Abstract
Amyotrophic lateral sclerosis is a devastating neurodegenerative disease caused by loss of motor neurons. Its pathophysiology remains unknown, but progress has been made in understanding its genetic and biochemical basis. Clinical trialists are working to translate basic science successes into human trials with more efficiency, in the hope of finding successful treatments. In the future, new preclinical models, including patient-derived stem cells may augment transgenic animal models as preclinical tools. Biomarker discovery projects aim to identify markers of disease onset and progression for use in clinical trials. New trial designs are reducing study time, improving efficiency and helping to keep pace with the increasing rate of basic and translational discoveries. Ongoing trials with novel designs are paving the way for amyotrophic lateral sclerosis clinical research.
Keywords: antisense oligonucleotide, continual reassessment model, futility design study, induced pluripotent stem cells, selection design study
Introduction to amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease caused primarily by motor neuron loss resulting in progressive weakness, disability and eventually death. The incidence of ALS is approximately 2/100,000 [1]. Although this incidence is comparable to that of multiple sclerosis, the prevalence of ALS is much lower at 5/100,000 because of its rapid progression [1]. Average age of onset is 55–65 years, mean survival is 3–5 years and onset before 30 years of age is uncommon [2].
Amyotrophic lateral sclerosis is diagnosed and monitored based on the clinical history, physical examination, disease course, electrodiagnostic findings and elimination of competing diagnoses. Consensus diagnostic criteria, known as the revised El Escorial Criteria, are typically used for study enrollment [3]. These criteria have been valuable to the field as diagnostic criteria; however, early diagnosis remains challenging. At the time of diagnosis, less than 60% fulfill El Escorial Criteria for definite or probable ALS and the average duration between symptom onset and diagnosis is approximately 1 year [4–6].
The pathophysiology of ALS is not understood. Even whether neurons or glial cells are the primary site of pathogenesis is the subject of debate. Current theories about pathogenesis center primarily on abnormal protein aggregation (e.g., neurofilament heavy chain aggregates, superoxide dismutase 1 (SOD1) aggregates and the inclusion of cystatin C in Bunina bodies), abnormal RNA processing (evidenced by the presence of causative mutations in genes for RNA-binding proteins, such as transactive response DNA-binding protein 43 [TDP-43] and fused in sarcoma/translated in liposarcoma), abnormal mitochondrial function, glutamate excitotoxicity and neuroinflammation [7]. Thus, candidate drugs targeting these mechanisms have received the most attention from trialists.
A history of challenges & innovation in ALS clinical research
Until the late 1980s and early 1990s, very litle was known about the etiology of ALS. As a result, few clinical trials for ALS were conducted. However, in the early 1990s a series of events led to an increase in the number of ALS clinical trials. The SOD1 gene encoding Cu/Zn superoxide dismutase became the first known gene causing familial ALS (FALS), riluzole gained US FDA approval for treatment of ALS, the first transgenic ALS animal models were developed and gains were made in supportive care for those with ALS. These breakthroughs led to optimism in the scientific community and fostered the development of regional clinical research networks.
Gene discovery
In 1991, linkage analysis localized a FALS gene to the long arm of chromosome 21 [8], and subsequently identified it to be in the SOD1 gene [9]. Numerous distinct SOD1 mutations have been described – substitution of alanine for valine at position 4 (SOD1A4V) is the most common in the USA and is associated with a rapid disease progression [10].
Since the discovery of the SOD1 gene mutation, multiple causative mutations have been identified [11–23], and the pace of these discoveries continues to increase due to the introduction of powerful new techniques for gene discovery. However, the vast majority of ALS cases (>90%) are still sporadic, without known genetic contribution.
Animal models
In 1994, the first transgenic mouse model of ALS was reported [24]. Initial investigations of humans and transgenic mice carrying SOD1 mutations suggested a reduction in SOD1 quantity in the cerebrospinal fluid (CSF) and activity in erythrocytes [25–27]. Subsequent investigations revealed an ALS phenotype in transgenic mice with an elevation in SOD1 concentration [24] and normal SOD1 enzymatic activity [28], while SOD1-deficient mice did not develop an ALS phenotype [29]. An effort to treat humans and transgenic sheep expressing the mutant SOD1 gene with recombinant wild-type SOD1 via intrathecal infusion failed to show disease improvement despite marked elevations in SOD1 [30]. This evidence suggests that SOD1 mutations confer a toxic gain-of-function, rather than a loss-of-function, fitting with the predominantly autosomal dominant inheritance pattern of SOD1 FALS. Potential treatments for ALS are often screened in the G93A mouse model prior to human trials.
