Abstract
Stressors can exert a wide variety of responses, ranging from adaptive responses to pathological changes; moreover, recent studies suggest that mild stressors can attenuate the response of a system to major stressful events. We have previously shown that two-week exposure to cold, a comparatively mild inescapable stressor, induced a pronounced reduction in ventral tegmental area (VTA) dopamine (DA) neuron activity, whereas restraint stress increases DA neuron activity. However, it is not known if these stressors differentially impact the VTA in a region-specific manner, if they differentially impact behavioral responses, or whether the effects of such different stressors are additive or antagonistic with regard to their impact on DA neuron firing. To address these questions, single-unit extracellular recordings were performed in anesthetized control rats and rats exposed to chronic cold and tested after delivery of a two-hour restraint session. Chronic cold stress strongly attenuated the number of DA neurons firing in the VTA, and this effect occurred primarily in the medial and central VTA regions that preferentially project to reward-related ventral striatal regions. Chronic cold exposure also prevented the pronounced increase in DA neuron population activity without affecting the behavioral sensitization to amphetamine produced by restraint stress. Taken together, these data show that a prolonged inescapable mild stressor can induce plastic changes which attenuate the DA system response to acute stress.
Keywords: rat, DA neuron population activity, ventral tegmental area, prefrontal cortex, amphetamine cross-sensitization
Introduction
Stress is generally defined as an outside force that leads to a response of the body to a demand for change (Selye, 1937; 1956) or a threat to the maintenance of homeostasis (Pacak & Palkovits, 2001). Stress involves a heightened arousal or excitability, with a perception of aversiveness and a feeling of lack of control over outcomes (Kim & Diamond, 2002). As such, stress can be either adaptive, to prepare for a response in the face of an apparent danger, or maladaptive, since it can trigger multiple pathological states. Thus, stressors can have multiple effects on individuals depending on the nature of the stressor and the susceptibility of the individual.
The DA system demonstrates complex responses to stressors, and likely plays a major role in adaptive and maladaptive responses particularly as they relate to motivation and psychopathology. Stress is known to affect mesolimbic DA levels (Abercrombie et al., 1989; Puglisi-Allegra et al., 1991; Finlay & Zigmond, 1997), although the direction of this effect is controversial, with studies showing either enhancement or reduction in DA release (for review see Cabib & Puglisi-Allegra, 2011). Moreover, different types of stressors appear to have markedly different effects on DA neurons. Potent stressors such as restraint stress are known to produce changes resembling a highly anxious state and have been associated with increased DA system response (Antelman et al., 1980; Piazza & Le Moal, 1998; Pacchioni et al., 2007). In contrast, exposure to inescapable mild stressors show decreased DA system activity (Moore et al., 2001) and symptoms related more to depression (Morilak et al., 2005; Kompagne et al., 2008). Indeed, the effects on chronic cold exposure (CCE) on rats (in terms of levels of stress-related hormones) appear to resemble that of depressed patient (Willner, 1995; Korte, 2001), whereas rats exposed to acute restraint exhibit stress-related hormone levels comparable to that occurring with anxiety and post-traumatic stress disorders (Yehuda & Antelman, 1993; Craig et al., 1995; Korte, 2001). Thus, these different stressors produce markedly different effects in the brain as well as on the DA system. Of course, whether the difference in effect is due to the severity of the stressor or the inescapable nature (Cabib & Publisi-Allegra, 2011) is not known.
Nonetheless, how these stressors impact DA system physiology is not completely understood. Thus, studies have shown that the VTA DA system does not respond uniformly to stimuli, but can show medial-lateral differences that are proposed to relate to reward versus stimulus response (Ikemoto, 2007; Lodge & Grace, 2011; Valenti et al., 2011). Moreover, there is evidence that some types of stress can markedly alter responses to other stressors; e.g., low levels of stress or controllable stressors have been reported to “immunize” a system to subsequent stressors (Williams & Maier, 1977; Gresch et al., 1994; Ortiz et al., 1996; Bhatnagar et al., 1998; Pardon et al., 2003; Dronjak et al., 2004; Ma & Morilak, 2005a; Belda et al., 2008). Therefore, in this study we examined whether there was a difference in the regional responsivity of VTA DA neurons to prolonged mild versus acute stressors, and furthermore whether CCE produced an additive effect or an attenuation with respect to DA system activity.
Materials and Methods
Subjects and materials
Upon arrival, 200–220g male Sprague-Dawley rats (Hilltop; Scottdale, PA) were pair-housed in a colony room with controlled temperature (22 °C), humidity (47%), and 12-hour light/dark cycle (lights on at 07:00 AM). All rats had ad libitum access to food (laboratory rodent diet 5001; PMI Feeds, St. Louis, MO) and water. All experiments were performed in accordance with the guidelines outlined in the USPHS Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
All chemicals and reagents were obtained from Sigma-Aldrich, unless otherwise specified.
Stress protocols
After 5–7 days acclimation in the colony room, rats from the same cage were separated and randomly assigned to control or stress groups, where stress consisted of inescapable exposure to chronic cold, acute restraint, or a combination of the above. The two stressors were chosen based on their propensity to induce activation of the hypothalamic-adrenal-cortical axis and heightened responses to stimuli (i.e., restraint stress) and an inescapable, mild stressor to which the rats accommodate (CCE).
For the chronic cold experiments, control rats were single-housed in the colony room and their cage-mates were prepared for cold stress by shaving the fur caudally from their hindlimb to the neck, a procedure that potentiates the effectiveness of cold stress on the catecholamine system while not preventing thermoregulatory adaptation (Fluharty et al., 1985; Moore et al., 2001). Rats were then transferred to a cold room (4 °C) on a light cycle similar to the colony room (12 hour cycle; lights on at 7AM), and housed singly in hanging wire mesh cages for 14–17 days (Jedema et al., 1999; Moore et al., 2001). Previous studies indicate that during the first few days, rats exposed to chronic cold did not increase their body weight to the same extent as their matched control (Folk, 1974; Jedema & Grace, 2003). However, adaptation to cold temperature occurred promptly and with continued exposure to cold, the body weight normalized (Folk, 1974; Mana & Grace, 1997; Jedema et al., 1999; Moore et al., 2001) and rats exhibited normal levels of spontaneous behavior (Van Zoeren & Stricker, 1976; Moore et al., 2001), body temperature and respiration (Moore et al., 2001). At the termination of the 2 week cold exposure, rats were removed from the cold room and transferred back to the colony room at ambient temperature, where they were housed singly for 18–20 hours before being tested. However, if during cold exposure rats showed any signs of distress, cold-induced injury or tissue damage they were immediately excluded from the study.
A subgroup of CCE rats and controls were housed in the colony room for an extra 7 days after removal from the cold room prior to single-unit extracellular recordings. In addition, one day after cold removal, another subgroup of CCE rats (and controls) received a single session of 2 hours restraint stress acute restraint (AR) in a custom-designed plexiglass tube (Valenti et al., 2011).
At termination of each restraint session, rats were immediately prepared for electrophysiology recording or behavioral testing (see below). All experimental procedure occurred within 4–5 hours following restraint session, as previously described (Valenti et al., 2011).
