Abstract
We report a case of hookworm-related cutaneous larva migrans diagnosed microscopically. Viable hookworm larvae were found by microscopic examination of a skin scraping from follicular lesions. Amplification and sequencing of the internal transcribed spacer 2 allowed the specific identification of the larvae as Ancylostoma braziliense.
Introduction
Hookworm-related cutaneous larva migrans (HrCLM) is a parasitic skin disease frequently seen in patients returning from travel abroad.1,2 It usually presents as a creeping dermatitis (often located on feet) and more seldomly, as folliculitis.3,4 HrCLM is considered as a clinical diagnosis,1,2 and the detection of hookworm larvae is rarely possible. We describe a patient in which culprit larvae could be recovered from a folliculitis lesion and also identified by polymerase chain reaction (PCR) and sequencing of the internal transcribed spacer 2 (ITS2) region.
Case Report
In August of 2011, a 34-year-old French woman presented in our department with a localized itchy rash after returning from a 2-week trip in Guadeloupe (French West Indies). Most of her time was spent lying on a towel on a sandy beach in a two-piece bathing suit. She did not see any stray cats or dogs on the beach. Two days before returning to France, she noticed pruriginous pimples on her trunk and left breast. She did not complain of any other symptom, and she did not recall any insect bites or stings; 7 days after she returned (9 days after the onset of symptoms), she visited her general practitioner and was treated for HrCLM with a single dose of oral ivermectine (12 mg), 10% based albendazole ointment, and topical corticosteroid.
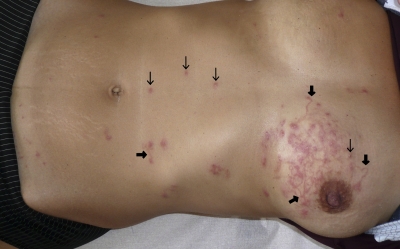
Two days after consultation with her general practitioner, the patient presented in our department with persistent pruritus and a rash located on her belly and left breast, which consisted of roughly 12 erythematous papules and pustules (about 3 mm in diameter) associated with multiple lesions of creeping dermatitis (about 2 mm in width and 20–50 mm in length), some of them emerging from the follicular canal (Figure 1).
Figure 1.
HrCLM presenting with papulo-pustular lesions of folliculitis (thin arrow) associated with multiple trails of creeping dermatitis (broad arrow).
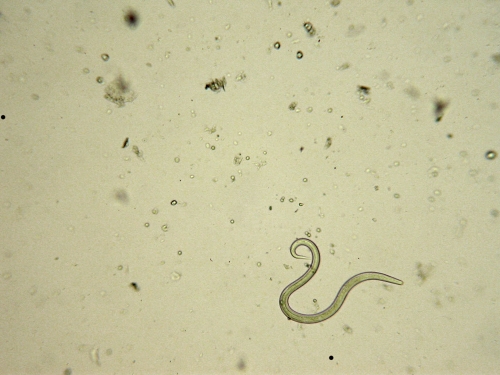
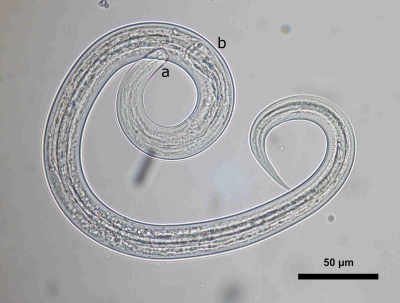
Skin scraping of approximately six follicular lesions revealed three living nematode larvae. The larvae all displayed the same phenotypical characteristics. The length and width of the larvae were approximately 650 and 20 μm, respectively (Figure 2) . The oral apparatus consisted of a small buccal capsule (3×15 μm). The esophagus extended as a longitudinal column to the enlarged posterior bulb. The length of the esophagus represented less than one-third of the total length of the larvae (Figure 3 ). All these morphological features were in accordance with the morphological criteria of Ancylostoma that were defined in the work by Nichols5 in the 1950s.
Figure 2.
Living hookworm larva recovered from a skin scraping of a lesion of folliculitis (Optical microscope,×10).
Figure 3.
Microscopic examination of a larva recovered from a scraping of folliculitis lesions. (A) buccal capsule, (B) esophagus with posterior bulb (Optical microscope).
