Abstract
Species identification of human hookworm infections among eight communities in rural areas of Peninsular Malaysia was determined during 2009–2011. Fecal samples were examined by microscopy and subsequently, the internal transcribed spacer 2 and 28S ribosomal RNA region of Necator americanus and Ancylostoma spp. were sequenced. Overall, 9.1% (58 of 634) were identified positive by microscopy for hookworm infection, and 47 (81.0%) of 58 were successfully amplified and sequenced. Sequence comparison found that N. americanus (87.2%) was the most predominant hookworm identified, followed by Ancylostoma ceylanicum (23.4%). No A. duodenale infection was detected in this study. Detection of A. ceylanicum in humans highlighted the zoonotic transmission among humans living near dogs. Thus, implementation of effective control measures for hookworm infections in future should seriously consider this zoonotic implication.
Introduction
Human hookworm infections have widespread socioeconomic and public health implications. Globally, an estimated 600 million persons are infected, resulting in up to 135,000 deaths annually.1 Human infection is primarily caused by two species of hookworm (Ancylostoma duodenale and Necator americanus).2 Geographic distribution of A. duodenale infections is mainly in the Middle East, northern Africa, India, Australia, and Europe, and N. americanus is more common in the Western Hemisphere, sub-Saharan Africa, eastern Asia, and southeast Asia.3
Clinically, infection in human causes iron-deficiency anemia, which may result in mental retardation and growth deficiencies, particularly in children.4,5 Besides the two human species, intestinal zoonotic infections with canine and/or feline hookworm such as A. ceylanicum, A. caninum, and Ancylostoma braziliense have also been reported in many parts of the world.6–8 More recently, zoonotic ancylostomiasis caused by A. ceylanicum was detected by using copro-molecular diagnostic tools in rural communities in Thailand9,10 and Laos.11
Accurate diagnosis by precise identification and differentiation of species involved is essential in monitoring the efficacy of mass treatment and effective control of hookworm infection. Currently, most diagnosis and research conducted on the epidemiology of human hookworm infection greatly relies on the use of a conventional method for the detection of eggs in fecal samples. The benefits of this method are mainly technical simplicity and low cost. Although microscopy is limited and hampered because N. americanus eggs are morphologically indistinguishable from Ancylostoma spp. and other strongylid nematodes, including Trichostrongylus spp. and Oesophagostomum spp., microscopy is still the gold standard technique for rapid diagnosis.
Frequently, mass treatment with anthelminthic drugs is performed without identification of the causative species of infection. Given that a clinical manifestation such as severity of anemia differs according to the hookworm species involved12 and the route of infection for each hookworm species also differs from species to species (e.g., N. americanus infection is mainly by skin penetration, and Ancylostoma spp. infections are more common by ingestion of infective third-stage larvae), species identification is paramount in designing appropriate and effective prevention and control strategies. Moreover, if a zoonotic hookworm is prevalent, the control target and strategies formulated also need to encompass animal hosts.
Although hookworm infection is still highly prevalent, especially in rural and remote areas of Peninsular Malaysia,13–15 information on the species of hookworm present in humans is lacking. Because of the importance of accurate identification of hookworm infection, this study was conducted as part of an ongoing epidemiologic investigation to provide genetic data on the species of hookworm infecting humans in Peninsular Malaysia.
Methods
Study area and population.
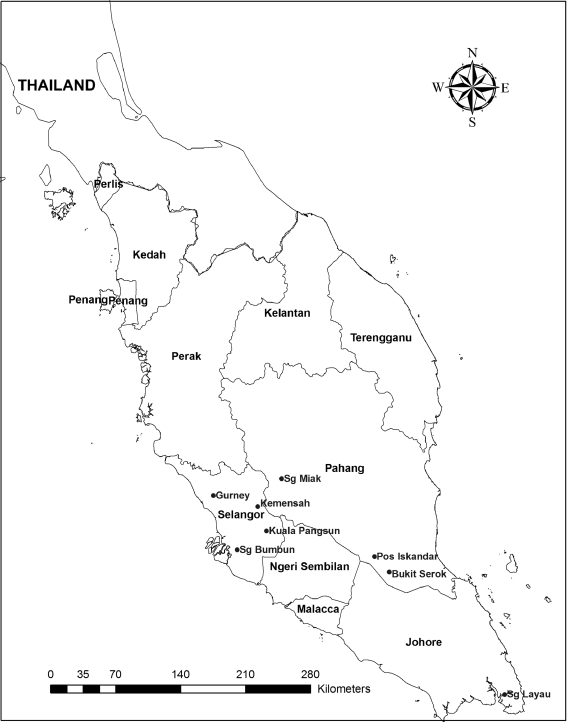
The study was carried out during April 2009–April 2011 in eight villages in West Malaysia, which have been recognized as geohelminth-endemic areas.14 The villages were Pos Iskandar (3.06°N, 102.65°E), Sungai Layau (1.53°N, 104.10°E), Bukit Serok (2.91°N, 102.82°E), Gurney (3.43°N, 101.44°E), Sungai Bumbun (2.85°N, 101.42°E), Kuala Pangsun (3.21°N, 101.88°E), Sungai Miak (3.52°N, 101.90°E), and Kemensah (3.21°N, 101.77°E) (Figure 1).