A total of 90 rats were used in this study. Given that the data obtained from match pairs of untreated CCE rats for rat tested either 18–20 hours after or 7 days after cold removal were not different, all data were combined. In order to limit the number of stress-exposed animals, data from an additional 3 acute restraint rats from a previous study (Valenti et al., 2011) were combined with data from the current investigation.
Surgery and single-unit extracellular recording
Single-unit extracellular recordings were performed from rats anesthetized by intraperitoneal (i.p.) injection of 8% chloral hydrate (400 mg/kg). The level of anesthesia was monitored for the duration of the experiment by testing for footpinch withdrawal reflex, and maintained by injection of supplemental doses of chloral hydrate as needed. Body temperature was monitored via a rectal probe and maintained at 37° C by a thermostatically-controlled heating pad (FHC; Bowdoinham, ME). Rats were placed in a stereotaxic apparatus (David Kopf Instruments; Tujunga, CA), and an incision was made on the scalp to expose the skull. A burr hole was drilled in the region overlying the VTA (in mm from bregma, AP: −5.3 to −5.7, ML: −0.6 to 1) or medial prefrontal cortex (mPFC, AP: +2.9 to +3.4, ML: −0.6 to1) (Paxinos & Watson, 1998). Omegadot glass microelectrode tubing (2mm o.d.; World Precision Instruments; Sarasota, FL) was pulled using a Narishige PE-2 Vertical Microelectrode Puller (Tokyo, Japan) and filled with 2M NaCl and 2% Pontamine Sky Blue dye, with in situ impedance of 8–12MΩ. The electrodes were lowered slowly into one of the recording regions using a hydraulic microdrive (David Kopf Instruments, model 640; Tujunga, CA). Neuronal activity was amplified and filtered (1000x gain, 100–4000 Hz band pass; Fintronics Inc.; Orange, CT), and fed to an audio monitor (Grass Instruments, model AM8; West Warwick, RI), an oscilloscope (BK Precision, model 2120; Yorba Linda, CA) for real-time monitoring, and to a computer interface with custom-designed acquisition and analysis software (Neuroscope; Brian Lowry, Pittsburgh, PA).
Recordings from the VTA were performed as previously described (Valenti & Grace, 2010; Valenti et al., 2011). VTA DA neurons were distinguished from other VTA neurons using open filter settings (low pass, 50 Hz; high pass 16 kHz) according to well-established criteria, including their location, unique long-duration waveform with prominent negative phase, and slow irregular firing rate (Grace & Bunney, 1983; Grace et al., 2007). The baseline activity of each DA neuron encountered during the electrode penetrations was recorded for 2–3 min to assess the baseline firing rate and percent of spikes fired in a burst pattern (i.e., burst firing). The effects of restraint stress and/or chronic cold on VTA DA neurons were assessed by recording the activity of all DA neurons encountered in either control or stress rats. The recording electrode was passed through the VTA for 6–9 electrode tracks in a predetermined grid-like pattern with each track separated by 0.2mm; recordings proceeded from the medial to central to the lateral VTA (2–3 tracks in each location; Valenti et al., 2011). Regions within the VTA were tentatively defined as medial, central and lateral on the basis of anatomical data showing the relative distribution with regard to target (Paxinos & Watson, 1998; Ikemoto, 2007). Antidromic activation was not carried out, since repetitive stimulation of target areas would confound the measure of DA neuron activity that was being assessed (Braszko et al., 1981).
For each rat, 3 parameters were obtained: 1) the population activity, defined as the number of spontaneously active VTA DA neurons encountered per electrode track; 2) average firing rate and 3) average percent burst firing, obtained by averaging the values of firing rate and percent of burst firing for each DA neuron recorded in each rat, respectively. Each parameter was then averaged across all of the rats tested in each group.
In addition, recordings of single units from prelimbic and infralimbic pyramidal neurons were conducted, as described previously (Valenti & Grace, 2009), to establish whether chronic cold stress affected the activity of the mPFC. Neurons were classified as putative pyramidal neurons if they exhibited firing frequencies < 5Hz and had spike durations of > 1.1 msec; this contrasted with the fast-spiking (FR> 10Hz), short spike duration putative interneurons (Bartho et al., 2004). Cortical pyramidal neurons exhibit 2 modes of firing: regular tonic firing and discrete burst firing (Connors & Gutnick, 1990; Baeg et al., 2001; Laviolette et al., 2005). In order to sample all neuronal populations, searching for mPFC neurons was done during stimulation of the BLA or entorhinal cortex; stimulation (0.1–0.8 mA; 0.5 Hz; 0.25msec duration) was delivered during electrode penetration using a Grass Instrument S88 stimulator connected to a NEX-100 bipolar concentric electrode (Rhodes Medical Instrument Inc.; Summerland, CA). However, all neurons encountered (and thus recorded) during the electrode tracks were spontaneously active and not activated by stimulation. Upon identification of a pyramidal neuron fitting the above criteria, 3 min of baseline activity was recorded to assess firing rates and percent of burst firing in control and CCE rats.
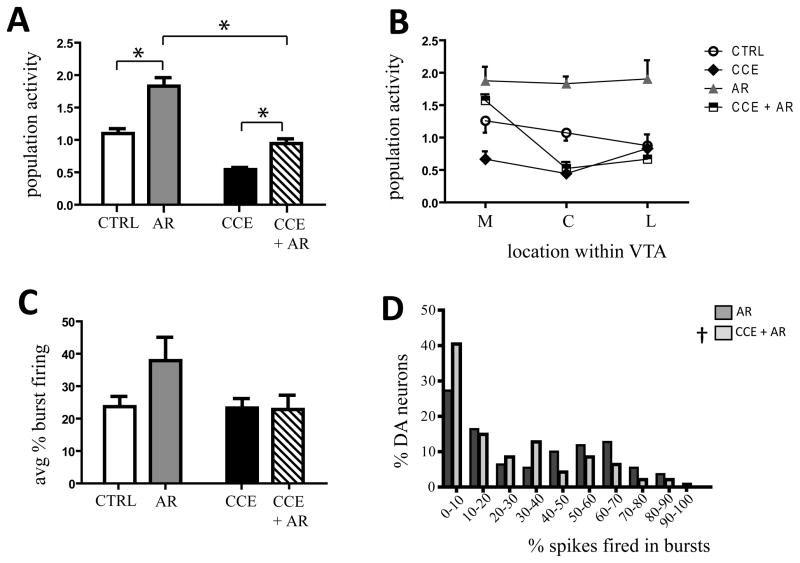
Figure 2. Chronic exposure to cold prevented the acute restraint-induced increase in VTA DA neuron population activity and burst firing.
