Abstract
OBJECTIVE
1) Determine differences in lifetime fluorescence between normal and malignant tissue of the upper aerodigestive tract. 2) Evaluate the potential of time-resolved laser-induced fluorescence spectroscopy (TR-LIFS) as a diagnostic instrument for head and neck squamous cell carcinoma (HNSCC).
STUDY DESIGN
Cross-sectional study.
SETTING
University-based medical center.
SUBJECTS AND METHODS
Nine patients with suspected HNSCC were included. In the operating room, a nitrogen pulse laser (337 nm, 700 ps pulse width) was used to induce tissue autofluorescence of normal tissue and suspected malignant lesions. Spectral intensities and time-domain measurements were obtained and compared to the histopathology at each site. A total of 53 sites were measured. The fluorescence parameters that provided the most discrimination were determined.
RESULTS
Differences in spectral intensities allowed for discrimination between malignant and normal tissue. The spectral intensity of malignant tissue was lower than the normal tissue, and a shift of peak intensity to a longer wavelength was observed in the normalized spectrum of malignant tissue in the range of 360~660 nm. Multiple time-resolved fluorescence parameters provided the best diagnostic discrimination between normal tissue and carcinoma, including average lifetimes (i.e., at 390 nm: 1.7±0.06 ns for normal and 1.3±0.06 ns for tumor, P=0.0025), and the Laguerre coefficients, LEC-2 (i.e., at 460 nm: 0.135±0.001 for normal and 0.155±0.007 for tumor, P<0.05).
CONCLUSION
These findings highlight some of the differences in lifetime fluorescence between normal and malignant tissue. TR-LIFS has potential as a non-invasive diagnostic technique for HNSCC.
Introduction
The National Cancer Institute predicts approximately 35,000 new cases of carcinoma of the oral cavity and pharynx in 2009.1 Over the past 10 years, the overall 5-year survival rate is approximately 61%.1 The survival rate from oral cavity cancer is significantly influenced by the stage at diagnosis. Over 80% of those diagnosed with localized disease will survive five years.1 However, this drops dramatically to just over 50% with regional disease, and to about 30% when distant disease is present at the time of diagnosis.1 Unfortunately, only one third of those with oral cavity cancer present with localized disease.1 Most already have regional or distant disease when they are diagnosed. Improved outcomes could be achieved by earlier detection. Improved diagnostic capabilities with simple and reliable techniques could increase the rate of early staged disease.
Fluorescence spectroscopy has potential as a noninvasive technique to aid in both the diagnosis and intra-operative assessment of head and neck cancer. This technique relies on the expression of either endogenous natural occurring fluorophores, such as collagen and NADH, present in all tissues or exogenous probes. Usually, a light source in the wavelength range of near-UV to visible light is used to induce excitation of these fluorophores.2 The fluorescence is recorded as an emission spectrum. Collagen and NADH, with peak emission wavelengths near 380 and 460 nm respectively, demonstrate different fluorescent properties throughout carcinogenesis. Peak intensity patterns and spectral line shapes using these fluorophores can be compared to distinguish between tissues.
Fluorescence spectroscopy is typically divided into steady-state (spectrally-resolved or intensity measurements) and time-resolved (time-domain and frequency-domain) techniques. Most studies using fluorescence spectroscopy in head and neck tumors focus on the spectral intensity measurements. 3-6 Much less investigation has been geared toward time-resolved techniques. Steady-state techniques measure the overall intensity, peak wavelength, and spectral shape. Time-resolved measurements examine the length of time that a given fluorophore emits light, and this fluorescence decay is expressed in terms of lifetimes. Time-resolved fluorescence for biological systems offers several distinct advantages.7 The lifetimes are sensitive to many variables within the biological microenvironment such as pH, ion concentration and binding, enzymatic activity, and temperature, allowing these factors to be analyzed. Also, biomolecules with overlapping fluorescence emission spectra but with different fluorescence decay times can be discriminated. Lifetime measurements are more robust to changes in fluorescence excitation-collection geometry, presence of endogenous absorbers (e.g. hemoglobin), photobleaching, and changes in fluorophores concentration, light scattering and excitation intensity, making this data more suitable for clinical investigations. Finally, a complete fluorescence emission spectrum (steady-state) can be obtained by recording the time-resolved fluorescence emission at a number of wavelengths across the emission spectrum.
