Abstract
This study was conducted to gain an understanding of the Angiostrongylus cantonensis infection status of rodent definitive host, snail intermediate host, and local residents in Guangzhou, China. A total of 430 rats were captured and 23 rats, from two species, were infected, with an average infection rate of 5.35%. A total of 795 Achatina fulica snails and 734 Pomacea canaliculata snails were collected. The average infection rates of these two species were 13.96% (111 of 795) and 1.50% (11 of 734), respectively. As for the seroprevalence of different occupations, the rate among the “general group” was significantly lower than the “occupational group.” From this survey, Guangzhou is implicated to be the natural focus of Angiostrongylus cantonensis. Rattus norvegicus and Achatina fulica play important roles in spreading this nematode in Guangzhou. Residents who live in Guangzhou, especially those working in certain industries such as agriculture, food-making, and aquaculture, face a higher risk of infection.
Introduction
Angiostrongylus cantonensis is the most important agent of eosinophilic meningitis in humans, which is primarily an epidemic of the tropical and subtropical zones. The life cycle of A. cantonensis involves rats as its definitive host, molluscs as intermediate hosts, and crustaceans (shrimps and crabs), frogs, and fish as paratenic hosts. Humans can be infected by direct contact with or consumption of intermediate or paratenic hosts, and even by drinking water contaminated with infective larvae.1
The first human case of angiostrongyliasis was reported in Taiwan in 1945.2 Since this discovery, more than 2,800 cases have been documented worldwide. The first case in mainland China was reported in Guangzhou in 1984.3 Although few cases were reported between 1984 and 1997, several outbreaks had been reported throughout Beijing, Yunnan, and Guangdong provinces in the past 10 years.4–6 With cases increasing rapidly in recent years, human angiostrongyliasis is becoming a new public health problem in mainland China.
Results from previous epidemiology surveys had shown the two main intermediate hosts (Pomacea canaliculata and Achatina fulica) were widely distributed in South China.7–10 This combined with the existence of positive definitive hosts, has implicated regions of some provinces such as Guangdong, Fujian, Hainan, and Zhejiang as the natural foci of A. cantonensis. Residents living in these areas face the constant risk of infection. A comprehensive illustration of the infection profiles of hosts is crucial for the government to assemble a wise control initiative.
To understand the current epidemical situation of A. cantonensis in Guangzhou and to provide the government with scientific advice in planning its course of action, our research group undertook an investigation on the infection profile of the definitive and intermediate hosts of this nematode and its seroprevalence in residents from 2008 through 2010 in the city of Guangzhou.
Materials and Methods
Introduction of the region investigated.
Guangzhou is the provincial capital of Guangdong province, which is located at the delta of the Pearl River in China.
Methods of sampling.
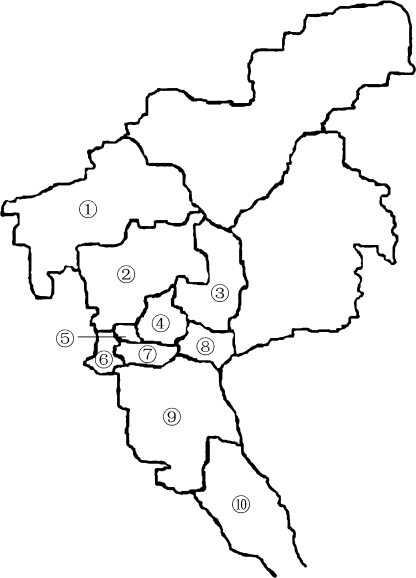
Ten districts of Guangzhou were divided into central and outskirt areas as shown in Figure 1. Rodents were randomly captured from different sites, such as farmland, hen and pigeon houses, near pools, and ditches using mousetraps. Mollusks such as A. fulica, P. canaliculata, and Limax maximus were collected from flower beds, farmlands, pools, and ditches.
Figure 1.
Investigated regions in Guangzhou. The surveyed regions were numbered. Four districts constituted the central area in this survey (④–⑦); other numbered districts constituted the outskirt area.