A) Acute restraint (grey bar) induced a marked increase in DA neuron population activity in untreated rats (white bar). Two week pre-exposure to cold stress attenuated the acute restraint-induced increase in DA neuron population activity (CCE, black bar; CCE + AR, hatched bar) (* two-way ANOVA, source of variation: CCE, F(1,26)= 99.3, P< 0.001; AR, F(1,26)= 62.9, P< 0.001; CCE x AR, F(1,1,26)= 7.01, P= 0.0136; see text for details). B) In CCE rats, restraint (black and white squares) induced a prominent increase in population activity in DA neurons located in the medial VTA (M), similar to the effect induced in rats that received only restraint (grey triangles) († CCE vs CCE + AR: two-way ANOVA, source of variation: CCE, F(1,21)= 4.52, P= 0.046; F(1,21)= 13.45, AR= 0.001; CCE x AR, F(1,1,21)= 0.45, P= 0.510). However, the increase in population activity in the central (C) and lateral (L) VTA was strongly attenuated compared to acute restraint, and did not differ from control levels (CCE, black diamonds) (* AR vs CCE +AR; central, two-way ANOVA, source of variation: F(1,21)= 50.42, CCE, P< 0.001; AR, F(1,21)= 12.07, P= 0.002; CCE x AR, F(1,21)= 8.24, P= 0.009; lateral, two-way ANOVA, source of variation: CCE, F(1,15)= 8.15, P= 0.012; AR, F(1,15)= 1.39, P= 0.256; CCE x AR, F(1,1.15)= 3.27, P= 0.091). C) Previous exposure to cold prevented the restraint-induced increase in percent burst firing (hatched bar) compared to restraint alone (grey bar) (control, white bars; CCE, black bar). D) The distribution of percent burst firing across neurons recorded revealed a significant leftward shift in percent burst firing when CCE preceded the acute restraint (AR, dark grey bars, CCE + AR, light grey bars). Two-way Kolmogorov-Smirnov test: ‡, P< 0.05 (see results for details)
Histology
At the cessation of single-unit recording, the electrode sites were marked via electrophoretic ejection of Pontamine sky blue dye from the tip of the recording electrodes (~ −20μA constant current for 20–30min; Fintronics Inc., bipolar constant current source). The rats were overdosed with anesthetic, and the brains removed and placed in 8% w/v paraformaldehyde in PBS for a minimum of 48 hours. Rat brains were then transferred to 25% w/v sucrose in PBS until saturated and were cut into 60μm coronal sections. Each slice was then mounted onto gelatin-chrom alum-coated slides, and stained with cresyl violet for histochemical verification of electrode location.
Behavioral response following psychostimulant administration
Psychostimulant-induced changes in locomotor responses of chronic cold rats or chronic cold + restraint rats were examined as previously described (Valenti et al., 2011). All tests were performed during the light phase of the diurnal cycle, to match the time of the electrophysiology recordings, on subjects randomly segregated into groups of 4: controls, chronic cold rats, acute restraint rats, and chronic cold + acute restraint rats. Rats that received acute restraint were housed in the same room in which restraint was induced for an additional 2 hours (Valenti et al., 2011), and were then transported to a different room for behavioral testing. Four rats were placed in 4 separate open-field arenas (Coulbourn Instruments Inc.; Whitehall, PA) where locomotor activity in the X-Y plane was assessed by automatic measurement of beam breaks. Baseline locomotor activity was recorded for 30 minutes; at that time, all rats were injected with 0.5mg/kg D-amphetamine (i.p.) and recording continued for an additional 120 minutes.
Data analysis and statistics
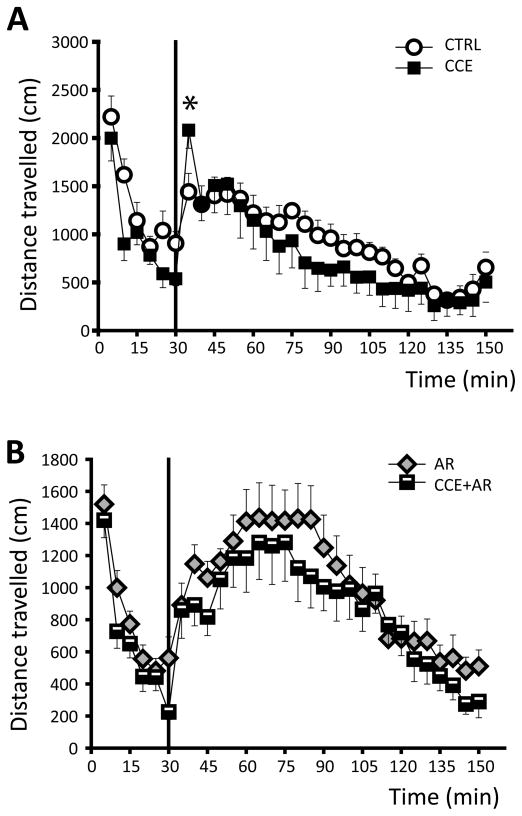
Data from single-unit extracellular recordings were analyzed off-line using the Neuroscope data analysis package (Brian Lowry, Pittsburgh, PA). Burst firing of VTA DA neurons was defined as the occurrence of 2 spikes with an interspike interval (ISI) of < 80ms indicating the initiation of a burst, and subsequent 2 spikes with an ISI > 160ms signaling burst termination (Grace & Bunney, 1984). Burst firing for mPFC pyramidal neurons was defined by the occurrence of spikes with an ISI < 45 ms (Laviolette et al., 2005). Locomotor activity data were acquired and analyzed on-line with TruScan Software (Coulbourn Instruments Inc.; Whitehall, PA). The effects of stress were evaluated for statistical significance with SigmaStat 3.1 (Systat Software Inc.; San Jose, CA) or MATLAB (The MathWorks Inc.; Natick, MA) software. One- or two-way ANOVA followed by Holm-Sidak method for All Pairwise Multiple Comparison Procedures or Kruskal-Wallis one-way ANOVA on Ranks followed by Dunn’s method (whenever the Shapiro-Wilks normality test on distribution failed) were employed to evaluate the effects of stress on neuronal activity. Two-sample Kolmogorov-Smirnov tests were performed to analyze whether stress affects the distribution of percent burst firing. The effects of stress on locomotor activity and the changes induced by amphetamine administration were evaluated by three-way ANOVA, with manipulation (controls vs stress), treatment (baseline vs amphetamine) and time as factors. Statistics on locomotor experiment were run comparing all 4 groups; however, figure displays raw data comparing only CTRL vs CCE (figure 3A) and CCE vs CCE + AR (figure 3A)
Figure 3. Effects of exposure to chronic cold on amphetamine administration-induced increase of locomotor activity.
A) Time course of locomotor activity before and after i.p injection of 0.5 mg/kg amphetamine (vertical line) to CCE rats (black diamonds) and matched controls (open circles), measured as distance travelled in cm.
B) Chronic cold exposure failed to significantly affect the locomotor response to amphetamine administration (vertical line) to rats that received restraint stress (AR, grey triangles; CCE + AR, black and white squares).
Three-way ANOVA, source of variation: CCE: F(1,864)= 4.275, P= 0.039; AR: F(1,864)= 1.485, P= 0.223; time: F(23,864)= 12.962, P< 0.001; for interactions: CCE x AR: F(1,1,864)= 0.905, P= 0,342; CCE x time, F(1,23,864)= 0.47, P= 0.985; AR x time, F(1,23,864)= 3.137, P< 0.001; CCE x AR x time, F(1,1,23,864)= 0.426, P= 0.992
When results from VTA or PFC recordings are presented for the first time, actual data (expressed as mean ± SEM), the number of rats and the number of DA neurons recorded are indicated, data for the same group are then omitted unless different.