Methods
This study was approved by the Institutional Review Board at the University of California Davis Medical Center. Patients who presented to the operating room with a presumed carcinoma of the oral cavity, pharynx, or larynx, and were scheduled to undergo a panendoscopy with biopsies or surgical resection were offered to participate in the study. With the patient under general anesthesia, designated sites in the aerodigestive tract were spectroscopically investigated with a prototype time-resolved laser-induced fluorescence spectroscopy (TR-LIFS) apparatus. This apparatus is similar to a previously used system described extensively in other studies.7,8 In brief, autofluorescence of the tissue under investigation was induced with a pulsed nitrogen laser (337 nm, 700 ps pulse width). The collected fluorescence was dispersed by an imaging spectrometer/monochromator (Horiba Jobin Yvon, Edison, NJ, Medel MicroHR, f/3.8, 600 g/mm grating) and then detected with a gated multichannel plate photo-multiplier tube. The laser triggering, wavelength scanning and data acquisition, storage and processing were controlled using a computer workstation and custom analytical software written in LabVIEW (National Instruments, Austin, TX) and MATLAB (Mathwork, Inc).
The fiber-optic probe was placed perpendicular to the surface of the mucosa. The probe was held in position by the surgeon, and each sample investigated was 1 mm in diameter. The apparatus and fiberoptic probe are depicted in Figure 1. Steady-state and time-resolved spectroscopy information was collected. The fluorescence emission of each sample was scanned in the 360-610 nm range at 10 nm intervals, with a time-resolution of 300 ps, and at a scanning speed of 0.8 s per wavelength. At each wavelength, 16 fluorescence pulses were collected and averaged by the oscilloscope. The total acquisition time was about 25 s across the scanned emission spectrum. After each measurement sequence, the monochromator was tuned to a wavelength slightly below the excitation laser line. The laser pulses reflected by the sample were measured and used to represent the temporal profile of the laser pulse. This profile was later used as input to the deconvolution algorithm for the estimation of fluorescence lifetimes.
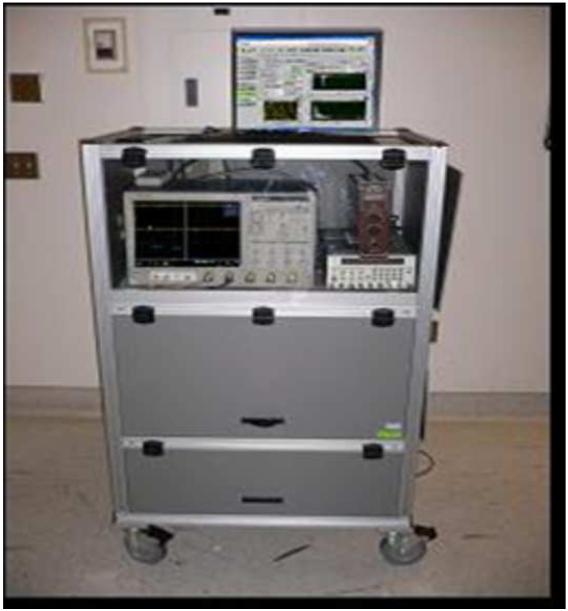
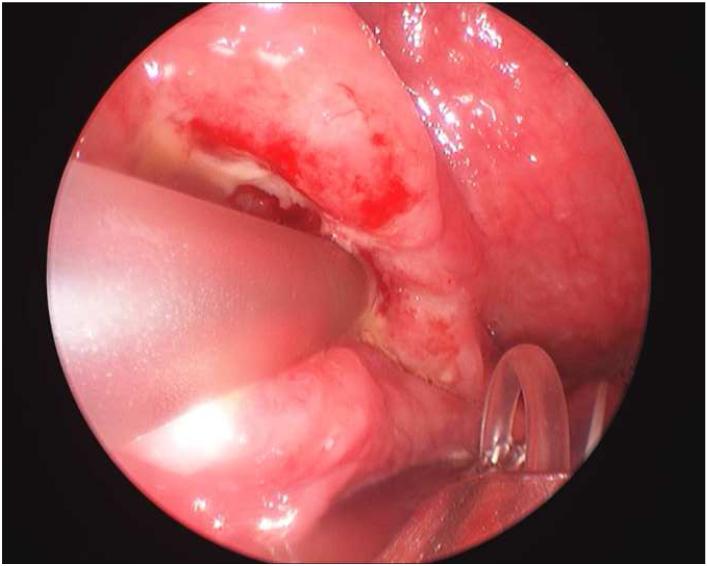
Figure 1.