Figure 1.
Location of the study areas in Peninsular Malaysia.
Each village had a small population, and the number of residents in each village was estimated to be 80–100 inhabitants. A total of 634 villagers, 2–82 years of age (276 males and 358 females) participated in this study. These communities lived in poor and socioeconomically deprived circumstances where overcrowding, poor environmental sanitation, low level of education, and poor provision of safe water are widespread. All houses have untreated tap water originating from a nearby river and there are no household-based sanitation facilities. The environmental condition of the village is generally poor with limited provision of latrine facilities therefore encouraging defecation in and around bushes or nearby rivers. Children usually defecated indiscriminately around their houses without parental supervision. In addition, it has been observed that it was common for villagers to walk barefooted while outdoors. The villagers also kept dogs, cats, monkeys, rabbits, and birds, and most of these domestic animals were left to roam freely.
Fecal sample collection and parasitologic procedures.
After persons had provided oral and written informed consent, pre-labeled plastic containers for fecal sample collection were handed out to all participants, and their ability to recognize their names was checked. The participants were instructed to scoop a thumb-size fecal sample by using a scoop provided into the container. Parents and guardians were instructed to monitor their children during sample collection to ensure that they placed their fecal samples into the correct container. Containers filled with samples were collected the next day.
Fresh fecal samples were stored at ambient temperature and transferred to the laboratory within 2–4 hours after collection. Samples were preserved in 2.5% potassium dichromate and refrigerated at 4°C until further analysis. Samples were processed using wet smear and formalin ethyl acetate sedimentation technique, followed by microscopic examination of iodine-stained samples for hookworms and other intestinal parasites.16 Samples that were microscopically positive for hookworm eggs were further characterized by using molecular procedures.
Genomic DNA extraction.
Genomic DNA was extracted directly from microscopically positive fecal samples by using the PowerSoil® DNA Kit (catalog no. 12888-100; MO BIO Laboratories, Carlsbad, CA) according to manufacturer's instructions. Before DNA extraction, approximately 0.2–0.3 g of fecal sample was added into a PowerBead Tube® and incubated at 70°C for 10 minutes with the presence of cell lysis and disruption agent provided by the manufacturer. Subsequently, the fecal sample was subjected to homogenization and lysis procedure for complete cell lysis by mechanical shaking (vortexing) with a vortex adapter (catalog. no. 13000-V1; MO BIO Laboratories). Final elution of DNA was performed in 50 μL of elution buffer instead of 200 μL. Extracted DNA was stored at –20°C until required for polymerase chain reaction (PCR) amplification.
DNA amplification by PCR.
A two-step semi-nested PCR was used for DNA amplification of hookworm species. For the first amplification, forward primer NC1 (5′-ACG TCT GGT TCA GGG TTC TT-3′) and reverse primer NC2 (5′-TTA GTT TCT TTT CCT CCG CT-3′)17 were used to amplify approximately 310-basepair and 420-basepair regions of internal transcribed spacer 2 and 28S ribosomal RNA region of N. americanus and Ancylostoma spp. The PCR was conducted in a 50 μL volume with the final mixture containing 10× PCR buffer, 2.5 mM dNTPs, 25 mM MgCl2, 10 pmol of each primer, 5 units of Taq polymerase, and 6 μL of DNA template. The sample was heated to 94°C for 5 minutes, followed by 30 cycles at 94°C for 30 seconds (denaturing), 55°C for 30 seconds (annealing), and 72°C for 30 seconds (extension), and a final extension at 72°C for 7 minutes. Control samples without DNA (DNase/RNase free water; catalog no. W4502; Sigma, St. Louis, MO) and samples containing N. americanus and Ancylostoma spp. genomic DNA (positive control) was included in each PCR.