Results
Exposure to chronic cold induced a long-lasting decrease in DA neuron population activity
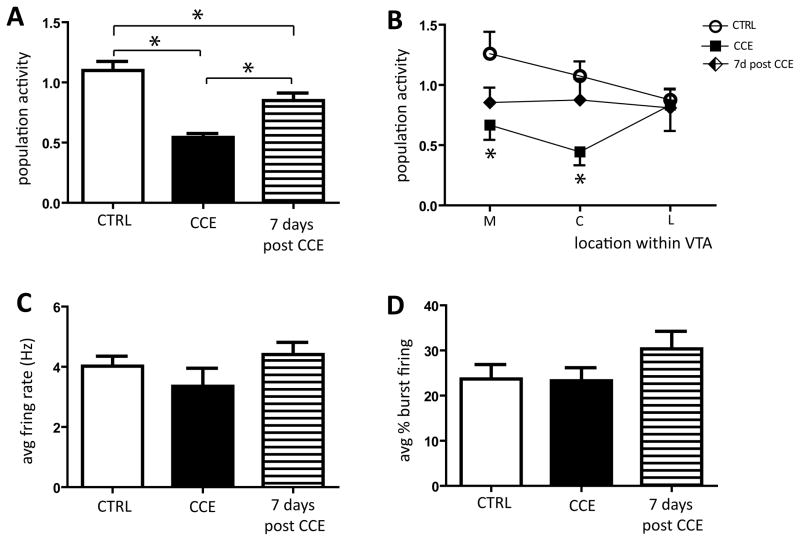
Rats were randomly assigned to either control or chronic cold groups, and the effect of stress on DA neuron activity was assessed. In control rats, the number of spontaneously active DA neurons encountered per electrode track was 1.1 ± 0.1 (n= 9 rats, 85 DA neurons), which is consistent with our previous studies (Valenti & Grace, 2010; Valenti et al., 2011). Two-week exposure to cold significantly reduced population activity by 46% to 0.5 ± 0.03 neurons/track (n= 6 rats, 27 DA neurons; CTRL vs CCE: one-way ANOVA, F(2,20)= 16.9, P< 0.001; Figure 1A), which is consistent with our previous report (Moore et al., 2001). To investigate whether chronic cold induced persistent changes in VTA DA neuron activity, following cold exposure a subgroup of CCE rats was housed in the ambient-temperature colony for an extra 7 days. In these rats, the number of spontaneously active DA neurons remained significantly reduced when compared to controls (15.1% below controls; CTRL vs 7d post CCE: 0.8 ± 0.1, n= 8 rats, 57 DA neurons; one-way ANOVA, F(2,20)= 16.9, P< 0.001; Figure 1A), nevertheless the population activity was significantly higher than observed in CCE rats tested 18–20 hours after removal from cold (CCE vs 7d post CCE: one-way ANOVA, F(2,20)= 16.9, P< 0.001; Figure 1A). We reported previously that different stressors can exert differential effects on DA neuron population activity depending on the relative location of the neurons within the VTA (Valenti et al., 2011). Thus, we further examined whether the effects of CCE were dependent on the location of the DA neurons across the medial-lateral extent of the VTA. We found that the CCE-induced decrease in population activity occurred primarily in the DA neurons located either in the medial (CTRL= 1.3 ± 0.2, n= 34 DA neurons; CCE= 0.7 ± 0.1, n= 12 DA neurons; one-way ANOVA, F(2,20)= 3.82, P= 0.039; Figure 1B) or in the central (CTRL= 1.1 ± 0.1, n= 29 DA neurons; CCE= 0.4 ± 0.1, n= 8 DA neurons; one-way ANOVA, F(2,20)= 4.51, P= 0.024; Figure 1B) part of the VTA for rats tested on the day after cold removal. However, no difference was observed in the lateral part of the VTA (one-way ANOVA, P= 0.939; Figure 1B). In contrast, DA neurons recorded from rats tested 7 days after CCE did not exhibited differences in activity in any of the 3 locations examined, when either compared to control or to CCE tested on the following day (CTRL vs 7d post CCE, one-way ANOVA: medial, P= 0.20; central, P= 0.953; lateral, P= 0.939; CCE vs 7d post CCE, one-way ANOVA: medial, P= 1.00; central, P= 0.180; lateral, P= 0.939; Figure 1B).
Figure 1. Chronic exposure to cold induced a persistent decrease in the number of spontaneously active VTA DA neurons.
A) Exposure to cold stress for 14–17 days induced a pronounced reduction in the number of spontaneously active VTA DA neurons (population activity; black bar) compared to controls (white bar). This attenuation in population activity persisted when examined 7 days following removal from cold exposure (7d post CCE, hatched bar) (* one-way ANOVA, P< 0.05; see text for details). B) Chronic cold selectively decreased the number of spontaneously active DA neurons located in the medial (M) and central (C) but not in lateral (L) VTA of rats tested at 18–20 hours after chronic cold exposure (black squares) compared to controls (white circles) (* CTRL vs CCE: one-way ANOVA, medial, P= 0.039; central, P= 0.024). In rats tested 7 days post CCE there was no significant change in the DA neuron population located in any subdivision of the VTA (black diamonds). No significant changes were observed either in average firing rate (C) or in average percent of burst firing (D).
Chronic exposure to cold did not significantly affect DA neuron average firing rate (CTRL: 4.0 ± 0.3Hz, n= 9 rats; CCE: 3.3 ± 0.6Hz, n= 6 rats one-way ANOVA, P= 0.2828; Figure 1C) or burst firing (CTRL: 23.7 ± 3.2%, n= 9 rats; CCE: 23.3 ± 2.9%, n= 6 rats; one-way ANOVA, P= 0.2935; Figure 1D) at either post-exposure time point. Moreover, there was no difference in the distribution of percent spikes in bursts for neurons recorded in either exposure group when compared to control (18–20 hours: two-sample Kolmogorov-Smirnov test, P= 0.5044; 7 days: two-sample Kolmogorov-Smirnov test, P= 0.3875).
Thus, these data suggest that a maintained inescapable mild stressor, i.e. a 2 week chronic exposure to cold, produced prominent changes in VTA DA neuron population activity, which were still present in an attenuated fashion a week after cold removal. Moreover, similar to the effects observed with repetitive footshock (Valenti et al., 2011), chronic cold stress affected VTA DA neuron spontaneous activity differentially depending on the location within the VTA.