Residents who had lived in Guangzhou for at least 3 years before the survey were considered to be local residents. A combined 906 blood samples were collected and assayed from both the general adult population, those involved in education, medical and disease prevention, industry, heavy manufacturing, and so on (the “general group”), and those involved in aquaculture or the processing of snails, A. fulica and P. canaliculata snails (the “occupational group”). The participants included 462 men and 444 women ranging in age from 18 to 59 years. The collection of blood samples was approved by the human research review board of Sun Yat-sen University. All participants were informed about the study procedures and gave their written informed consent or, in the case of illiterates, oral approval.
Examination of the hosts of A. cantonensis and seroprevalence of residents.
Each well in a Maxisorp 96-well microtiter plate (JET BIOFIL, Guangzhou Jet Bio-Filtration Products, Guangzhou, China) was coated overnight at 4°C with 10 μg/100 μL of crude somatic L4 antigen in carbonate-bicarbonate buffer. Wells were subsequently blocked for 2 hours at 37°C with 3% bovine serum albumin (BSA) in phosphate buffered saline (PBS) (pH 7.2) containing 0.05% Tween-20 (PBST). Human serum samples were 1:100 diluted and added to each well. To detect antibodies in serum samples, 1000 μL of horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG) secondary antibodies, diluted 1:3,000, was added and incubated for 45 minutes at 37°C. After washing the plates three times with PBST, 50 μL peroxidase substrate (3,3′,5,5′-tetramethylbenzidine) was added to each well. Plates were then incubated at 37°C for 10 minutes and 50 μL H2SO4 (0.2 M) was added to stop the reaction. The plates were read at wavelengths of 450/620 nm using an automated plate reader. The sensitivity and specificity was 100% and 93.7% shown in Table 1.
Table 1.
The sensitivity and specificity of enzyme-linked immunosorbent assay (ELISA)*
| Proven human angiostrongyliasis | Other parasitic diseases | Healthy control | |
|---|---|---|---|
| Positive | 12 | 2 | 0 |
| Negative | 0 | 20 | 10 |
| Total | 12 | 22 | 10 |
The positive reaction with other parasitic diseases was one for Trichinella spiralis and one for Clonorchis sinensis. The number of tests was 5 for Trichinella spiralis, 5 for Clonorchis sinensis, 5 for hookworm, 5 for Echinococcus multilocularis, 5 for Ascaris lumbricoides, and 2 for Schistosoma japonicum
Infection and examination of experimental animals.
The larvae separated from the snails were injected into Sprague-Dawley (SD) rats by gastric perfusion with 50 larvae per rat. The rats were euthanized on the 50th day post-infection, and their hearts and lungs were then dissected for detecting of adult A. cantonensis. The experiment of rats was approved by the animal research review board of Sun Yat-sen University. The maintenance and care of experimental animals complies with the guidelines for the humane use of laboratory animals of China.
Reagents, equipment, and experimental animals.
Pepsin (enzyme activity ≥ 1,200 U/g) was purchased from Ding Guo Chemical Reagent Co., Ltd (Beijing, China); Horseradish peroxidase-conjugated goat anti-human IgG secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The automated plate reader was manufactured by BioRad (Hercules, CA); Electrothermal incubator (DNP-9272) was manufactured by the Jing Hong Experimental Equipment Co., Ltd. (Shanghai, China); Anatomical Lens (X2520) was purchased from Teck Instrument Co., Ltd (Beijing, China). Other experimental materials and appliances were purchased from shops or supermarkets. The SD rats were provided by the experimental animal center of the School of Pharmaceutical Sciences at Sun Yat-sen University.
Data analysis.
All infection rates and intensity were calculated in Microsoft Excel (Microsoft, Redmond, WA) using functions provided by the software. Statistical analysis was done using SPSS (SPSS Inc., Chicago, IL) with proper statistical methods.
Results
Rat species composition.
Four hundred thirty rats of six different species were examined in this survey. Rattus norvegicus (R. norvegicus) comprised ∼50.93% (220 of 432) of the rodents captured, Suncus murinus ∼36.81% (159 of 432), R. flavipectus ∼4.63% (20 of 432), Mus musculus ∼5.56% (24 of 432), R. rattus ∼1.16% (5 of 432), and Rattus sludeni ∼0.93% (4 of 432), respectively.
