Abstract
West Nile virus (WNV) is the leading cause of mosquito-borne disease in the United States; however, risk factors for infection are poorly defined. We performed a case-control study to identify modifiable risk factors for WNV infection. Case-patients (N = 49) had laboratory evidence of recent WNV infection, whereas control-subjects (N = 74) had negative WNV serology. We interviewed participants, surveyed households, and assessed environmental data. WNV infection was associated with living in or near Water District X within Gilbert Township (adjusted odds ratio [aOR] 5.2; 95% confidence interval [95% CI] = 1.5–18.1), having water-holding containers in their yard (aOR 5.0; 95% CI = 1.5–17.3), and not working or attending school outside the home (aOR 2.4; 95% CI = 1.1–5.5). During this outbreak, WNV infection was likely primarily acquired peri-domestically with increased risk associated with potential mosquito larval habitats around the home and neighborhood.
Introduction
West Nile virus (WNV) is an arthropod-borne flavivirus maintained in nature in a bird-mosquito-bird cycle and transmitted to humans primarily through the bites of infective Culex mosquitoes. Since its introduction into the United States in 1999, WNV has become the leading cause of neuroinvasive arboviral disease and is responsible for focal seasonal outbreaks.1–3 Approximately 80% of human WNV infections are asymptomatic.4 Most symptomatic persons develop an acute undifferentiated febrile illness5–7; < 1% of infected persons develop WNV neuroinvasive disease (e.g., meningitis, encephalitis, or acute flaccid paralysis), which has a case fatality rate of ∼10%.1,4
Candidate WNV vaccines are being evaluated but none are licensed for use in humans. In the absence of a vaccine, prevention of WNV disease depends on community- and household-level mosquito control programs to reduce vector densities, personal protective behaviors to decrease exposure to mosquitoes, and screening of blood donors. Recommended personal protective behaviors include using mosquito repellents, wearing protective clothing (long-sleeved shirts and long pants), and limiting outdoor exposure when mosquitoes are most active. Applying insecticides, using air-conditioning, installing window and door screens, and reducing peri-domestic mosquito larval habitats are other public health actions or messages used to decrease the risk for WNV exposure.1
Despite annual focal outbreaks, modifiable risk factors for WNV infection are poorly defined. Only community-level mosquito control, avoiding outdoor exposure, and using insect repellent have been associated with reduced WNV disease or infection risk in the United States.4,8,9 Although several ecologic studies and geospatial models have been used to identify environmental risk factors for WNV disease,8,10–14 few controlled studies have been performed to look at risk factors for WNV infection.4,9,15,16 We performed a population-based, case-control study to identify modifiable environmental and behavioral risk factors for WNV infection during an outbreak in the East Valley of metropolitan Phoenix.
Materials and Methods
Setting.
In late spring and early Summer 2010, there was an outbreak of WNV noted in the East Valley of metropolitan Phoenix, as evidence by trapping of an unusual number of WNV-infected Culex quinquefasciatus mosquitoes and high numbers of human WNV disease cases reported to public health officials.17,18 The 2010 WNV activity in this area was unlike transmission patterns seen from 2005 to 2009, where the peak number of WNV cases were reported in late August to September.17 The last focal outbreak of WNV disease in the East Valley of metropolitan Phoenix occurred in 2004.19
Case definitions.
We defined a case-patient as an East Valley resident with laboratory-confirmed WNV infection diagnosed on a sample collected from May 25 to July 31, 2010. For the purposes of this investigation, the East Valley of metropolitan Phoenix was defined as the cities and towns of Apache Junction, Chandler, Gilbert, Mesa, Queen Creek, Tempe, Phoenix (zip code 85044 only), San Tan Valley (excluding zip code 85132), and other unincorporated areas occurring within the geographic boundaries of these towns and cities. Laboratory confirmation of WNV infection required detection of anti-WNV immunoglobulin (Ig) M antibodies or WNV ribonucleic acid (RNA) in serum or cerebrospinal fluid (CSF). Case-patients included symptomatic persons with neuroinvasive (N = 28) and non-neuroinvasive (N = 17) WNV disease, and asymptomatic viremic blood donors (N = 4). Control-subjects were East Valley residents with a negative result for anti-WNV IgM and IgG on CSF collected ≥ 4 days after symptom onset or serum collected ≥ 7 days after symptom onset. These criteria were used to prevent persons with either recent or previous WNV infections from being enrolled as control-subjects.20
Identification and enrollment of study participants.