Subsequently, samples that produced fragment approximately 310 and/or 420 basepairs in the first PCR were subjected to a second amplification. Amplification was conducted by using forward primer NA (5′-ATGTGCACGTTATTCACT-3′) for N. americanus18 and AD1 (5′-CGA CTT TAG AAC GTT TCG GC-3′) for Ancylostoma spp.19 and NC2 as a common reverse primer. The secondary amplification reagent concentrations were similar to those of the first round of PCR except that 6 μL of primary PCR product was added instead of DNA. The cycling conditions for the second round amplification were 94°C for 5 minutes, followed by 35 cycles at 94°C for 1 minute (denaturing), 55°C for 1 minute (annealing), and 72°C for 1 minute (extension), and a final extension at 72°C for 7 minutes. In both amplifications, samples were incubated in the MyCycler Thermal Cycler (Bio-Rad Laboratories, Hercules, CA).
Species identification of hookworm.
All PCR-positive amplicons were then purified using the QIAquick Gel Extraction Kit (catalog. no. 28104; QIAGEN, Hilden, Germany) according to the manufacturer's instructions. All purified amplicons were subjected to DNA sequencing in both directions (forward and reverse primers) using an ABI 3730XL sequencer (Bioneer Corporation, Daejeon, South Korea). Sequence chromatograms were viewed using Sequence Scanner version 1.0 (Applied Biosystems, Foster City, CA). Sequences were analyzed and aligned with each other and published sequences for all hookworms using BioEdit20 and MEGA4.21 Homology search was conducted using National Center for Biotechnology Information (Bethesda, MD) reference sequences with the Basic Local Alignment Search Tool as the means to determine hookworm species. All sequences generated in this study were deposited in GenBank under accession nos. HQ452515–HQ452517, HQ452537–HQ452543, and JF960362–JF960403.
Ethical considerations.
The study protocol was approved by the Ethics Committee of the University Malaya Medical Center, Malaysia (MEC Ref. No. 824.11). Before sample collection, an oral briefing to describe the objective and method of the study was given to the participants by the investigator. Consent was obtained either in written form (signed) or verbally and by thumb prints (for those who were illiterate) from participants or their parents/guardians (on behalf of their children).
Results
Overall hookworm infection.
Of 634 fecal samples examined, 58 (9.1%) were positive by microscopy for hookworm infection (Table 1 ). Size of ova ranged from 60 to 75 μm in length and from 36 to 40 μm in width. The highest prevalence was recorded in Gurney (19.1%), followed by Pos Iskandar (12.4%), Kuala Pangsun (11.1%), Bukit Serok (8.9%), Sungai Bumbun (3.7%), and Sungai Layau (1.1%). No positive sample was detected in Sungai Miak and Kemensah. Prevalence of other intestinal parasites detected in this study were 54.3% (344 of 634) for Trichuris trichiura, 26.7% (169 of 634) for Ascaris lumbricoides, 9.5% (60 of 634) for Giardia spp., and 9.1% (58 of 634) for Entamoeba histolytica/dispar.
Table 1.
Hookworm infections detected by microscopy and PCR* of human fecal samples in Peninsular Malaysia
| Location | No. examined | Microscopy | PCR | ||
|---|---|---|---|---|---|
| No. | % | No. | %† | ||
| Gurney | 141 | 27 | 19.1 | 23 | 85.2 |
| Pos Iskandar | 113 | 14 | 12.4 | 11 | 78.6 |
| Kuala Pangsun | 54 | 6 | 11.1 | 6 | 100 |
| Bukit Serok | 99 | 8 | 8.9 | 5 | 62.5 |
| Sungai Bumbun | 54 | 2 | 3.7 | 2 | 100 |
| Sungai Layau | 89 | 1 | 1.1 | 0 | 0 |
| Sungai Miak | 30 | 0 | 0 | 0 | 0 |
| Kemensah | 54 | 0 | 0 | 0 | 0 |
| Total | 634 | 58 | 9.1 | 47 | 81.0 |
PCR = polymerase chain reaction.
Based on the number positive by microscopy (n = 58).
Identification of hookworm species.