Depiction of the computer workstation required in operating room (a), fiberoptic probe with laser (b), and probe in vivo collecting data from oropharyngeal carcinoma (c).
Site directed biopsies and spectroscopic measurements were taken of the tumor and the surrounding area, either to map the extent of the tumor or confirm a negative margin. H&E slides of the formalin fixed tissue were evaluated by board certified pathologists. The spectroscopy data was compared to the histological findings. Spectroscopy measurements only were also taken of remote areas of normal appearing mucosa. A total of 53 measurements were taken in 9 patients. Anatomic subsites of the upper aerodigestive tract included the vocal fold, palate, buccal mucosa, floor of mouth, tongue, and lower lip. All 9 patients were male, and 7 of the 9 patients had a history of tobacco smoking. At the time of the study, 5 of the patients were undergoing evaluation for presumed recurrent disease, and 3 of these had received prior radiotherapy to the area under investigation.
Significant spectral intensity peaks were noted at wavelength bands 370-400 nm and 440-470 nm, which are corresponding to collagen and NADH fluorescence, respectively. At these wavelength bands, the ratios of the spectral intensity (I), lifetime (τ) values, and Laguerre expansion coefficients (LEC) were compared between the malignant and normal tissue using 2-way ANOVA. Statistical significance was determined by two-tailed student’s t-test, with significance determined at a P value < 0.05. The Laguerre expansion technique was used for deconvolution. This analytical approach for characterization of fluorescence decay was recently developed by our research group and described in detail elsewhere.9
Results
Histological examination confirmed normal tissue in 17 of the 18 biopsies presumed to be normal, with one specimen revealing chronic inflammation. In the 20 biopsies taken of suspicious lesions, 1 was found to have dysplasia, 1 was diagnosed as carcinoma in situ, and 18 were confirmed to be squamous cell carcinoma (SCCA). An additional 15 spectroscopic measurements of remote healthy appearing tissue were included in the analysis as normal tissue. In the 53 samples undergoing spectroscopic analysis, 14 samples measured from tongue (6 histologically classified as SCCA and 1 as carcinoma in situ), 13 from vocal cord or endolarynx (5 histologically classified as SCCA), 5 from buccal mucosa, (no carcinoma), 5 from floor of mouth, (1 with dysplasia, 1 diagnosed as SCCA), 4 from palate (no carcinoma) and 10 other miscellaneous sites.