Case-patients were identified through reports to the state or county health department of WNV disease cases or viremic blood donors. In addition, case-patients and control-subjects were identified through active surveillance for WNV testing performed at laboratories servicing the East Valley. Persons who tested negative for anti-WNV IgM and IgG on specimens collected too early in the illness to exclude WNV infection (i.e., CSF collected < 4 days after symptom onset or serum collected < 7 days after symptom onset) were offered a repeat blood draw and testing for anti-WNV IgM and were classified according to that result; persons who declined further testing were classified as having an indeterminate WNV test result and were excluded from the study. This investigation was determined to be part of the emergency public health practice response to the outbreak and did not require human subjects' review.
Data collection and definition of variables.
Variables previously identified as possible risk factors for WNV or St. Louis encephalitis virus infection or potentially associated with exposure to larval habitats were collected from a number of sources (Table 1). After obtaining verbal consent, a standardized telephone questionnaire was administered from July 29–August 18, 2010. Information collected included demographics and personal protective behaviors. Permission was then sought to conduct a household visit, during which an entomologist and an epidemiologist inspected the outside of the house, the yard, and the neighborhood within 100 m of the property for mosquito larval habitats and other risk factors that increase exposure to mosquitoes. To explore additional environmental features, we retrieved information from county databases, irrigation maps, and geographic information system (GIS) maps (Environmental Systems Research Institute [ESRI], Redlands, CA). To account for the estimated flight distance of Culex tarsalis and Cx. quinquefasciatus in urban habitats, these environmental features were evaluated within 500 m of each participant's home.21 Finally, GIS-based data were used for 2009 estimates of population density and proportion of vacant households by census block group.
Table 1.
Description of data sources and data collected
| Source | Data collected |
|---|---|
| Telephone interview | • Demographics • Behaviors that might affect exposure to mosquitoes such as outdoor activities and working or attending school outside the home |
| Household visit | • Household mosquito prevention measures such as door and window screens • Potential mosquito larval habitats in the yard such as water-holding containers* • Potential mosquito larval habitats ≤ 100 m from residence such as flood irrigation and catch basins† |
| County databases | • Year of house construction • Neglected pools that are capable of serving as mosquito larval habitats located ≤ 500 m from residence as reported to county environmental services, May–June, 2010 |
| Irrigation maps | • Irrigation service areas (water districts) and open irrigation canals ≤ 500 m from residence |
| Geographic Information System (GIS) | • Green space ≤ 500 m from residence such as parks, golf courses, and agricultural land • 2009 demographic information within census block groups such as population density and percent of houses that were vacant |
Includes common yard containers and discarded artificial containers such as pots, inflatable pools, and tires.
Catch basins are defined as man-made depressions in the ground where storm water from roadways and/or residential areas is directed. Large fences or other barriers occasionally limited the visibility of all areas within 100 m of a residence.
Data analysis.
Data were analyzed using SAS statistical software version 9.2 (SAS Institute, Cary, NC) and mapped with ArcMap 10.0 (ESRI). A P < 0.05 was considered significant. Univariate analysis was performed on collected variables using χ2 or Fisher's exact tests. Persons with missing data were excluded from the univariate analysis. Missing data was most often associated with participants who did not consent to a home visit or deceased or debilitated case-patients for whom surrogates were not able to provide the specific information. For multivariable analysis, such data were assumed to be missing at random and were imputed by modeling them as a function of the observed data for complete cases.22 Five data sets with imputed data were created using SAS PROC MI and then were analyzed using SAS PROC MIANALYZE. Variables with a P ≥ 0.1 on univariate analysis were entered into the logistic model in a stepwise manner. A variable must have had a score χ2 statistic with P < 0.05 to be retained in the model. Variables with a Wald P > 0.05 were removed from the model.
