Abstract
The causal agent of melioidosis, Burkholderia pseudomallei, has been cultured from paddy fields in the Lao PDR. We carried out a pilot study to examine the relationship between bacterial soil contamination and that of nearby surface waters in Saravane Province. Soil sampling was conducted at a depth of 30 cm (100 holes in a 45 × 45 m grid) at two sites, East and West Saravane. Moore's swabs were used for water sampling of paddy fields, lakes, rivers, boreholes, and storage tanks within 2 km of the two soil sampling sites. B. pseudomallei from soil and water were cultured on Ashdown's agar. Thirty-six percent and 6% of water samples collected around East and West Saravane, respectively, were culture positive for B. pseudomallei. Low pH and high turbidity were independently associated with culture of B. pseudomallei. Most positive water samples were from the Sedone River, downstream of the East Saravane site. Moore's swabs are simple and inexpensive tools for detecting B. pseudomallei in surface waters.
Introduction
Burkholderia pseudomallei, the causative agent of melioidosis, is an environmental bacterium, infecting humans by contact with contaminated soil or water.1,2 Widely distributed in Southeast Asia and northern Australia, this Gram-negative bacillus can cause a diversity of acute, sub-acute, and chronic clinical manifestations, which are frequently unrecognized because of the difficulties of accessible laboratory diagnosis. The treatment is prolonged and expensive, requiring antibiotics such as ceftazidime or carbapenems. Mortality remains high (20–50%) despite early and appropriate antibiotic therapy.2
The geographical distribution of human cases of melioidosis appears to be related to environmental reservoirs of B. pseudomallei.3 Human infections often occur after direct exposure to contaminated soil and/or water with a particularly high incidence in rice farmers. In Ubon Ratchathani Province, northeast Thailand, the estimated average incidence of melioidosis was 12.7 cases per 100,000 people per year4,5 and was the third leading cause of death after human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) and tuberculosis in 2006.5 In the Lao PDR (Laos), most (85%) of 483 patients diagnosed with melioidosis at Mahosot Hospital (until November 2011), since the first recorded patient in 1999,6 have had homes in Vientiane capital and Vientiane Province. Although these are close to the Mekong River, this finding may simply reflect the catchment population of the hospital. The first environmental survey in Laos detected B. pseudomallei in 36% of 110 soil samples collected from rice fields within a 150 km radius of Vientiane capital.7 Recently, it has been demonstrated that B. pseudomallei occurs in Lao soils beyond the immediate vicinity of the Mekong River, with the highest bacterial soil density ever reported occurring in Saravane Province, southern Laos.8 Which factors in soil influence bacterial density remains unclear. Muddy clay-rich soils and stagnant pond water of rice fields are probably important reservoirs.9,10 Factors promoting B. pseudomallei proliferation in soils in the laboratory are moisture content > 10%, pH 5–6, and temperature 37–42°C.11,12 Burkholderia pseudomallei can survive for many years in distilled water without added nutrients.13 Water can transmit the bacterium to humans by direct mucocutaneous inoculation, near drowning, and probably inhalation.14–16 Few studies have investigated the contamination of environmental water by B. pseudomallei.17–20 The Moore's swab method, introduced in 1948,21 uses a gauze swab as a filter to catch and concentrate microorganisms in flowing or stagnant water. This method has been successfully used to isolate Salmonella spp., Vibrio spp., and enteroviruses from sewage,22–24 but has only been used once, unsuccessfully, to detect B. pseudomallei in surface water.17 We therefore used Moore's swabs in a pilot study to examine the distribution of B. pseudomallei in surface water around two sites in southern Laos with high B. pseudomallei soil densities.
Materials and Methods
Soil and water sampling.