However, the disconnect between the frequent, yet small, successes in transgenic animal models and the negative human trial results is a topic of much debate. The G93A mouse has been shown to produce mutant human SOD1 mRNA at concentrations many times higher than wild type mouse SOD1 [31], which has led to hypotheses that overexpression could be an important driver of the difference between responses in mouse models and humans. This overexpression may confer different response to treatment in the mouse models than in humans. Furthermore, the presymptomatic span in rodents is very short, and once symptomatic the transgenic mice rapidly progress to paralysis and death. The early onset and rapidity of the decline may also explain the difference in response between mice and humans. Most patients have sporadic ALS, the pathophysiology of which may differ in critical ways from that of FALS. Even FALS due to different mutations may respond differently to potential therapies. Thus, the wisdom of screening potential therapies in the SOD1 model has come under scientific scrutiny and new screening models are needed.
Influential clinical trials
Riluzole trials
Riluzole is the only FDA-approved treatment for ALS. In two separate trials, at 12 or 18 months, the risk of tracheostomy or death was significantly decreased [32,33], although there was no effect on strength, vital capacity (VC) or functional scales. Subsequently, riluzole was shown to extend survival in SOD1G93A transgenic mouse models by 10–15 days [34].
Other important early trials
Ciliary neurotrophic factor, brain-derived neurotrophic factor and IGF were each tested in large clinical trials in the mid 1990s [35]. None of these trials were positive, but they led to the validation of a number of clinical end points. The most influential has been the ALS functional rating scale (ALSFRS), developed by the ciliary neurotrophic factor Study Group [36].
Lithium trials
In 2008, it was reported that treatment with lithium reduced spinal motor neuron loss, delayed symptom onset, prolonged survival after disease onset, conferred a 36% overall survival benefit in SOD1G93A transgenic mice and, in a small trial in humans, improved survival, slowed VC loss and preserved strength over 15 months [37]. Many ALS patients were eager to be treated with lithium and clinicians were likely to oblige, creating a challenging environment for further trials of lithium.
Two trials used innovative designs to study lithium for ALS quickly, especially given the off-label availability of lithium. First, a Northeast ALS (NEALS) Consortium trial guaranteed participants open-label treatment at the end of the study and employed a time-to-event design to speed the study [38]. Terminal study event was defined as a ALSFRS-revised (R) decrease of 6-points. Interim analyses with futility stopping rules were preplanned. The trial enrolled quickly and was stopped for futility at the first interim analysis. Thus, an innovative design overcame a specific challenge (recruitment) and provided important data. The time-to-event analysis sped enrollment at the expense of a decreased statistical power and ability to detect only large changes in the ALSFRS-R rate of decline.
Second, the Western ALS (WALS) Study Group performed an open-label, historical placebo, Phase II trial of lithium for ALS [39]. The placebo arm subjects from a prior trial of minocycline [40] acted as historical controls and were well matched. ALSFRS-R scores declined more rapidly in those receiving lithium. Eliminating the placebo arm sped enrollment and provided power to detect small changes, albeit at the expense of true randomization. The use of control patients from a prior clinical trial holds promise.
Challenges in ALS clinical trials
Challenges in ALS research include disease rarity and heterogeneity, a lack of pathophysiological understanding and the absence of reliable biomarkers. As discussed, animal models are a frequently used tool, but their use has not translated to success in humans.
Disease rarity
Amyotrophic lateral sclerosis is a rare and fatal disease, and even in multicenter trials, patient enrollment can be slow. Multidisciplinary ALS clinics confer survival and quality-of-life benefits [41–44] and may facilitate patient enrollment in trials. In future, telemedicine may allow immobile patients to participate in multidisciplinary clinics for care and research participation. For now, however, recruitment remains a looming obstacle for all clinical trials.
Disease heterogeneity
The rapidity of progression varies widely in ALS. Average survival is approximately 3 years [45], but some patients progress rapidly, succumbing in less than a 1 year, whilst others survive for more than a decade [46]. Survival is longer in patients with a longer delay between symptom onset and diagnosis and in younger patients, and is shorter in patients with bulbar onset of symptoms [46,47]. El Escorial Criteria stage at the time of diagnosis is inconsistently correlated with rate of disease progression [46,48]. No specific markers of disease onset or progression have been reproducibly identified.