Results
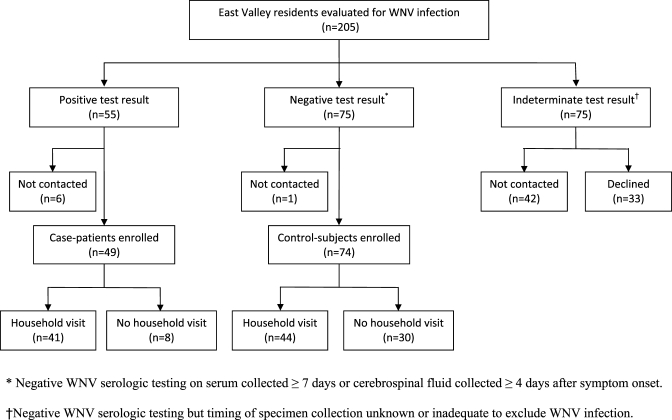
We identified 205 East Valley residents who were evaluated for WNV infection using specimens collected between May 25 and July 31, 2010. Of these, 55 (27%) tested positive for WNV infection and 150 (73%) tested negative. However, only 75 of the persons testing negative had enough information to exclude recent WNV infection (Figure 1). Forty-nine case-patients and 74 control-subjects were enrolled and completed the questionnaire; of these, 41 case-patients and 44 control-subjects agreed to the household assessment.
Figure 1.
Enrollment and household visits of residents of the East Valley of metropolitan Phoenix, Arizona evaluated for West Nile virus (WNV) infection on specimens collected May 25–July 31, 2010.
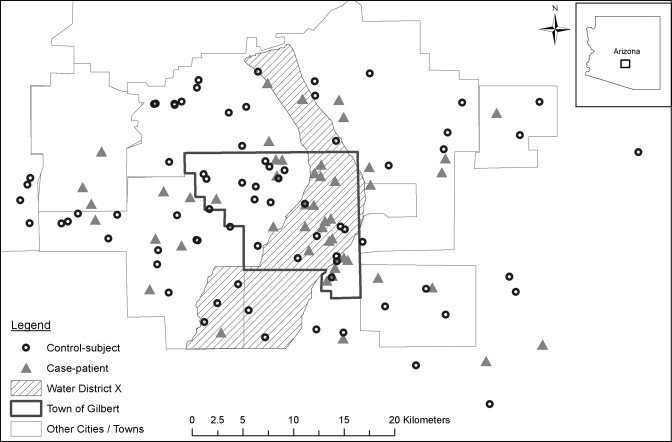
The median age of the 49 case-patients was 50 years (range: 15–80 years) and of the 74 control-subjects was 44 years (range: 4–89 years). Case-patients were significantly more likely than control-subjects to be ≥ 60 years of age (odds ratio [OR] 2.5; 95% confidence interval [95% CI] = 1.0–6.1) and to live in the Town of Gilbert (OR 2.4; 95% CI = 1.1–5.2) (Table 2 and Figure 2).
Table 2.
Univariate analysis of demographics of West Nile virus case-patients and control-subjects—East Valley of metropolitan Phoenix, Arizona, May 25–July 31, 2010
| Characteristics | Case-patients (N = 49) | Control-subjects (N = 74) | Univariate analysis | |||
|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | P value* | ||
| Sex | 0.08 | |||||
| Male | 27 | (55) | 29 | (39) | ||
| Female | 22 | (45) | 45 | (61) | ||
| Age group | 0.04† | |||||
| < 20 years | 3 | (6) | 3 | (4) | ||
| 20–39 years | 10 | (20) | 26 | (35) | ||
| 40–59 years | 21 | (43) | 34 | (46) | ||
| ≥ 60 years | 15 | (31) | 11 | (15) | ||
| Lives in Town of Gilbert | 0.02 | |||||
| Yes | 24 | (49) | 21 | (28) | ||
| No | 25 | (51) | 53 | (72) | ||
| Race | –‡ | |||||
| White | 35 | (71) | 60 | (81) | ||
| Other | 4 | (8) | 12 | (16) | ||
| Unknown | 10 | (20) | 2 | (3) | ||
| Ethnicity | – | |||||
| Non-Hispanic | 31 | (63) | 65 | (88) | ||
| Hispanic | 6 | (12) | 7 | (9) | ||
| Unknown | 12 | (24) | 2 | (3) | ||
| Education | – | |||||
| > High school | 29 | (59) | 56 | (76) | ||
| High school or less | 15 | (31) | 17 | (23) | ||
| Unknown | 5 | (10) | 1 | (1) | ||
| Household income | – | |||||
| < $50,000/year | 16 | (33) | 22 | (30) | ||
| ≥ $50,000/year | 24 | (49) | 38 | (51) | ||
| Unknown | 9 | (18) | 14 | (19) | ||
Persons with an “unknown” result excluded from univariate analysis.