Two locations in Saravane province (southern Laos), with the highest B. pseudomallei soil contamination in the 2009 survey8 were selected as reference sites (East Saravane and West Saravane) (Figure 1). To investigate relationships between contamination of soil and nearby water, in July 2010 we collected 100 samples of soil from each site, using the same methods as in 2009, and 100 water samples around each site.8 A GPS (Global Positioning System Garmin GPS Map 60CSx, Southampton, UK) was used to define the location of the SE corner of a grid of 45×45 m in which 100 holes were dug at a distance of 5 m from each other. Soil (100 g) was removed at a depth of 30 cm, and then cultured as previously described.8,25 The same grids as sampled in 2009 were resampled—with an estimated year-to-year hole location deviation of < 5 m.
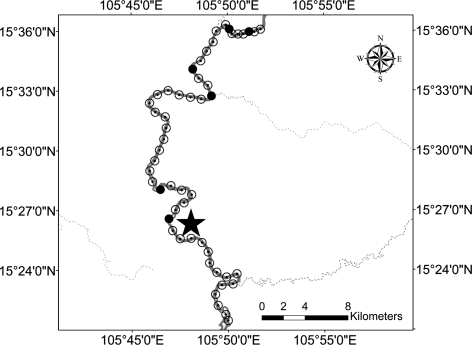
Figure 1.
Location of the reference sites in Saravane Province. Black stars are the soil sampling sites. Closed black circles are the locations where the Sedone River waters were sampled. Water flows to the south.
In July 2010, within a radius of 2 km around each reference site, we collected 50 water samples from lakes or ponds, rice fields, and boreholes or domestic water tanks. One hundred water samples were also collected in the Sedone River, downstream and upstream from each reference site. The Sedone is the main river of Saravane Province flowing into the Mekong River. The East and West Saravane reference sites are 100 and 600 m distant from the Sedone River, respectively, and the two sites are ∼140 km distant from each other along the Sedone River bank. At Saravane East, we placed 33 and 15 swabs downstream and upstream, respectively, from the reference site. At Saravane West, we placed 14 and 38 swabs downstream and upstream, respectively, from the reference site. Intervals between each water sampling point in the river were ∼1 km.
Moore swabs were prepared by cutting cotton gauze, with mean weight (95% confidence interval [CI]) of 38.9 (38.3–39.4) g/m2, into strips of 120×16 cm, folding the strips longitudinally 2–3 times and tying in the center with nylon fishing line. Swabs were autoclaved and kept in a sterile plastic bag until use. At each water sampling site, the swabs were immersed halfway between the surface and water body bottom for 24 hours. Swabs were then inserted in sterile plastic bags and transported to the Vientiane laboratory, within 24 hours, at ambient temperature.
Three physicochemical water parameters, temperature, turbidity, and pH, were recorded at each sampling point, at Moore's swab immersion and again on removal and the mean calculated. Temperature and pH were measured by Hanna Instruments HI98127 (pHep-4) Combo Waterproof pH Meter and Thermometer (Hanna Instruments, Ann Arbor, MI) and turbidity by a Pocket Turbidimeter (Hach, Loveland, CO), expressed in nephelometric turbidity units (NTU). The sampling points were recorded by GPS (above).
Cultures.
Soil samples were cultured using described semi-quantitative procedures.8,25 For the water analysis, the bags containing the Moore's swabs (with a mean volume of water/swab of 73.9 mL (95% IC: 72.2–75.7) mL), were vigorously shaken and 100 mL of aspirated water were directly plated on Ashdown agar. Each Moore's swab was then placed into 150 mL of selective modified Ashdown's broth (containing 10 g/L of tryptone soya broth, 5 mL/L of 0.1% crystal violet, 50 mg/L of colistin, and 40 mL/L of gylcerol). Selective broths were incubated aerobically at 40°C for 7 days and subcultured on Ashdown agar at Days 3 and 7. Burkholderia pseudomallei was identified by colonial morphology, the latex agglutination test, resistance to colistin, and susceptibility to co-amoxiclav as previously described.25
Data analysis.