Chronic cold exposure did not alter mPFC pyramidal neuron activity
The PFC is a region known to regulate stress responses subcortically (Abercrombie et al., 1989; Finlay et al., 1995; Cabib & Puglisi-Allegra, 1996), and recent studies suggested that mPFC attenuates stress responses following a controllable stressor (Amat et al., 2008). Thus, the effects of CCE on mPFC pyramidal neurons were examined. In control rats, putative pyramidal neurons exhibited an average firing rate of 1.1 ± 0.2Hz (n= 7 rats, 34 neurons). Two weeks of exposure to cold did not significantly alter pyramidal neuron firing rate (CCE: 0.9 ± 0.1Hz, n= 7 rats, 26 neurons; Kruskal-Wallis one-way ANOVA on Ranks, P= 0.704). In addition, chronic cold stress did not change mPFC pyramidal neuron percent burst firing (CTRL: 41.5 ± 3.0%, CCE: 40.0 ± 4.4%; one-way ANOVA, P= 0.769) or the average interspike interval (CTRL: 16.6 ± 1 msec, CCE: 16.6 ± 1 msec; Kruskal-Wallis one-way ANOVA on Ranks, P= 0.820). Thus, cold stress did not affect any of the parameters of mPFC pyramidal neuron activity measured.
Previous exposure to chronic cold prevented the acute restraint-induced increase in VTA DA neuron population activity
We have shown that restraint stress, given either acutely or repeatedly, increased DA neuron population activity (Valenti et al., 2011). Thus, the effect of restraint on DA neuron activity of untreated rats was opposite in direction from that found following chronic cold (Figure 1). Therefore, we examined whether the previous exposure to chronic cold affected the restraint-induced increase in VTA DA neuron population activity or restraint-induced amphetamine cross-sensitization of locomotor activity. A two-way ANOVA was applied to examine the effects of CCE, AR and the interaction between CCE and AR, followed by Holm-Sidak method for multiple comparisons (source of variation: CCE, F(1,26)= 99.3, P< 0.001; AR, F(1,26)= 62.9, P< 0.001; CCE x AR, F(1,1,26)= 7.01, P= 0.0136). Thus, an acute restraint stress session induced a pronounced activation of VTA DA neuron population activity (CTRL vs AR: 1.9 ± 0.1, n= 8 rats, 135 DA neurons; two-way ANOVA, F(1,26)= 99.3, P< 0.001; Figure 2A), as previously reported (Valenti et al., 2011). Acute restraint stress also increased DA neuron population activity in rats pre-exposed to chronic cold compared to CCE alone, restoring the CCE-induced decrease in population activity toward control levels (CCE vs CCE + AR: 0.9 ± 0.1, n= 7 rats, n= 54 DA neurons; two-way ANOVA, F(1,26)= 99.3, P< 0.001; Figure 2A). However, the restraint-induced increase in DA neuron population activity observed in CCE rats was by far less pronounced than in AR alone (AR vs CCE + AR: two-way ANOVA, F(1,26)= 62.9, P< 0.001; Figure 2A). In addition, there was a significant interaction among groups (CCE x AR: two-way ANOVA, F(1,1,26)= 7.01, P= 0.0136). Thus, pre-exposure to chronic cold appeared to protect the DA system from the effects of acute restraint stress.
In examining the location of VTA DA neuron population change within the VTA, there were marked differences observed following the stress protocol. Thus, acute restraint increased DA neuron firing across medial, central, and lateral portions of the VTA (CTRL vs AR, two-way ANOVA: medial, F(1,21)= 4.5, P= 0.045; central, F(1,21)= 50.4, P< 0.001; lateral, F(1,21)= 8.1, P= 0.012; Figure 2B), whereas chronic cold decreased population activity primarily in the medial and central VTA (Figure 1B and 2B). In CCE animals subsequently exposed to acute restraint, the increase in medial VTA DA neuron firing remained (AR: 1.9 ± 0.2, n= 8 rats, 45 DA neurons; CCE + AR: 1.6 ± 0.1, n= 7 rats, 33 DA neurons; two-way ANOVA, P= 0.258), which corresponded to a 135.1% increase from CCE alone (CCE vs CCE + AR; two-way ANOVA, F(1,21)= 13.4, P= 0.0014; Figure 2B). In contrast, previous exposure to chronic cold prevented the prominent increase induced by restraint stress in central (AR: 1.8 ± 0.1, n= 8 rats, 44 DA neurons; CCE + AR: 0.5 ± 0.1, n= 7 rats, 11 DA neurons; two-way ANOVA, F(1,21)= 50.4, P< 0.001) and lateral VTA (AR: 1.9 ± 0.3, n= 7 rats, 40 DA neurons; CCE + AR: 0.7 ± 0.1, n= 5 rats, 10 DA neurons; two-way ANOVA, F(1,15)= 8.15, P= 0.012) with population activity being not significantly different from that observed following CCE alone (CCE vs CCE + AR: two-way ANOVA, central, P= 0.658; lateral, P= 0.659; Figure 2B). In addition, a significant interaction was observed between the effects of cold and restraint stress only for DA neurons located in central VTA (two-way ANOVA, F(1,1,21)= 8.24, P= 0.0092). Therefore, CCE attenuated the ability of restraint stress to increase VTA neuron activity in regions of the VTA that project to more associative regions of the striatum, without affecting the increase in the reward-related medial VTA regions (Ikemoto, 2007; Lodge & Grace, 2011; Valenti et al., 2011).
No significant change in either average firing rate (for CTRL and CCE see above; AR: 4.3 ± 0.2Hz, n= 8 rats; CCE + AR: 3.8 ± 0.1Hz, n= 7 rats; two-way ANOVA, source of variation, CCE: P= 0.0841; AR, P= 0.2742) or average percent burst firing (for CTRL and CCE see above; AR: 35.3 ± 4.1%, n= 7 rats; CCE + AR: 22.8 ± 4.4%, n= 7 rats; two-way ANOVA, source of variation, CCE: P= 0.0995; AR: P= 0.1526; Figure 2C) was observed in any of the groups tested or when the interaction of the effect of the 2 stress protocols was examined (FR: two-way ANOVA, P= 0.8404; %B: two-way ANOVA, P= 0.1216). Given that acute restraint was shown to increase the average percent of burst firing in control rats (Valenti et al., 2011), we examined whether pre-exposure to chronic cold also prevented the restraint-induced increase in burst firing observed in untreated rats (Figure 2C). Further analysis revealed that pre-exposure to cold stress altered the distribution in percent burst firing, with many more neurons showing low levels of burst discharge following chronic cold + acute restraint (AR vs CCE + AR; two-sample Kolmogorov-Smirnov test, P= 0.0424; Figure 2D).