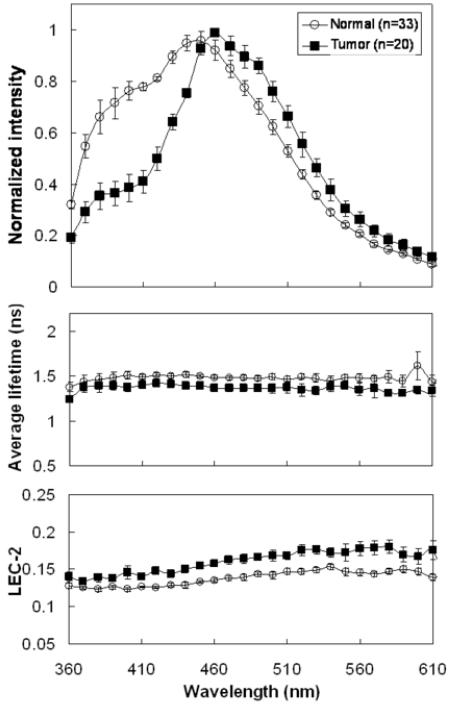
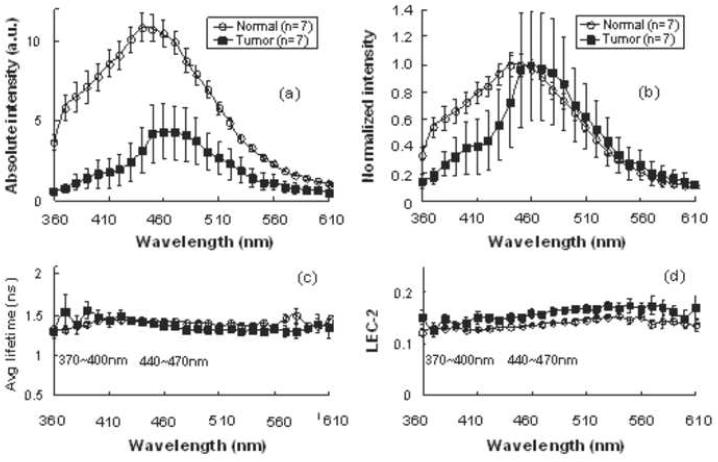
Significant spectral intensity peaks of normal and malignant tissue were noted at two spectral bands: 370-400 nm and 440-470 nm. Across all wavelengths between 360-610 nm, the absolute fluorescence intensity was significantly decreased in carcinoma compared to the normal mucosa. In the malignant tissue, the normalized spectral intensity shape revealed a much more prominent peak at the 440-470 nm spectral band than at the 370-400 nm band (Fig 2). The intensity spectrum showed a slight blue shift in the carcinoma specimens in the 440-470 nm band.
Figure 2.
Representative time-resolved fluorescence spectroscopy features of the averaged normal and tumor tissue of different anatomic sites: Normalized spectrum, Average fluorescence lifetime (τ), Laguerre coefficients of 2nd-order (LEC-2). Data are given as mean ± S.D.
Normalized spectral intensities, lifetime values, and Laguerre coefficient values were compared at the two spectral bands of interest (370-400 nm and 440-470 nm). When pooling all anatomical sites, the average lifetime at the 370-400 nm spectral band for normal tissue was 1.47±0.14 ns while the average lifetime for malignant lesions was 1.38±0.08 ns (not statistically significant). At the 440-470 nm spectral band, the average lifetime was 1.50±0.05 ns for normal areas and 1.38±0.05 ns for malignant tissue (P<0.001). The LEC-2 was significantly different between the normal tissue and carcinoma at both spectral bands (Table 1).
Table 1.
Statistical value of comparison of normal and malignant tissue at 370~400nm and 440~470nm (all sites). Data are given as mean ± S.D.