Intercooled Stata 8.2 (College Station, TX) was used for statistical analysis. Student's t test was used for comparing the means of bacterial soil concentration between 2010 and 2009 at each reference site. The χ2 and Fisher's exact tests were used to compare the isolation rates of B. pseudomallei. Correlations between water pH, temperature, and turbidity were analyzed using the Mann-Whitney test. Logistic regression was performed to calculate the odds ratios (OR) of B. pseudomallei occurrence in water, with adjustment for water pH, temperature, and turbidity. The Spearman's rank coefficient was calculated to test for correlation between these water parameters. A P value ≤ 0.05 was considered significant.
Results
Soil samples.
At East Saravane 76 of 100 soil samples were culture positive for B. pseudomallei and the geometric mean (95% CI) bacterial concentration was 501 (314–797) cfu/g. At West Saravane, 25 of 100 soil samples were positive for B. pseudomallei with geometric mean (95% CI) bacterial concentrations of 147 (48–452) cfu/g. As in 2009, the isolation rate and soil concentrations of B. pseudomallei at East Saravane remained significantly higher than that of West Saravane (P < 0.001). The mean (95% CI) rainfall at Saravane town during the 30 days before sampling in 2010 and 2009 were 5.3 (0.8–9.9) and 9.9 (2.6–17.1) mm/day, respectively.
Water samples.
Of water samples collected around the East Saravane and West Saravane sites, 35 of 98 (36%) and 6 of 102 (6%), respectively, were culture positive for B. pseudomallei—all by direct plating (Table 1). At both sites, river water samples were more frequently contaminated than rice field water samples. Only one sample collected from lakes and ponds and one sample collected from boreholes and domestic tanks were positive (Table 1). Three isolates from lake water samples collected at West Saravane were identified as B. thailandensis based on their colonial morphology, latex test negativity, resistance to colistin, ability to assimilate L-arabinose, and susceptibility to co-amoxiclav.
Table 1.
Number of water samples positive for Burkholderia pseudomallei for different sources
| Water sources | East Saravane | West Saravane | P | ||
|---|---|---|---|---|---|
| n | Positive n (%) | n | Positive n (%) | ||
| Boreholes and domestic water tanks | 3 | 1 (33) | 35 | 0 | 0.07* |
| Lakes and ponds | 10 | 1 (10) | 15 | 0 | 0.4* |
| Rice fields | 37 | 14 (38) | 0 | 0 | |
| Rivers | 48 | 19 (39) | 52 | 6 (11) | 0.003 |
| Total | 98 | 35 (36) | 102 | 6 (6) | |
Fisher's exact test.
Physicochemical water parameters.
Water pH, turbidity, and temperature varied between water types (Table 2). For river water the median (range) temperature was 29.9 (28.5–31.6)°C for positive samples and 30.7 (28.3–33.5)°C for negative samples (P = 0.006). The pH was significantly lower in positive river water samples (median [range] 7.80 [7.49–8.86]) than in negative ones (median [range] 8.47 (7.55–9.03]) (P < 0.001). The turbidity of water samples from rice fields was high in both positive and negative samples (P = 0.12) and these samples were more acidic than water samples from other sources, whether positive or negative for B. pseudomallei (Table 2). For all water samples the isolation of B. pseudomallei was more frequent in waters with low pH (median 7.62 versus 8.16, P < 0.001) and high turbidity (median 324 versus 236 NTU, P = 0.001). However, there was no significant difference in temperature between positive and negative samples (median 30.8 versus 30.9°C, P = 0.3). In multivariate analysis, with adjustment for water pH, temperature, and turbidity, low pH (OR [95% CI] = 0.5 [0.3–0.8], P = 0.03) and high turbidity (OR [95% CI] = 1.0039 [1.0006–1.0072], P = 0.02) were independently associated with the presence of B. pseudomallei. There was no correlation between temperature and the other two water parameters (pH and turbidity) (P > 0.05), however pH was significantly lower at higher turbidity (P < 0.001).
