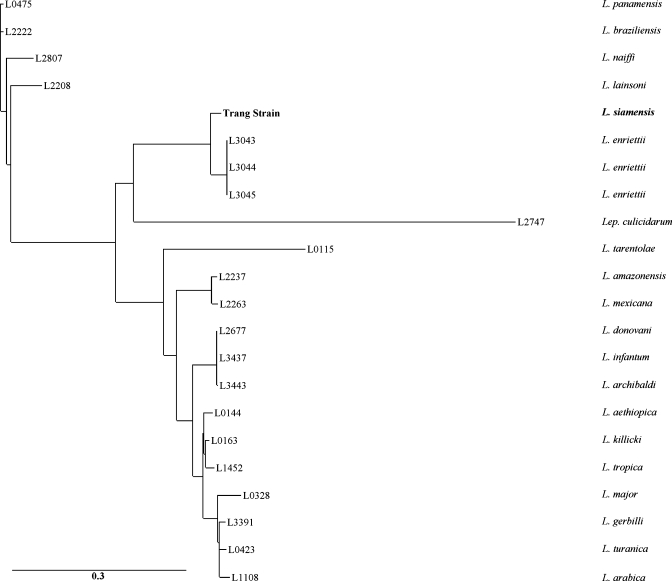
A phylogenetic analysis of the L. siamensis (Trang strain) was performed at the French Reference Center for Leishmaniasis (FRCL) by multilocus analysis of three protein coding DNA sequences (locus 03.0980, elongation initiation factor 2 α-subunit [JQ586200]; locus 04.0580, spermidine synthase 1 [JQ586201]; locus 31.2610, RNA polymerase II largest subunit [JQ586202]; they are located on L. major chromosomes 3, 4, and 31, respectively). The L. siamensis genotype was compared with 17 different Leishmania species genotypes representative of the genetic diversity within the Leishmania genus (Figure 2). From the 1,680 bp analyzed, 333 informative sites were identified, and the genetic similarity between L. siamensis and L. enrietti, a zoonotic Leishmania species infecting Brazilian guinea pigs, was confirmed. Currently considered as the standard approach for Leishmania taxonomy, the isoenzymatic profiling of the Trang strain was performed at FRCL. Unfortunately, some isoenzymes were not detectable, and the others were hardly comparable with the zymodemes described in the other Leishmania species, probably because of the genetic divergence of L. siamensis.
Figure 2.
Phylogenetic comparison of the Trang strain to 17 other Leishmania species. Concatenated sequences of three protein-coding genes (XM_001687340, XM_883450, and XM_001685196; 1,680 nt in all) located on three different chromosomes in the Leishmania genome were compared using the maximum likelihood method implemented in PhyML with a GTR + I + G model of nucleotide substitutions selected by the JModelTest. Three distinct and highly supported clusters were distinguishable. Bootstrap values (1,000 replicates) were given at the nodes. A well-individualized cluster included both the Trang strain (L. siamensis) and three L. enrietti strains, even when the two species were clearly distinct.
Conclusions
This report is the first report of an autochthonous disseminated dermal and visceral leishmaniasis caused by L. siamensis. Dermatological manifestations in this patient were similar to those manifestations previously described in AIDS patients who were infected with L. infantum and L. donovani.7,8 In this case, the cause of anemia, which might be caused by leishmaniasis, was not properly investigated. Dermal lesions were later developed after amphotericin B was not given. Increased awareness of leishmaniasis, especially for HIV-positive patients, is suggested in the endemic areas. Genetic characterization confirmed that L. siamensis is distinct from other reported Leishmania spp. Establishment of in vitro culture of L. siamensis will bring up additional study of important biological parameters of the organism. Our report raises concerns over this emerging disease in Thailand. Epidemiological studies, including disease surveillance, screening of people and animals in the affected areas, and also identification of natural vectors, are urgently needed and essential for prevention and control strategies in Thailand.
ACKNOWLEDGMENTS
We would like to thank Dr. Mohamed Kasbari and Dr. Francine Pratlong from the French Agency for Health and Safety and the French Reference Centre on Leishmaniasis, respectively, for the preliminary results of isoenzyme analysis. F.E.B. is funded by an InfectioPole Sud Fondation PhD grant.
Footnotes
Authors' addresses: Lertwut Bualert, Department of Dermatology, Trang Hospital, Trang, Thailand, E-mail: dr_lertwut_clinic@yahoo.com. Wiwat Charungkiattikul, Department of Medicine, Trang Hospital, Trang, Thailand, E-mail:wiwatmed@gmail.com. Paramee Thongsuksai, Department of Pathology, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand, E-mail: paramee.t@psu.ac.th. Mathirut Mungthin, Suradej Siripattanapipong, Rommanee Khositnithikul, Tawee Naaglor, and Saovanee Leelayoova, Department of Parasitology, Phramongkutklao College of Medicine, Bangkok, Thailand, E-mails: mathirut@pmk.ac.th, suradejs@rocketmail.com, kik_kuru@yahoo.com, tawee_narkloar@hotmail.com, and s_leelayoova@scientist.com. Christophe Ravel and Fouad El Baidouri, Academic Hospital of Montpellier, French Reference Centre on Leishmaniasis, Unité Mixte de Recherche 5290, Montpellier, France, E-mail: christophe.ravel@univ-montp1.fr.
References
- 1.Sukmee T, Siripattanapipong S, Mungthin M, Worapong J, Rangsin R, Samung Y, Kongkaew W, Bumrungsana K, Chanachai K, Apiwathanasorn C, Rujirojindakul P, Wattanasri S, Ungchusak K, Leelayoova S. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008;38:617–622. doi: 10.1016/j.ijpara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Suankratay C, Suwanpimolkul G, Wilde H, Siriyasatien P. Autochthonous visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected patient: the first in Thailand and review of the literature. Am J Trop Med Hyg. 2010;82:4–8. doi: 10.4269/ajtmh.2010.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller N, Welle M, Lobsiger L, Stoffel MH, Boghenbor KK, Hilbe M, Gottstein B, Frey CF, Geyer C, von Bomhard W. Occurrence of Leishmania sp. in cutaneous lesions of horses in central Europe. Vet Parasitol. 2009;166:346–351. doi: 10.1016/j.vetpar.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Lobsiger L, Muller N, Schweizer T, Frey CF, Wiederkehr D, Zumkehr B, Gottstein B. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol. 2010;169:408–414. doi: 10.1016/j.vetpar.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Uliana SR, Nelson K, Beverley SM, Camargo EP, Floeter-Winter LM. Discrimination amongst Leishmania by polymerase chain reaction and hybridization with small subunit ribosomal DNA derived oligonucleotides. J Eukaryot Microbiol. 1994;41:324–330. doi: 10.1111/j.1550-7408.1994.tb06085.x. [DOI] [PubMed] [Google Scholar]
- 6.le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, Rousseau D, Kubar J. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J Clin Microbiol. 1999;37:1953–1957. doi: 10.1128/jcm.37.6.1953-1957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, Gradoni L, Ter Horst R, Lopez-Velez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guimaraes LH, Machado PR, Lago EL, Morgan DJ, Schriefer A, Bacellar O, Carvalho EM. Atypical manifestations of tegumentary leishmaniasis in a transmission area of Leishmania braziliensis in the state of Bahia, Brazil. Trans R Soc Trop Med Hyg. 2009;103:712–715. doi: 10.1016/j.trstmh.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]