| 370~400nm | 440~470nm | |||
|---|---|---|---|---|
| Lifetime (ns) | LEC-2 | Lifetime (ns) | LEC-2 | |
| Normal (n=33) | 1.47 ± 0.14 | 0.12 ± 0.005 | 1.50 ± 0.05 | 0.13 ± 0.007 |
| Tumor (n=20) | 1.38 ± 0.08 | 0.14 ± 0.004 | 1.38 ± 0.05 | 0.16 ± 0.009 |
| P-value | 0.232 | 0.006 | 0.007 | 0.003 |
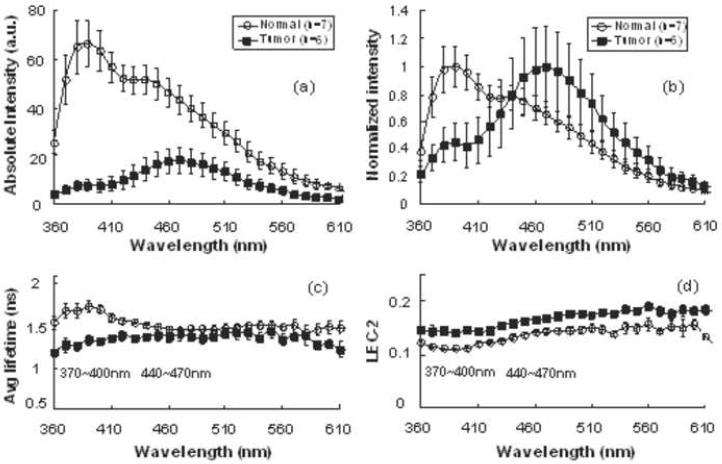
When examining individual anatomical subsites, various statistically significant differences were noted. Figures 3 and 4 depict the intensities, lifetimes, and Laguerre coefficient values across the spectrum for the vocal cord and tongue, respectively. In the vocal cord, the normalized intensity and average lifetime were significantly lower in the malignant tissue at the 370-400 nm band. A statistically significant difference in the LEC-2 at both the 370-400 nm and 440-470 nm bands was noted (Table 2). In the tongue, a significant difference was only noted at 440-470 nm between the cancer and normal tissue using the LEC-2 (Table 3).
Figure 3.
Representative time-resolved fluorescence spectroscopy features of the normal and tumor tissue of vocal cord: (a) Absolute spectrum, (b) normalized spectrum, (c) Average fluorescence lifetime (τf), (d) Laguerre coefficients of 2nd-order (LEC-2). Data are given as mean ± S.D.
Figure 4.
Representative time-resolved fluorescence spectroscopy features of the normal and tumor tissue of tongue: (a) Absolute spectrum, (b) normalized spectrum, (c) Average fluorescence lifetime (τf), (d) Laguerre coefficients of 2nd-order (LEC-2). Data are given as mean ± S.D.
Table 2.
Statistical value of comparison of normal and malignant tissue at 370~400nm and 440~470nm in the vocal cord. Data are given as mean ± S.D.
| 370~400nm | 440~470nm | |||
|---|---|---|---|---|
| Lifetime (ns) | LEC-2 | Lifetime (ns) | LEC-2 | |
| Normal (n=7) | 1.69 ± 0.19 | 0.11 ± 0.003 | 1.47 ± 0.09 | 0.14 ± 0.009 |
| Tumor (n=6) | 1.29 ± 0.13 | 0.14 ± 0.014 | 1.38 ± 0.12 | 0.16 ± 0.008 |
| P-value | 0.001 | 0.006 | 0.186 | 0.002 |
Table 3.
Statistical value of comparison of normal and malignant tissue at 370~400nm and 440~470nm in the tongue. Data are given as mean ± S.D.
| 370~400nm | 440~470nm | |||
|---|---|---|---|---|
| Lifetime (ns) | LEC-2 | Lifetime (ns) | LEC-2 | |
| Normal (n=7) | 1.37 ± 0.05 | 0.13 ± 0.003 | 1.42 ± 0.05 | 0.14 ± 0.003 |
| Tumor (n=7) | 1.49 ± 0.24 | 0.14 ± 0.016 | 1.38 ± 0.07 | 0.16 ± 0.016 |
| P-value | 0.779 | 0.318 | 0.418 | 0.049 |
Discussion
This study demonstrated many of the fluorescence spectroscopy characteristics that can be used to identify carcinoma of the upper aerodigestive tract. The results highlight the additional information that can be obtained from time-resolved parameters and is the first study to use the Laguerre expansion coefficients for head and neck carcinoma. The lifetime values at 440-470 nm, attributed to the NADH biomolecule, demonstrated the most significant difference between normal and malignant tissue. The second order Laguerre coefficient was beneficial at both the 370-400 nm band (collagen), and the 440-470 nm band (NADH).