Age ≥ 60 years compared with age < 60 years for univariate analysis.
P value > 0.1.
Figure 2.
Map of West Nile virus case-patients and control-subjects by place of residence—East Valley of metropolitan Phoenix, Arizona, May 25–July 31, 2010. Based on the estimated population density, 150 m buffer were created around each residence and then a point was randomly chosen within the buffer to protect individual privacy.
The only significant behavioral risk factor for WNV infection was not working or attending school outside the home (OR 2.6; 95% CI = 1.2–5.4) (Table 3). The majority of both case-patients and control-subjects did not adhere to WNV personal protection behaviors; for example, most reported never wearing long sleeves and pants when outdoors (78% case-patients and 64% control-subjects) and never wearing insect repellent when outdoors (86% and 82%). Conversely, some potentially protective behaviors were common in both groups, such as air-conditioner use (85% and 91%) and keeping windows closed (73% and 80%).
Table 3.
Univariate analysis of behavioral and environmental characteristics for West Nile virus case-patients and control-subjects—East Valley of metropolitan Phoenix, Arizona, May 25–July 31, 2010
| Characteristics | Case-patients | Control-subjects | Univariate analysis | ||
|---|---|---|---|---|---|
| n/N | (%) | n/N | (%) | P value | |
| Behavioral characteristics | |||||
| Does not work or attend school outside the home | 33/49 | (67) | 33/74 | (45) | 0.01 |
| Never wears long sleeves and pants when outdoors | 38/49 | (78) | 47/74 | (64) | 0.09 |
| Never uses insect repellent when outdoors | 42/49 | (86) | 61/74 | (82) | –* |
| Spends ≥ 1 hour/day outdoors | 31/46 | (67) | 42/73 | (58) | – |
| Always uses air-conditioner | 41/48 | (85) | 67/74 | (91) | – |
| Keeps windows closed | 36/49 | (73) | 59/74 | (80) | – |
| House and yard characteristics | |||||
| Water-holding containers in yard | 38/41 | (93) | 31/44 | (70) | < 0.01 |
| House constructed before year 2000 | 25/49 | (51) | 49/74 | (66) | 0.09 |
| Pool at residence | 27/49 | (55) | 30/74 | (41) | – |
| Deck or unscreened porch | 39/41 | (95) | 40/44 | (91) | – |
| Unscreened doors or windows | 32/41 | (78) | 35/44 | (80) | – |
| Irrigation and other environmental characteristics | |||||
| Lives within 500 m of Water District X | 16/49 | (33) | 12/74 | (16) | 0.03 |
| Neglected pools within 500 m of residence reported to county | 22/49 | (45) | 20/74 | (27) | 0.04 |
| Population density ≥ 1,000/sq. miles within census block group | 46/49 | (94) | 62/74 | (84) | 0.08 |
| ≥ 25% of houses vacant within census block group | 8/49 | (16) | 5/74 | (7) | 0.10 |
| Green area ≥ 10% of total area within 500 m of residence | 22/49 | (45) | 29/74 | (39) | – |
| Catch basins within 500 m of residence | 18/31 | (58) | 25/38 | (66) | – |
| Open irrigation canals within 500 m of residence | 21/47 | (45) | 38/67 | (57) | – |
| Flood irrigation observed within 100 m of residence | 10/41 | (24) | 8/44 | (18) | – |
P value > 0.10.
n = number affected; N = total number.
For environmental risk factors, case-patients were significantly more likely than control-subjects to have water-holding containers in their yard (OR 5.3; 95% CI = 1.4–20.3), live in or near (within 500 m) of Water District X (OR 2.5; 95% CI = 1.1–5.9), and have a neglected pool reported within 500 m of their home (OR 2.2; 95% CI = 1.0–4.7) (Table 3 and Figure 2). Because of interaction between the variables “lives in the Town of Gilbert” and “lives in or near Water District X”, these two variables were combined for the multivariable analysis to “lives in or near Water District X within Gilbert.” The majority of study participants lived in houses that were constructed > 10 years ago (51% case-patients and 66% control-subjects) and in settings with a population density ≥ 1,000 persons/square mile (94% and 84%).