The 58 hookworm-positive samples were then subjected to PCR and sequence analysis. The PCR amplicons were successfully obtained from 47 (81.0%) of 58 samples and genetically characterized on the basis of its DNA sequence of the internal transcribed spacer 2 region of the ribosomal RNA gene. In addition, 576 microscopy-negative results were also screened by using molecular technique, and PCR results confirmed that these samples were negative. Sequence comparison using the Basic Local Alignment Search Tool demonstrated that 87.2% (41 of 47) and 23.4% (11 of 47) were N. americanus and A. ceylanicum, respectively. Overall, 76.6% (36 of 47) and 12.8% (6 of 47) of humans had single infections with N. americanus and A. ceylanicum, respectively, and 10.6% (5 of 47) had mixed infections with both species (Table 2 ). The highest prevalence of N. americanus was recorded in Gurney (21 of 47, 44.7%), followed by Pos Iskandar (10 of 47, 21.3%), Kuala Pangsun (4 of 47, 8.5%), and Bukit Serok (4 of 47, 8.5%), and the lowest prevalence was recorded in Sungai Bumbun (1 of 47, 2.1%). As for A. ceylanicum, 10.6% (5 of 47) were detected in persons in Gurney, 4.3% (2 of 47) in Pos Iskandar and Kuala Pangsun, and 2.1% (1 of 47) in Bukit Serok and Sungai Bumbun respectively.
Table 2.
Necator americanus and Ancylostoma ceylanicum infections in human fecal samples detected by PCR* according to villages (n = 47) in Peninsular Malaysia
| Location | PCR positive | N. americanus only | A. ceylanicum only | N. americanus and A. ceylanicum | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Gurney | 23 | 18 | 78.3 | 2 | 8.7 | 3 | 13.0 |
| Pos Iskandar | 11 | 9 | 81.8 | 1 | 9.1 | 1 | 9.1 |
| Kuala Pangsun | 6 | 4 | 66.7 | 2 | 33.3 | 0 | 0 |
| Bukit Serok | 5 | 4 | 80.0 | 0 | 0 | 1 | 20.0 |
| Sungai Bumbun | 2 | 1 | 50.0 | 1 | 50.0 | 0 | 0 |
| Sungai Layau | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sungai Miak | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kemensah | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 47 | 36 | 76.6 | 6 | 12.8 | 5 | 10.6 |
PCR = polymerase chain reaction.
Discussion
In this study, microscopy examination showed that 9.1% (58 of 634) of the participants had hookworm infection. This finding is consistent with those of a recent study among similar rural communities in Malaysia (12.8%)14 and other local studies (prevalence range = 3.0–10.8%).13,22–24 However, the data must be interpreted cautiously because these studies were based on a single fecal examination, which possibly results in underestimation of the actual prevalent rates. In addition, light hookworm infections might have been underdiagnosed by microscopy.
Conventionally, hookworm detection by microscopy is not able to differentiate between N. americanus and Ancylostoma spp. In the present study, molecular analysis was used to confirm human infections of two species of hookworm, namely N. americanus and A. ceylanicum. It is assumed that the principal species of hookworm infecting human in Peninsular Malaysia is the most predominant human hookworm worldwide (N. americanus). Thus, it is not surprising that our molecular analysis showed that 87.2% of the infections were caused by N. americanus. Similarly, the only available species-specific hookworm study conducted in Sarawak (East Malaysia) also found one N. americanus isolate in human sample.25 Interestingly, no A. duodenale infection was found in these study areas, which also supports the geographic restriction of this species. This finding was consistent with those of other studies in countries in Southeast Asia, which reported that the distribution of N. americanus is far more prevalent than that of A. duodenale.
In the southern part of Thailand, a low prevalence for A. duodenale (0.1%) has been reported in humans compared with 99.9% for N. americanus.26 Recently, a study among persons living in central Thailand indicated that 92.0% of the persons were infected with N. americanus compared with 2.0% with A. duodenale.10 Conversely, a recent study in Laos found that Ancylostoma spp. infections (9.4%) were slightly more prevalent than N. americanus infections (5.9%).11 However this finding could be a biased representation because specific species of Ancylostoma was not elucidated in that study. Because this community lived near dogs, it is highly possible that the Ancylostoma spp. identified could be a mixture of A. ceylanicum and/or A. duodenale. Nonetheless, geographic variance in the distribution of the two human hookworm species is a multi-factorial phenomenon, given that factors such as human and parasite behavior, ethnicity, climate, temperature, and environmental factors are involved.12,27 Because Malaysia, Thailand, and Laos are neighboring countries with similar geographic conditions, human factors such as life style should be compared in future studies.