Infection profile of rats.
Twenty-three rats were infected by A. cantonensis with an average infection rate of 5.35%. A total of 265 A. cantonensis adults were harvested from these rats, the mean intensity of A. cantonensis infection in infected rats was 11.52. Two rat species were found to be infected by A. cantonensis: the infection rate was calculated to be 9.09% (20 of 220) for R. norvegicus and 15.00% (3 of 20) for R. flavipectus, with no significant difference (χ2 = 0.214, P > 0.05).
Infection profiles of Rattus norvegicus.
The infection rate and intensity in each of the two areas of the city are shown in Table 2. The infection rates were not significantly different between the two areas (χ2 = 0.764, P > 0.05), nor were the infection intensities (Wilcoxon W = 56.000; P > 0.05).
Table 2.
Infection profiles of Rattus norvegicus in different areas
| Area | No. | No. positive (%) | Total no. of larval infected | Intensity range of larvae per rat |
|---|---|---|---|---|
| Central | 76 | 6 (6.98) | 37 | 1–20 |
| Outskirt | 144 | 14 (10.45) | 216 | 1–77 |
Our group also investigated whether the size of the rat affects the infection profile. Rats collected were divided into three groups by body weight, namely a large group (≥ 270 g), medium group (181∼269 g), and small group (≤ 180 g). The infection rates of these groups were 19.67% (12 of 61), 6.85% (5 of 73), and 3.49% (3 of 86), respectively, with a significantly different (χ2 = 11.974, P < 0.01). The infection rate of the large group was significantly higher than the medium and small groups (χ2 = 4.933, P < 0.05; χ2 = 10.201, P < 0.01), whereas the intensities of the three groups did not significantly differ (χ2 = 4.760, P > 0.05).
Infection profiles of snails.
Achatina fulica and P. canaliculata were the two predominant species collected in this survey. Seven hundred ninety-five A. fulica snails were examined, and their infection rate was 13.96% (111 of 795); 734 P. canaliculata snails were examined and found to be infected at a rate of 1.50% (11 of 734). There was a significant difference in the infection rates of these two snail species (χ2 = 80.743, P < 0.01). In addition to the two main species, we also collected other species of mollusks such as Discotectonica acutissima and L. maximus. Infected animals of both these species were found, at a rate of 13.6% (8 of 59) for D. acutissima and 7.10% (3 of 42) for L. maximus. Infection rates for A. fulica and P. canaliculata did not differ in the central and outskirt areas of Guangzhou; i.e., 14.29% and 13.65% for A. fulica and 1.62% and 1.32% for P. canaliculata. The infection intensity also was not significantly different between the two areas (Wilcoxon W = 36.000, P > 0.05).
Infection of A. fulica of different sizes.
Snails collected were divided into three groups based on weight. The infection rates among different groups of these snails were significantly different (χ2 = 6.888, P < 0.05), the infection rate in the large group was significantly higher than the small group (χ2 = 6.888, P < 0.05); the intensities of infection among the three different groups were significantly different (χ2 = 9.883, P < 0.01), with the intensity of the large group significantly higher than the small group (Wilcoxon W = 478.000, P < 0.01). A summary of these results are shown in Table 3.
Table 3.
Infection profiles of Achatina fulica by its size
| Group | Weight (g) | No. | No. positive (%) | Total no. of larval infected | Intensity range of larvae |
|---|---|---|---|---|---|
| Small | ≤ 10 | 212 | 20 (9.43) | 6,168 | 13–1710 |
| Medium | 10.5–20 | 330 | 46 (13.94) | 31,800 | 1–4205 |
| Large | > 20 | 257 | 49 (19.07) | 58,655 | 1–8578 |
Examination of experimentally infected rats.
The A. cantonensis adults were harvested from the pulmonary arteries or right heart in all experimentally infected rats. The results indicated that larvae that were separated from the snails were the third-stage larvae of A. cantonensis.
Seroprevalence of angiostrongyliasis in residents.