Logistic regression analysis identified three independent risk factors for WNV infection: living in or near Water District X within Gilbert (adjusted OR [aOR] 5.2; 95% CI = 1.5–18.1), having water-holding containers in the yard (aOR 5.0; 95% CI = 1.5–17.3), and not working or attending school outside the home (aOR 2.4; 95% CI = 1.1–5.5) (Table 4).
Table 4.
Multivariable analysis of predictors of West Nile virus infection—East Valley of metropolitan Phoenix, Arizona, May 25–July 31, 2010
| Risk factor | OR* | [95% CI] | aOR | [95% CI] |
|---|---|---|---|---|
| Lives in or near Water District X within Gilbert | 5.0 | [1.6–15.1] | 5.2 | [1.5–18.1] |
| Water-holding containers in yard | 4.0 | [1.5–11.1] | 5.0 | [1.5–17.3] |
| Does not work or attend school outside the home | 2.6 | [1.2–5.4] | 2.4 | [1.1–5.5] |
Crude OR for water-holding containers in yard based on 5 complete datasets with imputed data; thus it differs slightly than the ORs that would be generated from the data presented in Table 3 where only observed data are presented.
OR = odds ratio; aOR = adjusted odds ratio; CI = confidence interval.
Discussion
Our case-control study identified several unique risk factors for WNV infection, which suggest that exposures around the home and in the surrounding neighborhood played an important role in this outbreak. Water-holding containers can act as mosquito larval habitats and are a known risk factor for other mosquito-borne diseases such as dengue.23–25 A number of man-made containers, such as combined sewer overflow canals and road-side catch basins, have been associated with Culex spp. breeding and are frequently targeted by WNV vector control programs.26,27 However, to our knowledge, this is the first time common yard or discarded artificial containers have been identified as a risk for WNV infection during an outbreak. This finding might have been influenced by the arid nature of the study area, making artificial water sources more important as potential larval habitats for Culex spp. mosquitoes. Culex quinquefasciatus commonly breed around human homes and in containers with high organic content water, whereas Cx. tarsalis is usually associated with irrigation runoffs (clean water) in agricultural fields away from human settlements.28,29 Therefore, the observed association between peridomestic water-holding containers and WNV infection and the high predominance of WNV-infected Cx. quinquefasciatus in the study area suggest that during this outbreak Cx. quinquefasciatus was primarily responsible for transmitting WNV to persons close to their home.18
The incidence of WNV disease has previously been associated with proximity to irrigated agriculture.11 We did not identify proximity to open irrigation canals or flood irrigation as risk factors but did identify residence in the area common to Water District X and the Town of Gilbert as a risk factor for WNV infection. This might reflect irrigation infrastructure or irrigation practices that are specific to this area and impact mosquito breeding.
The association between not working or attending school outside the home and WNV infection further supports the premise that WNV was likely acquired peri-domestically during this outbreak. However, those who stayed at home tended to be older and increased age is a known risk factor for WNV neuroinvasive disease, which affected > 50% of case-patients,1 raising the possibility that not working or attending school outside the home was a proxy for increased age. Despite this, not working or attending school outside the home remained a risk factor across all ages. Further study is warranted to examine whether staying at home increases the risk of WNV infection through peri-domestic mosquito exposure or if it is a marker for age or underlying medical conditions that predispose the household resident to symptomatic or severe WNV disease.
No modifiable behavioral characteristics were associated with increased risk of WNV infection in our study. The possible role of personal protective behaviors in preventing WNV infection has been examined in a limited number of serosurveys that were conducted soon after WNV was first detected in North America.4,9,16 Avoiding time outdoors when mosquitoes are most active, specifically dusk to dawn, has been identified in three studies as being protective against WNV infection.4,9,16 Use of insect repellent has been associated with a lower WNV seroprevalence among persons who spent at least 2 hours outdoors from dusk to dawn.4 Finally, a serosurvey from Canada reported persons were at lower risk for WNV infection if they used at least two of the following personal protective behaviors: mosquito avoidance, insect repellent, and protective clothing in the form of long sleeves and long pants when outdoors.16 Human infections with St. Louis encephalitis virus, a closely related flavivirus also transmitted by Culex spp. mosquitoes, also have been associated with time spent outdoors and not wearing long sleeves and long pants.30,31 One possible reason our study did not identify modifiable behavioral risk factors might be that few study participants actually adhered to recommended personal protective behaviors. For example, > 80% of case-patients and control-subjects reported never using insect repellent. Possible reasons for poor adherence to personal protective measures during this outbreak include: a lack of “nuisance” mosquitoes (e.g., mosquitoes that aggressively bite humans but do not transmit WNV), such as Psorophora columbiae or Aedes vexans, resulting in a decreased likelihood of using insect repellant; extreme heat making it impractical to wear long sleeves or pants when outside; and a belief that WNV no longer poses a threat.