Table 2.
Physicochemical parameters of water samples
| Water sources | Isolation of Burkholderia pseudomallei | P* | |
|---|---|---|---|
| Negative median (range) | Positive median (range) | ||
| Rivers | N = 75 | N = 25 | |
| pH | 8.47 (7.55–9.03) | 7.8 (7.49–8.86) | < 0.001 |
| Temperature (°C) | 30.7 (28.3–33.5) | 29.9 (28.5–31.6) | 0.006 |
| Turbidity† (NTU) | 237 (39.6–> 400) | 280 (101.65–> 400) | 0.12 |
| Rice fields | N = 23 | N = 14 | |
| pH | 6.54 (5.11–9.74) | 6.17 (5.23–6.79) | 0.1 |
| Temperature (°C) | 35.1 (31.1–40.8) | 35.2 (31.8–39.5) | 0.9 |
| Turbidity (NTU) | > 400 (22.15–> 400) | > 400 (301–> 400) | 0.6 |
| Lakes and ponds | N = 24 | N = 1 | |
| pH | 7.19 (6.2–8.8) | 6.59 | 0.3 |
| Temperature (°C) | 31.3 (29.4–39.6) | 33.4 | 0.4 |
| Turbidity (NTU) | > 400 (59.75–> 400) | > 400 | 0.5 |
| Water tanks and boreholes | N = 37 | N = 1 | |
| pH | 8.43 (5.81–9.35) | 5.19 | 0.09 |
| Temperature (°C) | 28.8 (26.6–35.6) | 35.75 | 0.09 |
| Turbidity (NTU) | 5.25 (1.65–> 400) | > 400 | 0.1 |
Mann-Whitney rank sum test.
Limits of turbidimetry: 0.1 to 400 nephelometric turbidity units (NTU).
B. pseudomallei in water in relation to the reference sites.
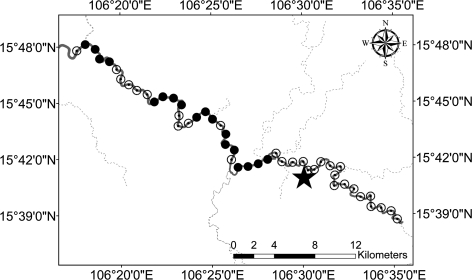
Most B. pseudomallei positive water samples were collected around the East Saravane site where the highest soil concentrations of B. pseudomallei were observed (Table 1). Within a 2 km radius from the reference site, 38% of rice field water samples were positive. In the Sedone River, none of the 15 swabs placed at 1 km intervals upstream (15 km) were positive. Conversely, most swabs immersed downstream from this site, up to 33 km, especially those closest, were positive (Figure 2).
Figure 2.
Sampling points on the Sedone River upstream and downstream from the East Saravane reference site (black star). Open black circles represent points that were negative for Burkholderia pseudomallei and closed black circles represent points that were positive for B. pseudomallei. Water flows to the north west.
At West Saravane, none of the 50 water samples collected within a 2 km radius of the reference site, 15 from lakes or ponds and 35 from storage tanks were positive for B. pseudomallei. In the Sedone River, upstream from this reference site, 6 of 38 swabs were positive up to 33 km away, whereas all the swabs taken up to 15 km downstream were negative (Figure 3). The distance between the reference site of East Saravane and the last positive sampling point for B. pseudomallei downstream on the Sedone River was about 140 km (estimated by using Google Earth Pro software) following the course of the river.
Figure 3.
Sampling points on the Sedone River upstream and downstream from the West Saravane reference site (black star). Open black circles represent points that were negative for Burkholderia pseudomallei and closed black circles represent points that were positive for B. pseudomallei. Water flows to the south.