Studies of homogeneous subpopulations within a disease can increase statistical power and decrease trial duration. Unfortunately, clinically evident subgroups are either not predictive of disease course or are not obvious until late in the disease. Causative mutations can confer reliable phenotypes; for example, rapid progression is a feature of the SOD1 A4V mutation and lower motor neuron predominance is a hallmark of fused in sarcoma (FUS) mutations. However, these mutations are rare, and different mutations in a single gene may have dissimilar clinical features [49]. Thus, genotyping has not been used to characterize subgroups for study, although some trialists have begun to collect and store DNA for future sequencing and subgroup analysis.
Lack of biomarkers
The NIH defines a biomarker as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes or pharmacologic responses to a therapeutic intervention’ [50]. There are ongoing biomarker discovery efforts [51], and such markers could hasten study entry or serve as surrogate outcome measures in clinical trials. In the absence of such markers, trialists have depended upon more traditional outcome measures discussed below, leading to the need for large, prolonged and expensive efficacy trials.
Outcome measures
Effective ALS clinical trial design depends upon the appropriate choice of outcome measure (Table 1). Over a decade of work by ALS clinical trialists has defined a useful set of outcome measures that are the current mainstays of ALS clinical trial design. At present, the FDA requires that the primary outcome of a registration trial for a medication, one used to support the approval of a medication for a given indication, must provide evidence of clinically meaningful change, such as survival or functional measures. The European Agency for the Evaluation of Medicinal Products provides more specific guidance on the use of end points in clinical trials, suggesting the use of two of the following three items as primary end points: survival, disability (including ALSFRS-R) and strength [52].
Table 1.
Commonly used outcome measures in amyotrophic lateral sclerosis clinical trials: descriptions, benefits and drawbacks, and surrogate outcome measures in development.
| Outcome measure |
Description | Benefits | Drawbacks |
|---|---|---|---|
| Part 1. Outcome measures currently used in ALS clinical trials | |||
| Tracheostomy-free survival | Percentage of patients surviving without the need for a tracheostomy | Clinically relevant, evaluates true effect of therapy | Increases trial duration and cost, no information about quality of life or disability |
| ALSFRS-R | Captures many important features of disease progression, focusing on disability caused by ALS | Widely accepted, reproducible, easily administered | Subjective, statistical handling can be complicated |
| VC | Decline in maximum VC of patients as disease progresses | Declines with ALS progression, clinically relevant, related to survival, easily followed in clinic | Site of onset affects when VC declines, bulbar weakness causes inaccuracy |
| Ashworth Spasticity scale | Measure of spasticity by examiner for a series of individual muscles | Easily performed, many muscles can be tested, evaluates an otherwise overlooked aspect of ALS | Subjective, clinical applicability unclear, must decide which muscles to test, summary score may not reflect individual muscle scores |
| HHD | Quantitative means of assessing reduction in muscle strength | Objectively measured, portable, reproducible | Relies on examiner resistance, requires rigorous training, relies upon patient effort |
| Part 2. Novel outcome measures in development & validation | |||
| EIM | Transdermal probes evaluate the electrical properties of muscle tissue by applying high-frequency, low-intensity electrical stimulation and nearby recording to derive a measure of muscle impedence | Highly reproducible, requires little training, shows promise as a proxy for a clinically relevant end point, evaluates the final common pathway of the disease, potential to become hand-held | Questions about implementation and specificity in human disease remain, must decide which muscles to test, summary score may not reflect individual muscle scores, costs |
| ATLIS | Quantitative strength test of 12 muscle groups in limbs, using fixed, wireless load cell | Eliminates need for examiner counterforce, detailed force curves, reproducible | Maintains some dependence on patient effort, still expensive |
ALS: Amyotrophic lateral sclerosis; ALSFRS-R: ALS functional rating scale – revised; ATLIS: Accurate test of limb isometric strength; EIM: Electrical impedence myography; HHD: Hand-held dynamometry; VC: Vital capacity.
One of the most universally accepted outcome measures is tracheostomy-free survival. Since the early/mid-1990s, common surrogate measures have included VC [53], the ALSFRS-R [54–57] and quantitative measures of strength testing such as hand-held dynamometry [58]. Secondary outcome measures such as the Modified Ashworth Spasticity Scale and numerous measures of cognitive ability and quality-of-life questionnaires are sometimes used to supplement primary outcome measures.