Absence of air-conditioners and window screens has been associated with increased risk of human St. Louis encephalitis virus infection, raising the possibility that these could also be risk factors for WNV infection.31,32 However, air-conditioner use and presence of door and window screens were common among both case-patients and control-subjects in our study and did not protect against WNV infection. A finding similar to ours was obtained during a WNV outbreak in New York in 1999.4 In contrast, during a WNV outbreak in Romania, mosquitoes in the home and flooded basements in apartment buildings were associated with infection risk; this study did not address the presence of air-conditioners or screens.15 Overall, this suggests that differences in living conditions and settings might influence risk factors for WNV infection and that public health messages and interventions need to be tailored for different settings.
Risk of WNV disease has previously been associated with the presence of neglected pools12,14 and we detected this association on univariate but not multivariable analysis. However, as only a fraction of neglected pools are reported to the county, we likely underestimated the true density and potential risk of neglected pools in the study area. In attempts to improve neglected pool capture, we obtained aerial photographs taken shortly after the study period but found the resolution of these images to be inadequate to identify neglected pools.
Our study has certain limitations. Case ascertainment relied primarily on WNV disease cases reported to public health authorities that resulted in most case-patients having severe WNV disease. This likely affected the representativeness of case-patients compared with all WNV-infected persons and might explain why case-patients were older than control-subjects. Incomplete enrollment also might have affected the representativeness of study participants, particularly control-subjects. Because of concerns about the representativeness of study participants, we elected not to calculate population attributable risk for independent risk factors identified in this study. Suboptimal data sources might have limited our ability to detect associations and imputation of missing data reduced the precision of the estimates. Finally, particular factors that were unique to the study area, such as the climate, irrigation methods, and mosquito species responsible for transmitting WNV, might prevent generalizing these results to other areas.
Our results indicate that WNV infection in this outbreak was likely acquired peri-domestically and the risk of infection was increased by the presence of potential mosquito larval habitats around the home and in the neighborhood. Public health messages during an outbreak should therefore target reduction of potential mosquito larval habitats, in particular elimination of water-holding containers from the yard. Environmental factors unique to Water District X within the Town of Gilbert need further evaluation to define interventions to reduce residents' WNV infection risk. The infrequent use of many personal protective behaviors aimed at reducing mosquito exposure was striking. Ways to improve compliance with personal protective behaviors should be investigated and the impact of these measures on prevention of infection with WNV and other mosquito-borne pathogens should be further evaluated.
ACKNOWLEDGMENTS
We thank Jamie Feld, Marvin Godsey, Jessica Mack, Tricia Wadleigh, and Naomi Wheeler for assistance with data collection and data entry; Peggy Collins for assistance with database management; Janeen Laven and Amanda Panella for performing serologic testing; Rebecca Eisen for assistance with analysis of environmental data; and Dan Damien, Eric Feldman, Andrea Julius, Tamra Schuler, Kirk Smith, Don Thomas, and John Townsend for providing mosquito and environmental data used in this study.
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Katherine B. Gibney, Department of Epidemiology and Preventive Medicine, Monash University, The Alfred Centre, Melbourne, Australia, E-mail: Katherine.Gibney@monash.edu. James Colborn, Division Of Parasitic Diseases And Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: fue6@cdc.gov. Steven Baty, U.S. Army, Public Health Command and Region-Europe, Landstuhl, Germany, E-mail: steven.baty@us.army.mil. Andrean M. Bunko Patterson, Tammy Sylvester, and Craig Levy, Maricopa County Department of Public Health, Phoenix, AZ, E-mails: AndreanBunkoPatterson@mail.maricopa.gov, tammysylvester@mail.maricopa.gov, and CraigLevy@mail.maricopa.gov. Graham Briggs, Pinal County Division of Public Health, Florence, AZ, E-mail: grahambriggs@pinalcountyaz.gov. Tasha Stewart, Tempe Police Department, Tempe, AZ, E-mail: stewarttasha@gmail.com. Ken Komatsu, Arizona Department of Health Services, Phoenix, AZ, E-mail: Ken.Komatsu@azdhs.gov. Katherine MacMillan, Mark J. Delorey, John-Paul Mutebi, Marc Fischer, and J. Erin Staples, Division of Vector-borne Disease, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: iky4@cdc.gov, esy7@cdc.gov, grv0@cdc.gov, mxf2@cdc.gov, and auv1@cdc.gov.