Discussion
We performed a pilot study to determine the occurrence of B. pseudomallei in surface water by using Moore's swabs and to investigate the relationship between soil and water contamination. As in 2009, the two sites in the Saravane province were still heavily contaminated with B. pseudomallei, with bacterial soil densities higher in East Saravane than in West Saravane. Moore's swabs were capable of detecting B. pseudomallei in environmental water. Of 200 water samples, 41 (21%) were positive for B. pseudomallei, mostly from East Saravane, suggesting that the waters draining highly contaminated soils are also highly contaminated.
This is the first description of the successful use of Moore's swabs to detect B. pseudomallei in surface waters. This method has been widely used to isolate viruses, mycobacteria, Salmonella, and vibrios in environmental waters.24 It was able to detect Salmonella typhi in 50% of samples of sewage draining from the houses of chronic carriers and detected Escherichia coli O157:H7 in rivers in California.26,27 Moore's swabs were tried during outbreaks of melioidosis among pigs in Australia but the pathogen was not isolated.17 The search for B. pseudomallei in environmental waters without a process of concentration is often negative, even in areas where the soil is contaminated.28 Different methods of sampling and concentration have been advocated, including intraperitoneal inoculation in hamsters, centrifugation, and filtration through Millipore membranes followed by agar culture or in enrichment broth.18–20 Compared with these methods, Moore's swabs are simple, convenient, inexpensive, and require less equipment. They are better suited to sampling large volumes of water, acting as a filter that fixes and concentrates the microorganisms on the fibers. It is particularly effective for detecting transient contaminations or low bacterial densities in flowing waters. However, there have been no studies of the comparative sensitivity of these diverse techniques in the detection of B. pseudomallei.
There is very little information as to how soil B. pseudomallei density changes through time. At the two soil sites bacterial densities did not differ significantly over a 12-month interval but the frequency of holes with B. pseudomallei detected was lower in 2010 than in 2009. Of note, in June 2009 the rice paddies were waterlogged while these same rice fields were dry in July 2010. An association between the start of the monsoon and rise in cases of melioidosis has been described, both in Thailand and Australia.29 Whether the rainy season promotes movement of the bacteria to the more superficial layers of the soil or the humidity enhances multiplication of bacteria maintained in a viable non-culturable state in the dry season has not been determined.
Although we only examined two sites, B. pseudomallei were more frequently isolated from water in and flowing through the site with the highest bacterial densities. Most of the Moore's swabs immersed in the Sedone River, which tested positive, were collected downstream from this site. The two soil sites were chosen at random and we may have selected a site with unusually high B. pseudomallei density at East Saravane. More soil and water sampling in the province would be required to determine the extent of soil density heterogeneity and to examine the relationship between B. pseudomallei in water and soil. Burkholderia pseudomallei culture positivity was significantly related to relative water acidity and turbidity. The decomposition of organic matter in surface waters leads to their acidification. Such conditions may provide a selective advantage to B. pseudomallei, which can survive in water with a pH of 3–7.30 Similarly, an Australian study has shown that the contamination of water bores was associated with acidic pH, low salinity, and high iron levels.31
Is the presence of B. pseudomallei in environmental water a potential risk to humans? In Australia, the same pulsotypes were isolated from human cases and from drinking water during investigation of two outbreaks of melioidosis.18,32 In our study, 38 boreholes were sampled: only one of the three water samples collected around the East Saravane site was positive. However, human infection by water ingestion has never been formally documented and percutaneous inoculation and inhalation are considered to be the most common routes. Partial immersion of the body in contaminated water is probably one of the main factors of exposure, whether occupational in paddy fields or accidental during flooding.25,33
Important limitations of this study include that only two sites in one Lao province were investigated, Moore's swabs have not been standardized and the number, type, and size of the pieces of gauze, the binding, and the duration of immersion vary between studies. A major drawback of Moore's swabs is that they do not provide a quantitative assessment of bacterial water density. Burkholderia pseudomallei water and soil density may be underestimated by viable but non-culturable bacteria or by being hidden in a biofilm or in free-living amoebae.34,35 Molecular real-time polymerase chain reaction detection techniques targeting B. pseudomallei in water and soil may overcome these difficulties.36 If B. pseudomallei water density reflects soil density, detecting B. pseudomallei in rivers could potentially locate draining land areas with high bacterial density. Sampling at the mouth of a river may give an index of B. pseudomallei density in the watershed. Water sampling also has the advantage of avoiding the risks of detonating unexploded ordnance, which has very high density in eastern Laos.8
ACKNOWLEDGMENTS
We are grateful to all staff at Microbiology Laboratory, Mahosot Hospital, Vientiane, especially V. Davong, P. Panyanouvong, M. Seephonelee, D. Sengdetka, A. Seupsavith, B. Sibounheuang, V. Sihalath, M. Simmalavong, and M. Vongsouvath, all those who assisted in the field soil sampling, the farmers who granted permission for digging on their land, the Saravane community for access to their water bores and storage tanks, and David Dance for comments on the draft. We are very grateful to the Minister of Health and the Director of the Curative Department for their support.