Effects of chronic exposure to cold on amphetamine-induced locomotor activity
Previous studies from our laboratory suggest that the increased level of VTA DA neuron population activity correlates with the increased locomotor response to amphetamine (Lodge & Grace, 2008; Valenti et al., 2011). Given that CCE induced a pronounced reduction of DA neuron population activity, and that CCE attenuates the electrophysiological response to restraint stress, this relationship was examined behaviorally. Thus, both spontaneous and amphetamine-induced locomotor activity was recorded in 4 groups of rats: control, chronic cold rats, restraint rats, chronic cold + restraint rats. Baseline locomotor activity was recorded for 30 min and measured in separated open-field arenas (Coulborne Instruments). Rats were then removed from the arenas and injected i.p. with 0.5 mg/kg amphetamine. A three-way ANOVA was applied to analyze the effects of CCE, AR, time or their interactions, and all groups were compared (source of variation: CCE: F(1,864)= 4.275, P= 0.039; AR: F(1,864)= 1.485, P= 0.223; time: F(23,864)= 12.962, P< 0.001; for interactions: CCE x AR: F(1,1,864)= 0.905, P= 0.342; CCE x time, F(1,23,864)= 0.47, P= 0.985; AR x time, F(1,23,864)= 3.137, P< 0.001; CCE x AR x time, F(1,1,23,864)= 0.426, P= 0.992). In addition, a pairwise multiple comparison procedure (Holm-Sidak method) was applied following the ANOVA to compare these factors. Thus, administration of amphetamine to CCE rats induced a rapid and transient increase in locomotor activation compared to matched controls for the first 5 min from drug administration (CTRL, n= 8 rats vs CCE, n= 9 rats; three-way ANOVA, P= 0.040; Figure 3); however, locomotor activity of CCE rats was slightly but not significantly lower than that of controls in the subsequent time points. Consistent with our previous study (Valenti et al., 2011), 0.5 mg/kg amphetamine induced a pronounced increase in locomotor activity of AR rats compared to controls during the first 15 min post-drug (CTRL, n= 8 rats; vs AR, n= 12 rats; three-way ANOVA, 35min: P< 0.001; 40min: P= 0.042; 45min: P= 0.003; 50min: P= 0.026). In contrast, in rats that received acute restraint on the day after cold removal, chronic cold stress failed to affect significantly the acute restraint-induced increase in the locomotor response to amphetamine (AR, n=12 rats vs CCE+AR n= 11 rats; three-way ANOVA, P= 0.374; Figure 3B).
Thus, the decrease in DA neuron population activity observed in the CCE rats was not found to correlate with a decrease in the amplitude of amphetamine-induced locomotion. Moreover, 2 weeks continuous exposure to cold attenuated the restraint stress-induced increase in DA neuron population activity but did not affect restraint-induced behavioral activation to amphetamine.
Discussion
Stressors are known to induce plastic changes in neuronal systems; however, the nature of the stressor will influence the type of homeostatic changes induced. In this study, we used two types of stressors; a long-term exposure to cold, and an acute restraint stressor, which had been found previously to exert opposite actions with respect to DA neuron activity (Moore et al., 2001; Valenti et al., 2011). Specifically, we examined whether pre-exposure to one stressor, CCE, was additive or antagonistic with AR with respect to DA neuron firing or amphetamine-stimulated behavior. We found that CCE and AR affected DA neuron population activity in opposite directions. Moreover, previous CCE potently attenuated the ability of AR to activate DA neurons. In addition, a novel and potentially exciting finding is that each stressor produced regionally-specific actions within the medial-lateral extent of the VTA. However, there was a dissociation between DA neuron population activity and amphetamine-induced locomotion in the CCE and CCE+AR rats. The source of this difference is unclear, since treatments that increase DA neuron population activity consistently increase amphetamine-induced locomotion. There are several possibilities: First, it may be that with CCE there are other systems activated that may counteract the effects of CCE-induced decreases in population activity in the accumbens. Secondly, given that the amphetamine locomotor studies were performed in the awake animal and the electrophysiology in the anesthetized animal, the anesthesia may have suppressed some of these CCE activated compensatory systems. Although chloral hydrate has been shown repeatedly to have limited effect on DA neuron electrophysiology when compared to the awake animal (Hyland et al., 2002), further studies comparing release after CCE in awake versus anesthetized rats will be required to fully evaluate this effect.
The differences in response to AR vs CCE were not due simply to the long-term nature of the stressor, since we found previously that repeated restraint for 10 days produced a similar activation of DA neuron population activity, although the results were somewhat more variable (Valenti et al., 2011). Instead, we believe that the long-term, inescapable milder stressor produced an adaptation of the DA system in a way that attenuated its response to subsequent challenge. This raises the issue as to what characteristic of the two stressors is particularly relevant to the DA response observed. One dimension relates to the relative intensity of the stressor. Thus, restraint stress increases the level of ACTH and CRH during delivery, and these effects continue until stress termination (Dallman et al., 1987; Vahl et al., 2005). Restraint or immobilization also significantly increases the level of plasma catecholamines (Eliason, 1984; Kvetnansky et al., 1992) and tyrosine hydroxylase (Kvetnansky et al., 1970; McMahon et al., 1992). Moreover, restraint was found to increase in DA neuron activity (Valenti et al., 2011), and this activation of the DA system does not accommodate following a week of repeated restraint (Valenti et al., 2011). In contrast, 6 hours cold exposure have been found to increase plasma levels of noradrenaline that stabilized when cold exposure was prolonged for several days (Benedict et al., 1979). In addition, numerous studies show that following 2 weeks of CCE stress there was no significant change in the level of adrenocorticotropin (ACTH), corticotrophin releasing hormone (CRH) or corticosterone in rats (Armario et al., 1986; Hauger et al., 1990; Bhatnagar et al., 1995) and decreased DA neuron activity (Moore et al., 2001), despite a sensitization in the noradrenergic system in rats (Gresch et al., 1994; Finlay et al., 1995; Jedema et al., 1999; Pardon et al., 2003). Indeed, restraint stress has been used as a model of anxiety disorder (Padovan & Guimaraes, 2000; Vahl et al., 2005), whereas chronic mild, inescapable stressors have been used as a model of depression (Willner, 2005). Taken together, these data indicate that CCE can be considered a comparatively “mild” inescapable stressor in that rats rapidly adapt during exposure, as contrasted with the more potent anxiogenic response to restraint stress. On the other hand, it has been argued (Cabib & Pugilisi-Allegra, 2004, 2011) that the relevant dimension in determining the dopaminergic impact of a stressor is escapability. Thus, two types of stressors can be interpreted two ways - either mild stressors lead to decreased DA and strong stressors activate the DA system, or that chronic inescapable stressors lead to decreased and acute stressors lead to increased DA activity (cf. Cabib & Puglisi-Allegra, 2011). While our data is somewhat consistent with both interpretations, the fact that repeated restraint stress still leads to increased DA neuron activity, and that restraint is also an inescapable (though more acute or repeated) stressor suggests that, at least as far as DA neuron firing, it is the perceived strength of the stressor and not the chronicity or escapability that is important. However, the situation when measuring extracellular DA levels in postsynaptic targets (see Cabib & Puglisi-Allegra, 2011 for review) may not be equivalent to what is observed with respect to DA neuron activity.
The finding that prior CCE attenuates the subsequent response to AR stress on DA neuron activity is of potential relevance with respect to the resiliency of the system to alterations. Such an effect has been referred to as a stress “immunization,” or a protective influence of a prior mild stressor (Williams & Maier, 1977; Gresch et al., 1994; Ortiz et al., 1996; Bhatnagar & Dallman, 1998; Bhatnagar et al., 1998; Mizoguchi et al., 2000; Pardon et al., 2003; Dronjak et al., 2004; Ma & Morilak, 2005a; Belda et al., 2008). On the other hand, rather than being protective, the attenuation of restraint stress-induced activation by CCE may instead reflect a resistance to homeostatic DA system activation. If the latter is indeed the case, then CCE-induced prevention of DA system activation could actually prevent the adaptive increase in DA neuron firing rather than protecting the system from further stress-induced pathology. To differentiate these outcomes, further studies involving other measures of stress are likely required, and are currently under way.