Previous spectrally-resolved studies have shown reduced overall intensity and a wavelength shift to longer wavelengths in malignant lesions of the head and neck.10,11 These findings were consistent with the results from this study. Multiple explanations have been suggested for this phenomenon. A few of the possible causes include increased loss of collagen crosslinking, thickening of the epithelium, and increased hemoglobin absorption.10
Data using steady-state techniques, or spectrally-resolved fluoroscopy, in the head and neck are well described. However, time-resolved fluorescence spectroscopy has not received the same amount of attention. Chen et al used lifetime measurements to distinguish normal oral mucosa from premalignant lesions.12 They were successfully able to distinguish all premalignant lesions from normal mucosa using the lifetime measurements at 633 nm.
A previous study by the authors using the hamster buccal pouch model found that TR-LIFS could reliably distinguish dysplastic and malignant lesions from normal mucosa.13 The most diagnostic emission wavelengths in that study were 380 nm, 460 nm, and 635 nm. The fluorescence at these wavelengths are believed to originate from collagen, NADH, and porphyrin, respectively. Unlike the animal model, in this human study there was no significant intensity peak at 635 nm. Betz et al believes that the porphyrin is synthesized by microbes living on the necrotic surface of ulcerated tumors and is not reliable as a diagnostic indicator.
In this study, the lifetime measurements at the 440-470 nm band were significantly different between the malignant and normal mucosa. The Laguerre expansion coefficients (LEC-2) were significantly different at both the 370-400 nm and the 440-470 nm bands. Adding the information gleaned from the Laguerre expansion coefficients contributes to the diagnostic capabilities of lifetime fluorescence spectroscopy.
The small sample size included in this study limits the ability to generalize this data to all patients with head and neck carcinoma. The purpose of this initial pilot study was to identify some of the obvious differences in lifetime fluorescence between normal and malignant tissue of the head and neck and confirm in our human patients the findings found in our previous hamster cheek pouch research. One challenge encountered in this study was the varying spectroscopic values among normal tissue in different subsites of the upper aerodigestive tract. Variability has been seen even among different sites of the oral cavity.14 This lack of uniformity presents difficulty in creating a normal range of values across all subsites. When creating diagnostic algorithms in the future, criteria will vary depending on the anatomical site of interest.
An additional limitation in this study was the lack of normal healthy controls. The authors wanted to compare histologic results with fluoroscopic findings and obtaining biopsies in normal healthy volunteers would be difficult to justify. The ‘controls,’ or ‘normal’ tissue used in this study were biopsy samples taken from the surrounding tissue. As mentioned previously, lifetime fluorescence is sensitive to the biological microenvironment. This microenvironment could be affected by infection, inflammation, and contact exposures such as tobacco and alcohol. In this study population, many patients had a strong tobacco history and one-third had prior external beam radiation. Although histologically ‘normal,’ many of these surrounding areas had exposure to tobacco and radiation. The effects of tobacco exposure and radiotherapy on the microenvironment and its impact on lifetime fluorescence have not been elucidated. Future studies comparing histologically normal tissue between smokers and nonsmokers, as well as a data set of normal healthy volunteers, could help answer some of these questions.
This study adds to the current fluorescence spectroscopy studies by revealing the significance of the shorter lifetime between 440-470 nm in malignant tissues of the aerodigestive tract. Also, we introduce the concept of using the Laguerre expansion coefficient in fluorescence spectroscopy for head and neck cancer. Time-resolved fluorescence spectroscopy provides additional information to spectrally-resolved techniques and warrants further development to achieve its potential as a non-invasive diagnostic instrument for head and neck squamous cell carcinoma. Current limitations include lack of access to the relatively expensive fiberoptic probe and increased data to provide definitive diagnostic algorithms.
Acknowledgements
This work was supported in part by the NIH Grants R01-HL67377, UL1 RR024146; the AAO-HNSF Resident Research Grant, and the NSF Center for Biophotonics Science and Technology and the Cancer Center at UC Davis.