The seroprevalence was 1.30% (4 of 308) in the central area and 2.51% (15 of 598) in the outskirt areas, with no significant difference (χ2 = 1.449, P > 0.05). In addition, there was no significant difference (χ2 = 2.921, P > 0.05) between genders, as the seroprevalence was 1.30% (6 of 462) for men and 2.93% (13 of 444) for women. The seroprevalence for the “occupational group” was 3.28% (15 of 458), which was significantly higher (χ2 = 6.26, P < 0.05) than that of the “general group” 0.89% (4 of 448). The composition of sex and age in the two groups are list in Tables 4 and 5.
Table 4.
Sex composition of two groups*
| Groups | Male (%) | Female (%) | Total |
|---|---|---|---|
| General | 224 (50.0) | 224 (50.0) | 448 |
| Occupational | 238 (52.0) | 220 (48.0) | 458 |
The difference between two groups was not significant (χ2 = 0.350, P > 0.05).
Table 5.
Number of different age ranges of two groups*
| Groups | 18–27 (%) | 28–37 (%) | 38–47 (%) | Above 48 (%) | Total |
|---|---|---|---|---|---|
| General | 98 (21.9) | 170 (37.9) | 134 (29.9) | 46 (10.3) | 448 |
| Occupational | 105 (22.9) | 170 (37.1) | 140 (30.6) | 43 (9.4) | 458 |
The difference between two groups at 4 years of age range was not significant (χ2 = 0.350; χ2 = 0.066; χ2 = 0.046; χ 2 = 0.198, respectively, P > 0.05).
Discussion
In recent years, surveys from other cities in Guangdong have shown the wide distribution of hosts of A. cantonensis and the existence of positive hosts.7,8,11,12 The infection profile of rats in Guangzhou was reported by a previous survey7; however, there has not been thorough data about the infection profiles of snails and residents in Guangzhou. We conducted a survey, which included seroprevalence and analysis of intermediate and definitive hosts, to ascertain the status of A. cantonensis infection in Guangzhou.
The average infection of rodents was 5.35%, which was similar to the rate found in Yanjiang (5.43%), but lower than that found in other areas of Guangdong province and evaluated in previous studies; i.e., Shenzhen (12%), Maoming (10.47%), Jiangmen (9.82%), and Qingyuan (9.56%).7,8,11,12 Only two species of rats showed signs of infection, i.e., R. norvegicus and R. flavipectus with similar infection rates. However, R. norvegicus was found in a higher number and broader distribution in Guangzhou. Therefore, R. norvegicus can be considered the A. cantonensis definitive host with higher public health impact in this area.
We further investigated the effect, if any, of rat body weight on the infection, or vice versa. Data from this survey show that a greater body weight in R. norvegicus corresponded to both higher larvae intensity and a higher positive rate. This may be related to the differences in tolerance to infection and the opportunity to contact intermediate hosts.
In other surveyed regions of China, A. fulica and P. canaliculata were the major intermediate hosts of A. cantonensis.13,14 The infection rate of A. fulica was lower than Maoming (38.41%), Zhanjiang (31.94%), Jiangmen (30.52%), and Yunfu (18.78%), comparable to Shenzhen (10.3%), Zhaoqing (12.55%), and higher than Yangjiang (7.71%) and Qingyuan (2.00%).7,8,11 On the other hand, the infection rate of P. canaliculata was comparable to Yunfu (2.07%) and Yangjiang (0.86%), and lower than Shenzhen (20.7%), Maoming (20.14%), and Jiangmen (5.14%).7,8,11 Reasons for such a disparity may lie in differences in the natural conditions of each region and the snails' composition, which in turn may affect the snails' tolerance in different places and sample number.
The infection rate of A. fulica was significantly higher than P. canaliculata, which was consistent with the results from other regions.9,10,12 One reason for this discrepancy may be the difference in the habitats of these two snails. Achatina fulica is a land-bound species, whereas P. canaliculata must live near water. Achatina fulica therefore possess a greater chance of contact with the definitive hosts of A. cantonensis under natural conditions. That is, the life cycle of this nematode can be more easily maintained by traveling from rats to A. fulica than to P. canaliculata. Another reason could be related to the natural conditions of Guangzhou. Most P. canaliculata were found in pools rather than in paddy fields, which makes them unlikely to have opportunities for physical contact with the definitive host.