A novel finding is that, unlike AR, the impact of CCE on the medial-lateral distribution of DA neurons in the VTA was not uniform. Thus, AR induced an increase in DA neuron population activity in the medial, central, and lateral VTA. In contrast, CCE produced a potent decrease in DA neuron population activity in the medial and central VTA. We had shown previously that changes in population activity correspond to the amplitude of the DA response to inputs that drive burst firing (Lodge & Grace, 2006), with burst firing shown to be a correlate of exposure to reward-related stimuli (Schultz et al., 1997). Each neuron recorded was not identified by antidromic activation, since stimulation of the target site is known to alter DA neuron properties (Braszko et al., 1981; Floresco et al., 2001a). Nonetheless, anatomical data show that the substantial majority of medial VTA DA neurons project selectively to the reward-related ventromedial striatum (Ikemoto, 2007); therefore, the decrease in medial VTA DA neuron activity with CCE is consistent with an anhedonic response. This is also consistent with the region of the VTA that is activated following amphetamine sensitization (Lodge & Grace, 2011), which is associated with increased reward-related behaviors (White & Wang, 1984; Henry et al., 1989; Kalivas & Stewart, 1991). Indeed, the inescapable nature and long-term impact of CCE are similar in nature to inescapable stressors known to induce depressive-like states in rats (Williams & Maier, 1977; Gresch et al., 1994; Finlay et al., 1995; Ortiz et al., 1996). This is in marked contrast to the impact of AR on DA neuron activity, in which there is a pronounced increase in population activity across the medial-lateral extent of the VTA. In contrast to the medial VTA reward-related projections, the central and lateral VTA project to more dorsolateral areas of the ventral striatum/nucleus accumbens, which are areas known to play a larger role in stimulus salience (Ikemoto, 2007). Such an activation is likely related to a heightened state of responsivity, such as that associated with stress-induced anxiety or post-traumatic stress disorder (Yehuda & Antelman, 1993; Craig et al., 1995), rather than depression (Willner, 1995). This activation within lateral VTA would thus be consistent with a heightened response to sensory stimuli. Indeed, these lateral areas correspond to the regions of the VTA that are preferentially activated in a rodent developmental disruption model of psychosis (Lodge & Grace, 2011). Furthemore, the medial-lateral differences with respect to reward and salience are in line with a recent report of posteriorly located VTA neurons recorded in vitro (Lammel et al., 2011).
An important finding of this study was the ability of CCE to attenuate the sensitization of the DA system produced by AR. Thus, prior CCE attenuated the AR-induced increase in DA neuron population activity but not the sensitized locomotor response to amphetamine. Interestingly, the CCE-induced attenuation was most evident in the more lateral VTA regions, and not in the medial VTA. This would be consistent with a protective effect of CCE on the psychotogenic actions of stress, but not on the reward-related aspects, which remain closer to control levels following CCE + AR. In our previous study (Valenti et al., 2011), we found that AR-induced activation of VTA DA neuron population activity was dependent on the vHPC, since AR induced c-fos expression in the vHPC, and moreover inactivation of the ventral hippocampus (vHPC) attenuated the AR-induced increase in DA neuron population activity and locomotor sensitization to amphetamine. However, the vHPC is not likely to play a role in CCE-induced decreases in DA neuron population activity, since even total inactivation of the vHPC fails to decrease this parameter in control rats (Lodge & Grace, 2007). The fact that CCE also attenuated AR-induced increases in burst firing, that does not involve ventral subiculum-nucleus accumbens-ventral pallidum pathway, is further evidence for another region to be involved. One area that is known to regulate subcortical stress-related responses is the PFC, since inactivation of the PFC increases subcortical DA release (Finlay et al., 1995) and behavioral (Deutch et al., 1990; Deutch & Roth, 1990; Carlson et al., 1996) and neurochemical (Cabib & Puglisi-Allegra, 2004) response to stressors, and the PFC has been shown to attenuate stress responses following a controllable stressor (Amat et al., 2008). However, our findings suggest that the PFC may not be involved directly, since there was no observed change in pyramidal neuron activity in this region.
The mechanism underlying the adaptive effects of CCE on the DA system is not clear. However, our previous studies have shown that CCE will cause multiple changes within the noradrenergic system and the BLA. Thus, CCE was found to cause noradrenergic locus coeruleus neurons to be hyper-responsive to stimuli, ranging from membrane depolarization (Jedema & Grace, 2003) to noxious stimulation (Mana & Grace, 1997; Jedema et al., 1999; Jedema & Grace, 2004). Moreover, following CCE, the response of the BLA to NE was found to shift from a balanced alpha-adrenergic inhibition and beta-adrenergic excitation to one in which noradrenaline produced a predominantly excitatory effect (Buffalari & Grace, 2007). Although CCE attenuated DA system activity, it did cause a sensitization of the noradrenergic-amygdala system. We have recently found that blockade of NE beta receptors in the BLA can attenuate post-stress-induced inhibition of the DA system (Chang & Grace, 2011), supporting a role for NE in the BLA in attenuation of DA neuron activity. Moreover, studies by Morilak and colleagues have shown that the adaptive response of the noradrenergic system to cold exposure can attenuate activation to a subsequent acute stress (Pardon et al., 2003; Ma & Morilak, 2005a; b; Morilak et al., 2005). Therefore, it is possible that the noradrenergic system also plays a role in the stress immunization (Williams & Maier, 1977) following CCE observed here. However, the site of the CCE-induced attenuation in DA neuron responsivity is not yet known.
In conclusion, these studies show that a prolonged mild stressor that is known to sensitize the NE system to stressors actually attenuates the DA system response to acute stress. The fact that the attenuation occurs primarily in regions associated with animal models of psychosis rather than reward suggests that CCE produces an immunization of the DA system to the most deleterious effects of acute stress. Thus, all stressors are not equal in their impact on the brain; instead, the nature of the stressor can have dramatically different impacts on the DA system. Such responses highlight the differential nature of responses to stressors, be they adaptive or pathological.
Acknowledgments
The authors thank Dr. Hank P. Jedema and Dr. Daniel J. Lodge for their valuable comments during the preparation of this manuscript, Hugo Malagon-Vina for helpful discussions on statistical analysis, Nicole MacMurdo and Nadina Bembic for technical assistance, and Brian Lowry for the development of the custom-designed computer software Neuroscope. Brian Lowry was affiliated with the Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA and employed by Dr. Grace at the time this work was completed.
This work was supported by a research grant from United States Public Health Service Grant DA15408 to Anthony A. Grace.