Supported in part by a Resident Research Grant from the American Academy of Otolaryngology – Head and Neck Surgery Foundation.
Footnotes
Presented at the American Academy of Otolaryngology – Head and Neck Surgery Foundation Annual Meeting and Oto Expo. San Diego, California, October 4-7, 2009.
Sponsorships or competing interests that may be re levant to content are disclosed at the end of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006. National Cancer Institute; [accessed October 3, 2009]. Nov, 2008. http://seer.cancer.gov/statfacts/html/oralcav.html. [Google Scholar]
- 2.De Veld DC, Witjes MJ, Sterenborg HJ, et al. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41:117–31. doi: 10.1016/j.oraloncology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Gillenwater A, Jacob R, Ganeshappa R, et al. Noninvasive diagnosis of oral neoplasia based on fluorescence spectroscopy and native tissue autofluorescence. Arch Otolaryngol Head Neck Surg. 1998;124:1251–1258. doi: 10.1001/archotol.124.11.1251. [DOI] [PubMed] [Google Scholar]
- 4.Chen CT, Chiang HK, Chow SN, et al. Autofluorescence in normal and malignant human oral tissues and in DMBA-induced hamster buccal pouch carcinogenesis. J Oral Pathol Med. 1998;27:470–474. doi: 10.1111/j.1600-0714.1998.tb01914.x. [DOI] [PubMed] [Google Scholar]
- 5.Heintzelman DL, Utzinger U, Fuchs H. Optimal excitation wavelengths for in vivo detection of oral neoplasia using fluorescence spectroscopy. Photochem and Photobiol. 2000;72:103–113. doi: 10.1562/0031-8655(2000)072<0103:OEWFIV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Mallia RJ, Thomas SS, Mathews A. Laser-induced autofluorescence spectral ratio reference standard for early discrimination of oral cancer. Cancer. 2008;112:1503–1512. doi: 10.1002/cncr.23324. [DOI] [PubMed] [Google Scholar]
- 7.Fang Q, Papaioannou T, Jo J, et al. Time-domain laser-induced fluorescence apparatus for clinical diagnostics. Rev Sci Instrum. 2004;75:151–62. doi: 10.1063/1.1634354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcu L, Jo JA, Fang Q, et al. In-vivo detection of macrophages in a rabbit atherosclerotic model by time-resolved laser-induced fluorescence spectroscopy. Atherosclerosis. 2005;181:295–303. doi: 10.1016/j.atherosclerosis.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo JA, Fang Q, Papaioannou T, et al. Fast model-free deconvolution of fluorescence decay for analysis of biological systems. J Biomed Opt. 2004;9:743–52. doi: 10.1117/1.1752919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz RA, Gao W, Daye D, et al. Autofluorescence and diffuse reflectance spectroscopy of oral epithelial tissue using a depth-sensitive fiber-optic probe. Applied Optics. 2008;47:825–834. doi: 10.1364/ao.47.000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badizadegan K, Backman V, Boone CW, et al. Spectroscopic diagnosis and imaging of invisible pre-cancer. Faraday Discuss. 2004;126:254–279. doi: 10.1039/b305410a. [DOI] [PubMed] [Google Scholar]
- 12.Chen HM, Chiang CP, You C, et al. Time-resolved autofluorescence spectroscopy for classifying normal and premalignant oral tissues. Lasers Surg Med. 2005;37:37–45. doi: 10.1002/lsm.20192. [DOI] [PubMed] [Google Scholar]
- 13.Farwell DG, Meier JD, Park J, et al. Time-resolved fluorescence spectroscopy as a diagnostic technique of oral carcinoma: validation in the hamster buccal pouch model. Arch Otolaryngol Head Neck Surg. doi: 10.1001/archoto.2009.216. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betz CS, Mehlmann M, Rick K, et al. Autofluorescence imaging and spectroscopy of normal and malignant mucosa in patients with head and neck cancer. Lasers Surg Med. 1999;25:323–34. doi: 10.1002/(sici)1096-9101(1999)25:4<323::aid-lsm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]