Zoonotic hookworm infections in humans have been overlooked because of lack of molecular tools to identify hookworm species. In the present study, A. ceylanicum infections (23.4%, 11 of 47), a common canine and feline hookworm, were found in human. This result clearly indicated that A. ceylanicum may be more common than previously thought. Ancylostoma ceylanicum is the only species of zoonotic hookworm known to produce patent infections in humans. This finding has been demonstrated experimentally28,29 and naturally. Natural infections with A. ceylanicum have been reported in servicemen from The Netherlands returning from West New Guinea, who had heavy infections with concurrent anemia.30 Mostly light infections have been reported in humans in the Philippines,31 Taiwan,32 Thailand,33 and India.34 More recently, zoonotic ancylostomiasis caused by A. ceylanicum has been reported in rural communities in Thailand9,10 and Laos.11 Although emerging zoonotic case reporting is increasing worldwide, these parasites are still regarded as rare and abnormal hookworms of humans and largely overlooked in human parasite surveys.
Dogs and cats were the most common domestic animals in the studied communities. These animals were roaming freely and defecating around the neighborhood. Transmission of hookworm is aggravated in these areas because mud and damp soils favor development of infectious stage larvae. A study among persons living near semi-domesticated dogs in Bangkok, Thailand identified A. ceylanicum in persons where 77.0% of the dogs harbored this species.9 Additionally, a recent study among persons in a rural area in central Thailand showed that 6.0% of hookworm egg–positive persons harbored A. ceylanicum.10 Such human-animal cohabitation puts humans at risk for an array of zoonotic parasitic diseases. Because children usually defecate indiscriminately around their houses without parental supervision and in some cases even adults defecated indiscriminately in bushes and the nearby river near their houses, this behavior may facilitate transmission of parasites from humans to humans, humans to animals, or animals to humans.
Although all microscopy-positive samples that were negative by PCR (11 samples) were retested several times by PCR, amplification was still unsuccessful. The inability to amplify these samples was believed to be associated with inhibitor substances that were not eliminated before the PCR. Further study to optimize reduction of these inhibitors is necessary during the extraction process to increase PCR sensitivity.
Failure to amplify the samples mentioned above could also be caused by the fact that hookworm eggs identified were not from hookworms but from strongylid nematodes because they are morphologically indistinguishable by microscopy. One such strongylid is Trichostrongylus spp., which is also known as hookworm-like eggs. Human infection with Trichostrongylus spp. has been reported in other countries in Southeast Asia, such as Laos11 and Thailand,35 and in South Korea36 and Iran.37 Although there are no published reports of human infection with this species in Malaysia, Trichostrongylus spp. infection in humans cannot be disregarded because molecular identification of other strongylid nematode such as Trichostrongylus spp.-specific PCR has not been used in studies in Malaysia. Therefore, this analysis should be incorporated in future studies.
In conclusion, our study demonstrated that N. americanus was the most predominant hookworm species infecting humans in Malaysia. Cases of infection with A. duodenale were not found, which supports the geographic restriction of this species. Interestingly, A. ceylanicum, a canine hookworm, was also detected in humans, which shows that humans are at high risk of acquiring infection with A. ceylanicum, especially those living near dogs. Thus, implementation of effective control measures for hookworm infections should seriously consider this zoonotic implication.
ACKNOWLEDGMENTS
We thank the Ministry of Rural Development, Malaysia, headmasters, teachers, and heads of villages for providing permission to collect samples; Saidon Ishak, Shukri Jaffar, Wan Hafiz Wan Ismail, and Kaharmuzakir Ismail for providing technical assistance; and all villages for voluntarily participating in this study.
Footnotes
Financial support: This study was supported by University of Malaya high impact research grant (J-00000-73587) and research grant (RG221-10HTM).
Authors' addresses: Romano Ngui, Lee Soo Ching, Tan Tiong Kai, Muhammad Aidil Roslan, and Yvonne A. L. Lim, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: skyromano@gmail.com, eustacialee@gmail.com, stanley.monas@gmail.com, aidil_lanang@ymail.com, and limailian@um.edu.my.