Genomic DNA of two larvae was extracted using the DNA IQ system and Tissue and Hair Extraction kit (Promega, Madison, WI) according to the manufacturer's instructions. Amplification of ITS2 was performed in a final volume of 50 μL containing GC reaction buffer, Phusion High-Fidelity DNA polymerase (1 unit; Finnzymes, Espoo, Finland), forward and reverse primers (NC1: 5′-ACGTCTGGTTCAGGGTTGTT-3′; NC2: 5′-TTAGTTTCTTTTCCTCCGCT-3′; 0.8 μM each), 2′-deoxynucleoside 5′-triphosphate (dNTPs; 200 μM each), and extracted DNA. The amplification program was 1 cycle of denaturation (94°C, 5 minutes), 30 cycles of denaturation (94°C, 30 seconds), annealing (55°C, 30 seconds), and polymerization (72°C, 30 seconds) followed by 1 cycle of final extension (72°C, 7 minutes). PCR product was confirmed by a 2% agarose gel electrophoresis and sent for purification and sequencing to MWG Eurofins Operon (Ebersberg, Germany). Nucleotide sequence was entered into the Basic Local Alignment Search Tool (BLAST)6 and queried against the whole nucleotide collection. The resulting evidence identified the culprit larvae as A. braziliense (GenBank sequence database accession number DQ438061.1, 711 bp, e-value of 10–137, 100% of identity from nucleotide 443 to nucleotide 711).
Because of the extent of skin lesions and the presence of still living nematode larvae, the patient was treated with a second dose of oral ivermectine (12 mg). Topical treatment was stopped. In a follow-up phone interview 2 weeks later, the patient described a dramatic decrease in pruritus 2 days after consulting our department, but she stated that she self-administered another dose of oral ivermectine (12 mg) 2 days after the second dose. The clinical lesions present on the patient persisted much longer than the pruritus.
Discussion
To the best of our knowledge, this case is the first case where the HrCLM culprit larvae were found by skin scraping and identified at the species level with molecular techniques as A. braziliense.
Most cases of HrCLM are reported in tourists who have visited beaches in regions where A. braziliense is endemic in dogs and cats,7 such as the French West Indies. HrCLM does not occur after beach exposure in regions like the Pacific coast of the United States8 and Mexico, where A. braziliense is not present in cats and dogs. The species A. braziliense was not found in a survey of a large number of dogs and cats in Australia, which could explain the lack of HrCLM in people frequenting beaches in that country.9 This finding is concordant with the results of old studies, where human volunteers presented with creeping dermatitis after having been experimentally infected with larvae of A. braziliense.10
In the current study, we found three hookworm larvae by microscopic examination of the skin scrapings from a follicular skin lesion, which has previously only been described in three other instances.11–13 HrCLM hallmark is the presence of creeping dermatitis. Another rare but documented symptom of HrCLM is the presence of folliculitis lesions. Contrary to lesions of creeping dermatitis, it is possible to extract hookworm larvae from folliculitis lesions. In cases of folliculitis where histological data are available, it has been shown that the hookworm larvae are trapped in the follicular canal and therefore, can be extracted from the lesions.11,12,14–16 Conversely, in cases of creeping dermatitis, the culprit larvae are located somewhere beyond the extremity of the cutaneous trail. Therefore, the ability to extract the larva is only possible when the creeping dermatitis is associated with folliculitis. However, folliculitis has only been described in 3% of the patients with HrCLM.4
According to old experimental studies, the hookworm species A. braziliense and A. caninum were considered to be the leading causes of HrCLM.17 However, there are existing data in patients with CLM referring to the identification of the culprit larva after skin scrapping11–13 or cutaneous biopsy.11,14–16,18 In such circumstances, only A. caninum14 and uncharacterized Ancylostoma spp.13,15,18 have been diagnosed according to morphologic criteria as well as Pelodera strongyloides.11,12,16 A. caninum is the only species that has been identified according to the morphological criteria defined in the work by Nichols5 in the 1950s in a single case of HrCLM.14 Given the morphological similarities between A. ceylanicum and A. braziliense, the previous literature is difficult to use as a reference for identification of the species.7 The advent of biomolecular techniques has shown that morphological criteria are not reliable enough to distinguish between different species of Ancylostoma larvae.7 Therefore, this case is the first case of HrCLM in which a hookworm species could be identified by molecular techniques.
In a typical case of HrCLM, a single dose of ivermectin cures more than 95% of the patients.4 It is noteworthy that we found three viable hookworm larvae in the skin, although our patient had received a 12-mg oral dose of ivermectin 2 days previously. However, in patients where hookworm folliculitis is present, the need for prolonged treatment has been previously described.2,3 The need for prolonged treatment in such instances is confirmed in this case report.
Conclusion
Physicians should pay attention to the existence of lesions of folliculitis when they examine a patient with creeping dermatitis. As shown in this case study, the diagnosis of HrCLM can be confirmed by microscopic examination of a skin scraping, and molecular techniques can aid in the identification of the specific hookworm species responsible for HrCLM.
ACKNOWLEDGMENTS
The authors thank Aurélie Heckmann (UMR BIPAR) for managing the biomolecular techniques and Kasie Raymann for editing the manuscript.