References
- 1.Centers for Disease Control and Prevention Surveillance for West Nile virus disease—United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17. [PubMed] [Google Scholar]
- 2.Reimann C, Hayes E, DiGuiseppi C, Hoffman R, Lehman J, Lindsey N, Campbell G, Fischer M. Epidemiology of neuroinvasive arboviral disease in the United States, 1999–2007. Am J Trop Med Hyg. 2008;79:974–979. [PubMed] [Google Scholar]
- 3.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 4.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 5.Campbell G, Marfin A, Lanciotti R, Gubler D. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 6.Hayes E, Sejvar J, Zaki S, Lanciotti R, Bode A, Campbell G. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson J, Pertel P, Jones R, Siston A, Paul W, Austin C, Gerber S. Clinical characteristics and functional outcomes of West Nile fever. Ann Intern Med. 2004;141:360–365. doi: 10.7326/0003-4819-141-5-200409070-00010. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz M, Tedesco C, McTighe T, Austin C, Kitron U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr. 2004;3:8. doi: 10.1186/1476-072X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaBeaud A, Kile J, Kippes C, King C, Mandalakas A. Exposure to West Nile virus during the 2002 epidemic in Cuyahoga County, Ohio: a comparison of pediatric and adult behaviors. Public Health Rep. 2007;122:356–361. doi: 10.1177/003335490712200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownstein J, Rosen H, Purdy D, Miller J, Merlino M, Mostashari F, Fish D. Spatial analysis of West Nile virus: rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne Zoonotic Dis. 2002;2:157–164. doi: 10.1089/15303660260613729. [DOI] [PubMed] [Google Scholar]
- 11.Eisen L, Barker C, Moore C, Pape WJ, Winters A, Cheronis N. Irrigated agriculture is an important risk factor for West Nile virus disease in the hyperendemic Larimer-Boulder-Weld area of north central Colorado. J Med Entomol. 2010;47:939–951. doi: 10.1603/me10036. [DOI] [PubMed] [Google Scholar]
- 12.Harrigan R, Thomassen H, Buermann W, Cummings R, Kahn M, Smith T. Economic conditions predict prevalence of West Nile virus. PLoS ONE. 2010;5:e15437. doi: 10.1371/journal.pone.0015437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaBeaud AD, Gorman A-M, Koonce J, Kippes C, McLeod J, Lynch J, Gallagher T, King C, Mandalakas A. Rapid GIS-based profiling of West Nile virus transmission: defining environmental factors associated with an urban-suburban outbreak in northeast Ohio, USA. Geospat Health. 2008;2:215–225. doi: 10.4081/gh.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisen W, Takahashi R, Carroll B, Quiring R. Delinquent mortgages, neglected swimming pools, and West Nile virus, California. Emerg Infect Dis. 2008;14:1747–1749. doi: 10.3201/eid1411.080719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han LL, Popovici F, Alexander JP, Laurentia V, Tengelsen LA, Cernescu C, Gary HE, Ion-Nedelcu N, Campbell GL, Tsai TF. Risk factors for West Nile virus infection and meningoencephalitis, Romania, 1996. J Infect Dis. 1999;179:230–233. doi: 10.1086/314566. [DOI] [PubMed] [Google Scholar]
- 16.Loeb M, Elliott S, Gibson B, Fearon M, Nosal R, Drebot M, D'Cuhna C, Harrington D, Smith S, George P, Eyles J. Protective behavior and West Nile virus risk. Emerg Infect Dis. 2005;11:1433–1436. doi: 10.3201/eid1109.041184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maricopa County Department of Public Health West Nile Virus in Maricopa County: January 1, 2010–December 31, 2010. 2010. http://www.maricopa.gov/publichealth/Services/EPI/pdf/wnv/10/WNVEOY2010.pdf Available at. Accessed November 10, 2011.