Footnotes
Financial support: This study was funded by the Wellcome Trust-Mahosot-Oxford Tropical Medicine Research Collaboration and by Institut de la Francophonie pour la Médecine tropicale (IFMT) - Agence Universitaire de la Francophonie (AUF).
Disclosure: Most of this work was submitted by Khamsing Vongphayloth as an MSc thesis (Institut de la Francophonie pour la Médecine Tropicale, Vientiane, Lao PDR).
Authors' addresses: Khamsing Vongphayloth and Yves Buisson, Institut de la Francophonie pour la Médecine Tropicale, Vientiane, Lao PDR, E-mails: khamsing-v@live.com and yves.buisson@auf.org. Sayaphet Rattanavong, Amphonesavanh Sengdouangphachanh, and Phonlavanh Phouminh, Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR, E-mails: sayaphet@tropmedres.ac, amphone@tropmedres.ac, and phonelavanh@tropmedres.ac. Catrin E. Moore, Rattanaphone Phetsouvanh, and Paul N. Newton, Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR, and Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Nuffield Department of Medicine, University of Oxford, UK, E-mails: catrin@tropmedres.ac, rattanaphone@tropmedres.ac, and paul@tropmedres.ac. Vanaporn Wuthiekanun, Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: lek@tropmedres.ac.
Reprint requests: Paul N. Newton, Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR, Tel: (856) 21 242168, E-mail: paul@tropmedres.ac.
References
- 1.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 3.Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008;102((Suppl 1)):S1–S4. doi: 10.1016/S0035-9203(08)70002-6. [DOI] [PubMed] [Google Scholar]
- 4.Vuddhakul V, Tharavichitkul P, Na-ngam N, Jitsurong S, Kunthawa B, Noimay P, Noimay P, Binla A, Thamlikitkul V. Epidemiology of Burkholderia pseudomallei in Thailand. Am J Trop Med Hyg. 1999;60:458–461. doi: 10.4269/ajtmh.1999.60.458. [DOI] [PubMed] [Google Scholar]
- 5.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg. 2010;82:1113–1117. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phetsouvanh R, Phongmany S, Newton PN, Mayxay M, Ramsay A, Wuthiekanun V, White NJ. Melioidosis and Pandora's box in Lao PDR. Clin Infect Dis. 2001;32:653–654. doi: 10.1086/318713. [DOI] [PubMed] [Google Scholar]
- 7.Wuthiekanun V, Mayxay M, Chierakul W, Phetsouvanh R, Cheng AC, White NJ, Day NP, Peacock SJ. Detection of Burkholderia pseudomallei in soil within the Lao People's Democratic Republic. J Clin Microbiol. 2005;43:923–924. doi: 10.1128/JCM.43.2.923-924.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rattanavong S, Wuthiekanun V, Langla S, Amornchai P, Sirisouk J, Phetsouvanh R, Moore CE, Peacock SJ, Buisson Y, Newton PN. Randomized soil survey of the distribution of Burkholderia pseudomallei in rice fields in Laos. Appl Environ Microbiol. 2011;77:532–536. doi: 10.1128/AEM.01822-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suputtamongkol Y, Hall AJ, Dance DA, Chaowagul W, Rajchanuvong A, Smith MD, White NJ. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int J Epidemiol. 1994;23:1082–1090. doi: 10.1093/ije/23.5.1082. [DOI] [PubMed] [Google Scholar]