Footnotes
Authors' addresses: Alexandre Le Joncour, Groupe Hospitalier, Universitaire de la Pitié-Salpêtrière, Maladies Infectieuses et Tropicale, 47 bd de l'hopital, Paris 75013, France, E-mail: lejoncour.alexandre@gmail.com. Sandrine A. Lacour, ANSES, Laboratoire de Santé Animale, Maisons‐Alfort, France, E-mail: sandrine.lacour@anses.fr. Gabriel Lecso, Hôpital Universitaire de la Pitié-Salpêtrière – Parasitologie, Paris, France, E-mail: gm.lecso@free.fr. Stéphanie Regnier, Hôpital Universitaire de la Pitié-Salpêtrière, Maladies Infectieuses et Tropicales, Paris, France, E-mail: stephregnier1@yahoo.fr. Jacques Guillot, Ecole Nationale Vétérinaire d'Alfort – Laboratoire de Santé Animale, UMR BIPAR, Maisons‐Alfort, France, E-mail: jguillot@vet-alfort.fr. Eric Caumes, Hôpital Universitaire de la Pitié-Salpêtrière, Maladies Infectieuses et Tropicales, Paris, France, E-mail: eric.caumes@psl.aphp.fr.
References
- 1.Caumes E, Danis M. From creeping eruption to hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2004;4:659–660. doi: 10.1016/S1473-3099(04)01178-8. [DOI] [PubMed] [Google Scholar]
- 2.Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302–309. doi: 10.1016/S1473-3099(08)70098-7. [DOI] [PubMed] [Google Scholar]
- 3.Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol. 2002;146:314–316. doi: 10.1046/j.0007-0963.2001.04531.x. [DOI] [PubMed] [Google Scholar]
- 4.Caumes E, Carriere J, Guermonprez G, Bricaire F, Danis M, Gentilini M. Dermatoses associated with travel to tropical countries: a prospective study of the diagnosis and management of 269 patients presenting to a tropical disease unit. Clin Infect Dis. 1995;20:542–548. doi: 10.1093/clinids/20.3.542. [DOI] [PubMed] [Google Scholar]
- 5.Nichols RL. The etiology of visceral Larva migrans. II. Comparative larval morphology of Ascaris lumbricoides, Necator americanus, Strongyloides stercoralis, and Ancylostoma caninum. J Parasitol. 1956;42:363–399. [PubMed] [Google Scholar]
- 6. National Center for Biotechnology Information (NCBI) Nucleotide BLAST Program Available atW http://blast.ncbi.nlm.nih.gov/Blast.cgi Accessed November 26, 2011.
- 7.Traub RJ, Hobbs RP, Adams PJ, Behnke JM, Harris PD, Thompson RC. A case of mistaken identity–reappraisal of the species of canid and felid hookworms (Ancylostoma) present in Australia and India. Parasitology. 2007;134:113–119. doi: 10.1017/S0031182006001211. [DOI] [PubMed] [Google Scholar]
- 8.Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010;26:162–167. doi: 10.1016/j.pt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Palmer CS, Traub RJ, Robertson ID, Hobbs RP, Elliot A, While L. The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum. Vet Parasitol. 2007;145:304–313. doi: 10.1016/j.vetpar.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Dove WE. Further studies on Ancylostoma braziliense and the etiology of creeping eruption. Am J Epidemiol. 1932;15:664–711. [Google Scholar]
- 11.Pasyk K. Dermatitis rhabditidosa in an 11-year-old girl: a new cutaneous parasitic disease of man. Br J Dermatol. 1978;98:107–112. doi: 10.1111/j.1365-2133.1978.tb07340.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones CC, Rosen T, Greenberg C. Cutaneous larva migrans due to Pelodera strongyloides. Cutis. 1991;48:123–126. [PubMed] [Google Scholar]
- 13.Kim TH, Lee BS, Sohn WM. Three clinical cases of cutaneous larva migrans. Korean J Parasitol. 2006;44:145–149. doi: 10.3347/kjp.2006.44.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller AC, Walker J, Jaworski R, de Launey W, Paver R. Hookworm folliculitis. Arch Dermatol. 1991;127:547–549. [PubMed] [Google Scholar]
- 15.Purdy KS, Langley RG, Webb AN, Walsh N, Haldane D. Cutaneous larva migrans. Lancet. 2011;377:1948. doi: 10.1016/S0140-6736(10)61149-X. [DOI] [PubMed] [Google Scholar]
- 16.Ginsburg B, Beaver PC, Wilson ER, Whitley RJ. Dermatitis due to larvae of a soil nematode, Pelodera strongyloides. Pediatr Dermatol. 1984;2:33–37. doi: 10.1111/j.1525-1470.1984.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 17.Hunter GW, 3rd, Worth CB. Variations in response to filariform larvae of Ancylostoma caninum in the skin of man. J Parasitol. 1945;31:366–372. [PubMed] [Google Scholar]
- 18.Manning L, Chambers S, Paltridge G, Maurice P. Cutaneous larva migrans (hookworm) acquired in Christchurch, New Zealand. N Z Med J. 2006;119:U1910. [PubMed] [Google Scholar]