- 18.Arizona Department of Health Services Arizona 2010 West Nile Virus Statistics. 2010. http://www.westnileaz.com/pdf/wnv2010.pdf Available at. Accessed November 10, 2011.
- 19.Centers for Disease Control and Prevention West Nile virus activity–United States; November 9–16, 2004; MMWR; 2004. pp. 1071–1072. [Google Scholar]
- 20.Tilley PA, Walle R, Chow A, Jayaraman G, Fonseca K, Drebot M, Preiksaitis J, Fox J. Clinical utility of commercial enzyme immunoassays during the inaugural season of West Nile virus activity, Alberta, Canada. J Clin Microbiol. 2005;43:4691–4695. doi: 10.1128/JCM.43.9.4691-4695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisen WK, Milby MM, Meyer RP, Pfuntner AR, Spoehel J, Hazelrigg JE, Webb JP., Jr Mark-release-recapture studies with Culex mosquitoes (Diptera: Culicidae) in Southern California. J Med Entomol. 1991;28:357–371. doi: 10.1093/jmedent/28.3.357. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 23.Ashford D, Savage H, Hajjeh R, McReady J, Bartholomew D, Spiegel R, Vorndam V, Clark G, Gubler D. Outbreak of dengue fever in Palau, Western Pacific: risk factors for infection. Am J Trop Med Hyg. 2003;69:135–140. [PubMed] [Google Scholar]
- 24.Heukelbach J, de Oliveira FA, Kerr Pontes LR, Feldmeier H. Risk factors associated with an outbreak of dengue fever in a favela in Fortaleza, north-east Brazil. Trop Med Int Health. 2001;6:635–642. doi: 10.1046/j.1365-3156.2001.00762.x. [DOI] [PubMed] [Google Scholar]
- 25.Koopman JS, Prevots DR, Vaca Marin MA, Gomez Dantes H, Zarate Aquino ML, Longini IM, Sepulveda Amor J. Determinants and predictors of dengue infection in Mexico. Am J Epi. 1991;133:1168–1178. doi: 10.1093/oxfordjournals.aje.a115829. [DOI] [PubMed] [Google Scholar]
- 26.Kronenwetter Koepel T, Meece J, Miller C, Reed K. Surveillance of above- and below-ground mosquito breeding habitats in a rural midwestern community: baseline data for larvicidal control measures against West Nile virus vectors. Clin Med Res. 2005;3:3–12. doi: 10.3121/cmr.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez-Prokopec G, Vanden Eng J, Kelly R, Mead D, Kolhe P, Howgate J, Kitron U, Burkot T. West Nile virus infection risk is associated with combined sewer overflow streams in urban Atlanta, Georgia. Environ Health Perspect. 2010;118:1382–1388. doi: 10.1289/ehp.1001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reisen WK, Milby MM, Meyer RP. Population dynamics of adult Culex mosquitoes (Diptera: Culicidae) along the Kern River, Kern County, California, in 1990. J Med Entomol. 1992;29:531–543. doi: 10.1093/jmedent/29.3.531. [DOI] [PubMed] [Google Scholar]
- 29.Vinogradova E. Culex pipiens pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control. Sofia, Bulgaria: Pensoft Publishers; 2000. [Google Scholar]
- 30.Marfin AA, Bleed DM, Lofgren JP, Olin AC, Savage HM, Smith GC, Moore PS, Karabatsos N, Tsai TF. Epidemiologic aspects of a St. Louis encephalitis epidemic in Jefferson County Arkansas, 1991. Am J Trop Med Hyg. 1993;49:30–37. doi: 10.4269/ajtmh.1993.49.30. [DOI] [PubMed] [Google Scholar]
- 31.Meehan PJ, Wells DL, Paul W, Buff E, Lewis A, Muth D, Hopkins R, Karabatsos N, Tsai TF. Epidemiological features of and public health response to a St. Louis encephalitis epidemic in Florida, 1990–1. Epi Infect. 2000;125:181–188. doi: 10.1017/s0950268899004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson BE, Pigford CA, Work T, Wende RD. Serologic survey for St. Louis encephalitis and other group B arbovirus antibodies in residents of Houston, Texas. Am J Epi. 1970;91:87–98. doi: 10.1093/oxfordjournals.aje.a121116. [DOI] [PubMed] [Google Scholar]