References
- 1.Hotez PJ. One world health: neglected tropical diseases in a flat world. PLoS Negl Trop Dis. 2009;3:405. doi: 10.1371/journal.pntd.0000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan MS, Medley GF, Jamison D, Bundy DA. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology. 1994;109:373–387. doi: 10.1017/s0031182000078410. [DOI] [PubMed] [Google Scholar]
- 3.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Albonico M, Crompton DW, Savioli L. Control strategies for human intestinal nematode infections. Adv Parasitol. 1999;42:277–341. doi: 10.1016/s0065-308x(08)60151-7. [DOI] [PubMed] [Google Scholar]
- 5.Crompton DW. The public health importance of hookworm disease. Parasitology. 2000;121:39–50. doi: 10.1017/s0031182000006454. [DOI] [PubMed] [Google Scholar]
- 6.Croese J, Loukas A, Opdebeeck J, Prociv P. Occult enteric infection by Ancylostoma caninum: a previously unrecognized zoonosis. Gastroenterol. 1994;106:3–12. doi: 10.1016/s0016-5085(94)93907-1. [DOI] [PubMed] [Google Scholar]
- 7.Khoshoo V, Craver R, Schantz P, Loukas A, Prociv P. Abdominal pain, pan-gut eosinophilia, and dog hookworm infection. J Pediatr Gastroenterol. 1995;21:481. doi: 10.1097/00005176-199511000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Landmann JK, Prociv P. Experimental human infection with the dog hookworm, Ancylostoma caninum. Med J Aust. 2003;20:67–69. doi: 10.5694/j.1326-5377.2003.tb05222.x. [DOI] [PubMed] [Google Scholar]
- 9.Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RC. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet Parasitol. 2008;155:67–73. doi: 10.1016/j.vetpar.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, Piyaraj P, Naaglor T, Taamari P, Leelayoova S. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg. 2011;84:594–598. doi: 10.4269/ajtmh.2011.10-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato M, Sanguankiat S, Yoonuan T, Pongvongsa T, Keomoungkhoun M, Phimmayoi I, Boupa B, Moji K, Waikagul J. Copro-molecular identification of infections with hookworm eggs in rural Lao PDR. Trans R Soc Trop Med Hyg. 2010;104:617–622. doi: 10.1016/j.trstmh.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Beaver PC, Jung RC, Cupp EW. Clinical Parasitology. Philadelphia: Lea and Febiger; 1984. [Google Scholar]
- 13.Lim YA, Romano N, Colin N, Chow SC, Smith HV. Intestinal parasitic infections amongst Orang Asli (indigenous) in Malaysia: has socioeconomic development alleviated the problem? Trop Biomed. 2009;26:110–122. [PubMed] [Google Scholar]
- 14.Ngui R, Saidon I, Chow SC, Rohela M, Lim YA. Prevalence and risk factors of intestinal parasitism in rural and remote West Malaysia. PLoS Negl Trop Dis. 2011;5:e974. doi: 10.1371/journal.pntd.0000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jex AR, Lim YA, Bethony JM, Hotez PJ, Young ND, Gasser RB. Soil-transmitted helminths of humans in southeast Asia: towards integrated control. Adv Parasitol. 2011;74:231–265. doi: 10.1016/B978-0-12-385897-9.00004-5. [DOI] [PubMed] [Google Scholar]
- 16.Cheesbrough M. Parasitological Tests: District Laboratory Practice in Tropical Countries, Part 1. Cambridge, United Kingdom: Cambridge University Press; 1998. [Google Scholar]
- 17.Gasser RB, Chilton NB, Hoste H, Beveridge I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminthes. Nucleic Acids Res. 1993;21:2525–2526. doi: 10.1093/nar/21.10.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verweij JJ, Pit DS, van Lieshout L, Baeta SM, Dery GD, Gasser RB, Polderman AM. Determining the prevalence of Oesophagostomum bifurcum and Necator americanus infections using specific PCR amplification of DNA from faecal samples. Trop Med Int Health. 2001;6:726–731. doi: 10.1046/j.1365-3156.2001.00770.x. [DOI] [PubMed] [Google Scholar]