- 10.Kaestli M, Mayo M, Harrington G, Ward L, Watt F, Hill JV, Cheng AC, Currie BJ. Landscape changes influence the occurrence of the melioidosis bacterium Burkholderia pseudomallei in soil in northern Australia. PLoS Negl Trop Dis. 2009;3:e364. doi: 10.1371/journal.pntd.0000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palasatien S, Lertsirivorakul R, Royros P, Wongratanacheewin S, Sermswan RW. Soil physicochemical properties related to the presence of Burkholderia pseudomallei. Trans R Soc Trop Med Hyg. 2008;102((Suppl 1)):S5–S9. doi: 10.1016/S0035-9203(08)70003-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen YS, Chen SC, Kao CM, Chen YL. Effects of soil pH, temperature and water content on the growth of Burkholderia pseudomallei. Folia Microbiol (Praha) 2003;48:253–256. doi: 10.1007/BF02930965. [DOI] [PubMed] [Google Scholar]
- 13.Pumpuang A, Chantratita N, Wikraipa C, Saiprom N, Day NP, Peacock SJ, Wuthiekanun V. Survival of Burkholderia pseudomallei in distilled water for 16 years. Trans R Soc Trop Med Hyg. 2011;105:598–600. doi: 10.1016/j.trstmh.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng AC, Hanna JN, Norton R, Hills SL, Davis J, Krause VL, Dowse G, Inglis TJ, Currie BJ. Melioidosis in northern Australia, 2001–02. Commun Dis Intell. 2003;27:272–277. doi: 10.33321/cdi.2003.27.52. [DOI] [PubMed] [Google Scholar]
- 15.Limmathurotsakul D, Chaowagul W, Chierakul W, Stepniewska K, Maharjan B, Wuthiekanun V, White NJ, Day NP, Peacock SJ. Risk factors for recurrent melioidosis in northeast Thailand. Clin Infect Dis. 2006;43:979–986. doi: 10.1086/507632. [DOI] [PubMed] [Google Scholar]
- 16.Barnes JL, Ketheesan N. Route of infection in melioidosis. Emerg Infect Dis. 2005;11:638–639. doi: 10.3201/eid1104.041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ketterer PJ, Webster WR, Shield J, Arthur RJ, Blackall PJ, Thomas AD. Melioidosis in intensive piggeries in south eastern Queensland. Aust Vet J. 1986;63:146–149. doi: 10.1111/j.1751-0813.1986.tb02953.x. [DOI] [PubMed] [Google Scholar]
- 18.Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O'Reilly L, Cameron B. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis. 2000;6:56–59. doi: 10.3201/eid0601.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanetti F, De LG, Stampi S. Recovery of Burkholderia pseudomallei and B. cepacia from drinking water. Int J Food Microbiol. 2000;59:67–72. doi: 10.1016/s0168-1605(00)00255-5. [DOI] [PubMed] [Google Scholar]
- 20.Ellison DW, Baker HJ, Mariappan M. Melioidosis in Malaysia. I. A method for isolation of Pseudomonas pseudomallei from soil and surface water. Am J Trop Med Hyg. 1969;18:694–697. [PubMed] [Google Scholar]
- 21.Moore B. The detection of enteric carriers in towns by means of sewage examination. J R Sanit Inst. 1951;71:57–60. doi: 10.1177/146642405107100109. [DOI] [PubMed] [Google Scholar]