Footnotes
Disclosure/Conflicts of interest
All authors declare no direct conflict of interest. AAG receives support from Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, and EMD Serrono.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Armario A, Lopez-Calderon A, Jolin T, Balasch J. Response of anterior pituitary hormones to chronic stress. The specificity of adaptation. Neurosci Biobehav Rev. 1986;10:245–250. doi: 10.1016/0149-7634(86)90011-4. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Baffi JS, Palkovits M. Fine topography of brain areas activated by cold stress. A fos immunohistochemical study in rats. Neuroendocrinology. 2000;72:102–113. doi: 10.1159/000054577. [DOI] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Belda X, Fuentes S, Nadal R, Armario A. A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Horm Behav. 2008;54:654–661. doi: 10.1016/j.yhbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Benedict CR, Fillenz M, Stanford C. Noradrenaline release in rats during prolonged cold-stress and repeated swim-stress. Br J Pharmacol. 1979;66:521–524. doi: 10.1111/j.1476-5381.1979.tb13689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman MF, Roderick RE, Basbaum AI, Taylor BK. The effects of prior chronic stress on cardiovascular responses to acute restraint and formalin injection. Brain Res. 1998;797:313–320. doi: 10.1016/s0006-8993(98)00382-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Mitchell JB, Betito K, Boksa P, Meaney MJ. Effects of chronic intermittent cold stress on pituitary adrenocortical and sympathetic adrenomedullary functioning. Physiol Behav. 1995;57:633–639. doi: 10.1016/0031-9384(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Braszko JJ, Bannon MJ, Bunney BS, Roth RH. Intrastriatal kainic acid: acute effects on electrophysiological and biochemical measures of nigrostriatal dopaminergic activity. J Pharmacol Exp Ther. 1981;216:289–293. [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. Different effects of repeated stressful experiences on mesocortical and mesolimbic dopamine metabolism. Neuroscience. 1996;73:375–380. doi: 10.1016/0306-4522(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed]
- Carlson JN, Visker KE, Keller RW, Jr, Glick SD. Left and right 6-hydroxydopamine lesions of the medial prefrontal cortex differentially alter subcortical dopamine utilization and the behavioral response to stress. Brain Res. 1996;711:1–9. doi: 10.1016/0006-8993(95)01290-7. [DOI] [PubMed] [Google Scholar]
- Chang CH, Grace AA. Beta noradrenergic receptors modulate acute restraint-mediated down-regulation of dopamine system activity. Society for Neuroscience Abstract. 2011 Program 901.31.
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Craig KJ, Brown KJ, Baum A. Environmental factors in the etiology of anxiety. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 1325–1340. [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521:311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. discussion 402–363. [DOI] [PubMed] [Google Scholar]
- Dronjak S, Gavrilovic L, Filipovic D, Radojcic MB. Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav. 2004;81:409–415. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Eliason K. Stress and catecholamines. Acta Medica Scandinavica. 1984;215:197–204. doi: 10.1111/j.0954-6820.1984.tb04994.x. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ. The effects of stress on central dopaminergic neurons: possible clinical implications. Neurochem Res. 1997;22:1387–1394. doi: 10.1023/a:1022075324164. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001a;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. Journal of Neuroscience. 2001b;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fluharty SJ, Snyder GL, Zigmond MJ, Stricker EM. Tyrosine hydroxylase activity and catecholamine biosynthesis in the adrenal medulla of rats during stress. J Pharmacol Exp Ther. 1985;233:32–38. [PubMed] [Google Scholar]
- Folk GEJ. Textbook of environmental physiology. Lea & Febiger; 1974. [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. Journal of Neuroscience. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem. 1994;63:575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251:833–839. [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114(2):475–92. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Chronic exposure to cold stress alters electrophysiological properties of locus coeruleus neurons recorded in vitro. Neuropsychopharmacology. 2003;28:63–72. doi: 10.1038/sj.npp.1300020. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Sved AF, Zigmond MJ, Finlay JM. Sensitization of norepinephrine release in medial prefrontal cortex: effect of different chronic stress protocols. Brain Res. 1999;830:211–217. doi: 10.1016/s0006-8993(99)01369-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kompagne H, Bardos G, Szenasi G, Gacsalyi I, Harsing LG, Levay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008;193:311–314. doi: 10.1016/j.bbr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25:117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Goldstein DS, Weise VK, Holmes C, Szemeredi K, Bagdy G, Kopin IJ. Effects of handling or immobilization on plasma levels of 3,4-dihydroxyphenylalanine, catecholamines, and metabolites in rats. J Neurochem. 1992;58:2296–2302. doi: 10.1111/j.1471-4159.1992.tb10977.x. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Weise VK, Kopin IJ. Elevation of adrenal tyrosine hydroxylase and phenylethanolamine-N-methyl transferase by repeated immobilization of rats. Endocrinology. 1970;87:744–749. doi: 10.1210/endo-87-4-744. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Divergent activation of ventromedial and ventrolateral dopamine systems in animal models of amphetamine sensitization and schizophrenia. Int J Neuropsychopharmacol. 2011:1–8. doi: 10.1017/S1461145711000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005a;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Norepinephrine release in medial amygdala facilitates activation of the hypothalamic-pituitary-adrenal axis in response to acute immobilisation stress. J Neuroendocrinol. 2005b;17:22–28. doi: 10.1111/j.1365-2826.2005.01279.x. [DOI] [PubMed] [Google Scholar]
- Mana MJ, Grace AA. Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience. 1997;81:1055–1064. doi: 10.1016/s0306-4522(97)00225-x. [DOI] [PubMed] [Google Scholar]
- McMahon A, Kvetnansky R, Fukuhara K, Weise VK, Kopin IJ, Sabban EL. Regulation of tyrosine hydroxylase and dopamine beta-hydroxylase mRNA levels in rat adrenals by a single and repeated immobilization stress. J Neurochem. 1992;58:2124–2130. doi: 10.1111/j.1471-4159.1992.tb10954.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology. 2001;24:410–419. doi: 10.1016/S0893-133X(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–692. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Padovan CM, Guimaraes FS. Restraint-induced hypoactivity in an elevated plus-maze. Braz J Med Biol Res. 2000;33:79–83. doi: 10.1590/s0100-879x2000000100011. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971:55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1998. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res. 1991;554:217–222. doi: 10.1016/0006-8993(91)90192-x. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Selye H. The Significance of the Adrenals for Adaptation. Science. 1937;85:247–248. doi: 10.1126/science.85.2201.247. [DOI] [PubMed] [Google Scholar]
- Selye H. What is stress? Metabolism. 1956;5:525–530. [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Valenti O, Grace AA. Entorhinal cortex inhibits medial prefrontal cortex and modulates the activity states of electrophysiologically characterized pyramidal neurons in vivo. Cereb Cortex. 2009;19:658–674. doi: 10.1093/cercor/bhn114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Grace AA. Antipsychotic drug-induced increases in ventral tegmental area dopamine neuron population activity via activation of the nucleus accumbens-ventral pallidum pathway. Int J Neuropsychopharmacol. 2010;13:845–860. doi: 10.1017/S1461145709990599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zoeren JG, Stricker EM. Thermal homeostasis in rats after intrahypothalamic injections of 6-hyroxydopamine. American Journal of Physiology. 1976;230:932–939. doi: 10.1152/ajplegacy.1976.230.4.932. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment. Brain Res. 1984;309:283–292. doi: 10.1016/0006-8993(84)90594-8. [DOI] [PubMed] [Google Scholar]
- Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:240–252. [Google Scholar]
- Willner P. Dopamine mechanisms in depression and mania. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 921–932. [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry. 1993;33:479–486. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]