- 19.de Gruijter JM, van Lieshout L, Gasser RB, Verweij JJ, Brienen EA, Ziem JB, Yelifari L, Polderman AM. Polymerase chain reaction-based differential diagnosis of Ancylostoma duodenale and Necator americanus infections in human in northern Ghana. Trop Med Int Health. 2005;10:574–580. doi: 10.1111/j.1365-3156.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- 20.Hall TA. BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Series. 1999;41:95–98. [Google Scholar]
- 21.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 22.Nor Aini U, Al-Mekhlafi HM, Azlin M, Shaik A, Sa'iah A, Fatmah MS, Ismail MG, Firdaus MS, Aisah MY, Rozlida AR, Norhayati M. Serum iron status in Orang Asli children living in endemic areas of soil-transmitted helminth. Asia Pac J Clin Nutr. 2007;16:724–730. [PubMed] [Google Scholar]
- 23.Hakim SL, Gan CC, Malkit K, Azian MN, Chong CK, Shaari N, Zainuddin W, Chin CN, Sara Y, Lye MS. Parasitic infections among Orang Asli (aborigine) in the Cameron Highlands, Malaysia. Southeast Asian J Trop Med Public Health. 2007;38:415–419. [PubMed] [Google Scholar]
- 24.Al-Mekhlafi MH, Surin J, Atiya AS, Ariffin WA, Mohammed Mahdy AK, Che Abdullah H. Pattern and predictors of soil-transmitted helminth reinfection among aboriginal schoolchildren in rural Peninsular Malaysia. Acta Trop. 2008;107:200–204. doi: 10.1016/j.actatropica.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Romstad A, Gasser RB, Nansen P, Polderman AM, Chilton NB. Necator americanus (Nematoda: Ancylostomatidae) from Africa and Malaysia have different ITS-2 rDNA sequences. Int J Parasitol. 1998;28:611–615. doi: 10.1016/s0020-7519(97)00213-0. [DOI] [PubMed] [Google Scholar]
- 26.Anantaphruti MT, Maipanich W, Muennoo C, Pubampen S, Sanguankiat S. Hookworm infections of schoolchildren in southern Thailand. Southeast Asian J Trop Med Public Health. 2002;33:468–473. [PubMed] [Google Scholar]
- 27.Hoagland KE, Schad GA. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36–49. doi: 10.1016/0014-4894(78)90078-4. [DOI] [PubMed] [Google Scholar]
- 28.Wijers DJ, Smit AM. Early symptoms after experimental infection of man with Ancylostoma braziliense var. ceylanicum. Trop Geogr Med. 1966;18:48–52. [PubMed] [Google Scholar]
- 29.Carroll SM, Grove DI. Experimental infection of human with Ancylostoma ceylanicum: clinical, parasitological, haematological and immunological findings. Trop Geogr Med. 1986;38:38–45. [PubMed] [Google Scholar]
- 30.Anten JF, Zuidema PJ. Hookworm infection in Dutch servicemen returning from West New Guinea. Trop Geogr Med. 1964;64:216–224. [PubMed] [Google Scholar]
- 31.Velasquez CC, Cabrera BC. Ancylostoma ceylanicum (Looss 1911) in a Filipino woman. J Parasitol. 1968;54:430–431. [PubMed] [Google Scholar]
- 32.Yoshida Y, Okamoto K, Chiu JK. Ancylostoma ceylanicum infection in dogs, cats, and man in Taiwan. Am J Trop Med Hyg. 1968;17:378–381. doi: 10.4269/ajtmh.1968.17.378. [DOI] [PubMed] [Google Scholar]
- 33.Areekul S, Radomyos P, Viravan C. Preliminary report of Ancylostoma ceylanicum infection in Thai people. J Med Assoc Thai. 1970;53:315–321. [PubMed] [Google Scholar]
- 34.Chowdhury AB, Schad GA. Ancylostoma ceylanicum: a parasite of man in Calcutta and environs. Am J Trop Med Hyg. 1972;21:300–301. doi: 10.4269/ajtmh.1972.21.300. [DOI] [PubMed] [Google Scholar]
- 35.Panasoponkul C, Radomyos P, Singhasivanon V. Trichostrongylus infection in a Thai boy. Southeast Asian J Trop Med Public Health. 1985;16:513–514. [PubMed] [Google Scholar]
- 36.Yong TS, Lee JH, Sim S, Lee J, Min DY, Chai JY, Eom KS, Sohn WM, Lee SH, Rim HJ. Differential diagnosis of Trichostrongylus and hookworm eggs via PCR using ITS-1 sequence. Korean J Parasitol. 2007;45:69–74. doi: 10.3347/kjp.2007.45.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghadirian E, Arfaa F. Present status of trichostrongyliasis in Iran. Am J Trop Med Hyg. 1975;24:935–941. doi: 10.4269/ajtmh.1975.24.935. [DOI] [PubMed] [Google Scholar]