- 22.Barrett TJ, Blake PA, Morris GK, Puhr ND, Bradford HB, Wells JG. Use of Moore swabs for isolating Vibrio cholerae from sewage. J Clin Microbiol. 1980;11:385–388. doi: 10.1128/jcm.11.4.385-388.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duff MF. Isolation of ether-resistant enteroviruses from sewage: methodology. Appl Microbiol. 1970;19:120–127. doi: 10.1128/am.19.1.120-127.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly SM, Clark ME, Coleman MB. Demonstration of infectious agents in sewage. Am J Public Health Nations Health. 1955;45:1438–1446. doi: 10.2105/ajph.45.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuthiekanun V, Limmathurotsakul D, Chantratita N, Feil EJ, Day NP, Peacock SJ. Burkholderia pseudomallei is genetically diverse in agricultural land in Northeast Thailand. PLoS Negl Trop Dis. 2009;3:e496. doi: 10.1371/journal.pntd.0000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sears SD, Ferreccio C, Levine MM. Sensitivity of Moore sewer swabs for isolating Salmonella typhi. Appl Environ Microbiol. 1986;51:425–426. doi: 10.1128/aem.51.2.425-426.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooley M, Carychao D, Crawford-Miksza L, Jay MT, Myers C, Rose C, Keys C, Farrar J, Mandrell RE. Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS ONE. 2007;2:e1159. doi: 10.1371/journal.pone.0001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma G, Zheng D, Cai Q, Yuan Z. Prevalence of Burkholderia pseudomallei in Guangxi, China. Epidemiol Infect. 2010;138:37–39. doi: 10.1017/S0950268809990264. [DOI] [PubMed] [Google Scholar]
- 29.Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis. 2003;9:1538–1542. doi: 10.3201/eid0912.020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson J, Levy A, Sagripanti JL, Inglis TJ. The survival of Burkholderia pseudomallei in liquid media. Am J Trop Med Hyg. 2010;82:88–94. doi: 10.4269/ajtmh.2010.09-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Draper AD, Mayo M, Harrington G, Karp D, Yinfoo D, Ward L, Haslem A, Currie BJ, Kaestli M. Association of the melioidosis agent Burkholderia pseudomallei with water parameters in rural water supplies in northern Australia. Appl Environ Microbiol. 2010;76:5305–5307. doi: 10.1128/AEM.00287-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Currie BJ, Mayo M, Anstey NM, Donohoe P, Haase A, Kemp DJ. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am J Trop Med Hyg. 2001;65:177–179. doi: 10.4269/ajtmh.2001.65.177. [DOI] [PubMed] [Google Scholar]
- 33.Su HP, Yang HW, Chen YL, Ferng TL, Chou YL, Chung TC, Chen CH, Chiang CS, Kuan MM, Lin HH, Chen YS. Prevalence of melioidosis in the Er-Ren River Basin, Taiwan: implications for transmission. J Clin Microbiol. 2007;45:2599–2603. doi: 10.1128/JCM.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawasdidoln C, Taweechaisupapong S, Sermswan RW, Tattawasart U, Tungpradabkul S, Wongratanacheewin S. Growing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistance. PLoS ONE. 2010;5:e9196. doi: 10.1371/journal.pone.0009196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inglis TJ, Rigby P, Robertson TA, Dutton NS, Henderson M, Chang BJ. Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect Immun. 2000;68:1681–1686. doi: 10.1128/iai.68.3.1681-1686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaestli M, Mayo M, Harrington G, Watt F, Hill J, Gal D, Currie BJ. Sensitive and specific molecular detection of Burkholderia pseudomallei, the causative agent of melioidosis, in the soil of tropical northern Australia. Appl Environ Microbiol. 2007;73:6891–6897. doi: 10.1128/AEM.01038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]