From previous discussions, we conclude that A. fulica plays a more important role than P. canaliculata in spreading A. cantonensis in Guangzhou. However, the recent outbreaks of A. cantonensis were most frequently attributed to consumption of raw or undercooked P. canaliculata in China.4–6,15,16 Thus, local government should also work to control and regulate human contact with this potentially dangerous snail.
Earlier reports mentioned that the infection intensity and infection rate varied by body size in A. fulica.17,18 In this survey, the recorded larvae intensity of infected A. fulica samples was consistent with the previous conclusion. Body weight and infection intensity appear to be positively related. Although the infection rates across different body weights did not match previous data, this may be related to differences in the grading standards of snails in size and positive rate of definitive hosts and so on.
Considering the life cycle of A. cantonensis it is known that humans are infected by being exposed to or by ingesting the intermediate mollusks host. It can be concluded that the residents in closer contact with the intermediate host species are at a greater risk for acquiring the infection. In this survey, the difference in seroprevalence between different occupational groups supports such hypothesis. Residents working in certain industries such as agriculture, food production, and aquaculture are particularly in need of elevated prevention measures.
In conclusion, Guangzhou is the natural focus of A. cantonensis and the local residents are at risk of contacting this pathogen. This investigation will help us preliminarily understand the current epidemic situation of A. cantonensis in Guangzhou and soon lay a foundation for the scientific control of this emerging infectious disease.
ACKNOWLEDGMENTS
We thank Wen Yang for assistance in improving the manuscript.
Footnotes
Financial support: This study was supported by grants from National Basic Research Program of China (973 Program 2010CB530004 entitled Mechanisms of Immunoregulation in Angiostrongylus cantonensis), the National Natural Science Foundation of China, and the Guangdong Provincial People's Government of Joint Natural Science Fund (u0632003).
Authors' addresses: Xiao Yang, Zhenyu Qu, Xiaoying Zheng, Ai He, Yu Wu, Qian Liu, Dongjing Zhang, Zhongdao Wu, Zhuoya Li, and Ximel Zhan, Department of Parasitology, Zhongshan School of Medicine, Sun Yat-sen University, Key Laboratory for Tropical Diseases Control, Ministry of Education, Sun Yat-sen University, Guangzhou, Guangdong Province, P.R. China, E-mails: yangx2004@yahoo.com.cn, 64108580@qq.com, zhengxy@mail.sysu.edu.cn, heai19@sina.com, wuyu@mail.sysu.edu.cn, liuqian_8686@yahoo.com.cn, zhangdongjing06@163.com, wuzhd@mail.sysu.edu.cn, lizhuoya@21cn.com, and zhanximei@yahoo.com.cn. Hualiang He, Department of Plant Protection, Zhongkai University of Agriculture and Engineering, Guangzhou, Guangdong Province, P.R. China, E-mail: hehual@mail2.sysu.edu.cn.
References
- 1.Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- 2.Rosen L, Laigret J, Bories S. Observation on an outbreak of eosinophilic meningitis on Tahiti, French Polynesia. Am J Hyg. 1961;74:26–42. doi: 10.1093/oxfordjournals.aje.a120198. [DOI] [PubMed] [Google Scholar]
- 3.He JZ, Zhu SH, Yang SJ, Yu BW, Chen YS, Hu QX, Wang XB, Wang L. First discover and evidence of Angiostrongylus cantonensis in the cerebrospinal fluid from the case of the population of the mainland China. Acad J Guangzhou Med Coll. 1984;12:1–4. [in Chinese] [Google Scholar]
- 4.Wang J, Qi H, Diao Z, Zheng X, Li X, Ma S, Ji A, Yin C. An outbreak of Angiostrongylus cantonensis in Beijing. J Parasitol. 2010;96:377–381. doi: 10.1645/GE-2214.1. [DOI] [PubMed] [Google Scholar]
- 5.Lv S, Zhang Y, Chen SR, Wang LB, Fang W, Chen F, Jiang JY, Li YL, Du ZW, Zhou XN. Human angiostrongyliasis outbreak in Dali, China. PLoS Negl Trop Dis. 2009;3:e520. doi: 10.1371/journal.pntd.0000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng ZH, Cai JS, Lin RX, Pei FQ, Cui HE, Ou Y, Gao XX, Wang F, Zhou SQ, Xie YM. The first local outbreak of Angiostrongylus cantonensis infection in Guangdong province. S China J Prev Med. 2007;33:17–20. [in Chinese] [Google Scholar]
- 7.Chen D, Zhang Y, Shen H, Wei Y, Huang D, Tan Q, Lan X, Li Q, Chen Z, Li Z, Ou L, Suen H, Ding X, Luo X, Li X, Zhan X. Epidemiological survey of Angiostrongylus cantonensis in the west-central region of Guangdong Province, China. Parasitol Res. 2011;109:305–314. doi: 10.1007/s00436-011-2255-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang RL, Chen MX, Gao ST, Geng YJ, Huang DN, Liu JP, Wu YL, Zhu XQ. Enzootic angiostrongyliasis in Shenzhen, China. Emerg Infect Dis. 2008;14:1955–1956. doi: 10.3201/eid1412.080695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X, Du J, Tong C, Wang S, Liu J, Li Y, He C. Epidemic status of Angiostrongylus cantonensis in Hainan Island, China. Asian Pac J Trop Med. 2011;4:275–277. doi: 10.1016/S1995-7645(11)60085-0. [DOI] [PubMed] [Google Scholar]
- 10.Li FH, Zhou XM, Li YZ, Tao H. Investigation on epidemic focus of Angiostrongylus cantonensis in Yunnan Province, China. J Pathogen Biol. 2008;3:53–56. [in Chinese] [Google Scholar]
- 11.Qu ZY, Yang X, Cheng M, Lin YF, Liu XM, He A, Wu ZD, Zhan XM. Enzootic angiostrongyliasis, Guangdong, China, 2008–2009. Emerg Infect Dis. 2011;17:1335–1336. doi: 10.3201/eid1707.100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang D, Chen DX, Zhang Y, Li XM, Zhan XM. Investigation of the infection of Angiostrongylus cantonen in Yangchun City of Guangdong. J Pathogen Biol. 2008;3:695–696. [Google Scholar]
- 13.Lv S, Zhang Y, Steinmann P, Zhou XN. Emerging angiostrongyliasis in Mainland China. Emerg Infect Dis. 2008;14:161–164. doi: 10.3201/eid1401.061529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang QP, Chen XG, Lun ZR. Invasive freshwater snail, China. Emerg Infect Dis. 2007;13:1119–1120. doi: 10.3201/eid1307.061360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JX, Li YS, Zhu K, Chen BJ, Cheng YZ, Lin JC, Cao Y, Chen RZ. Epidemiological study on group infection of Angiostrongylus cantonensis in Changle City. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Xue Za Zhi. 2003;21:110–112. [in Chinese] [PubMed] [Google Scholar]
- 16.Xue DY, Ruan YZ, Lin BC, Zheng RY, Fang JQ, Zhao QY, Li MF, Pan CW. Epidemiological investigation on an outbreak of Angiostrongylus cantonensis in Wenzhou. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Xue Za Zhi. 2000;18:176–178. [in Chinese] [PubMed] [Google Scholar]
- 17.Liang HK, Sheng HX, Xu BK. Survey on the infection situation of definitive host, intermediate host and transport host of Angiostrongylus cantonensis in Guangzhou City. Zhonghua Liu Xing Bing Xue Za Zhi. 1984;5:245–248. [in Chinese] [PubMed] [Google Scholar]
- 18.Sithithaworn P, Brockelman WY, Brockelman C. Transmission of Angiostrongylus cantonensis through the giant African snail Achatina fulica: an experimental study. Southeast Asian J Trop Med Public Health. 1991;22:S200–S205. [PubMed] [Google Scholar]