Each of these outcome measures has strengths, but none is perfect [53]. Tracheostomy-free survival is undeniably clinically relevant. At the same time, it increases trial duration and cost, and provides no information about quality of life or disability. It can be confounded by the nonuniform application of life-extending interventions, such as noninvasive ventilation, feeding tube, riluzole therapy, and participation in a multidisciplinary ALS clinic. Successful randomization and blinding are necessary to overcome these potential confounders.
VC declines with ALS progression, is clinically relevant, related to survival and can be easily followed in the clinic. Many trials have used a pre-determined decline in forced VC as the primary outcome measure. However, VC decline can be a late feature in patients with limb-onset disease and is known to be affected early in patients with bulbar-onset ALS [58]. Furthermore, patients with prominent bulbar symptoms often have difficulty performing these tests due to facial weakness, leading to inaccuracies in measurement.
ALSFRS-R has become one of the most widely accepted measures of ALS progression. It captures many clinically relevant features of disease progression, focuses on disability caused by ALS, has been shown to be reproducible and is easily administered. However, despite all its potential benefits, it remains a subjective score. In addition, statistical handling of mortality in conjunction with ALSFRS-R has proven stubbornly complicated.
Hand-held dynamometry is a quantitative means to assess reduction in muscle strength, the primary effect of ALS. It is objectively measured, easily transported and reproducible. However, the test is subject to error introduced by examiner technique; thus, extensive outcome training is required to ensure intra- and inter-rater reliability, particularly when it is used as an outcome measure in multicenter studies. Furthermore, like other quantitative measures of strength, its results can vary based on patient effort.
Innovations & new directions in ALS clinical research
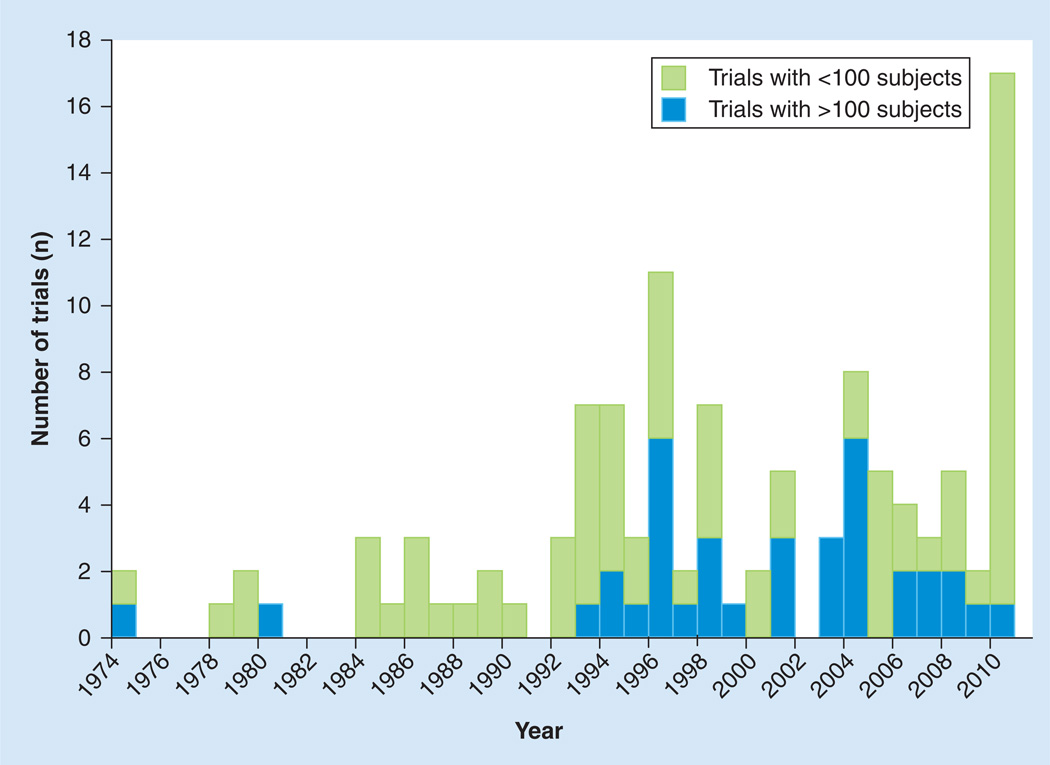
In 1979, Lasagna described numerous challenges in general clinical trial design [59]. These included a failure to publish negative results, recruitment and retention issues, statistical handling of subjects lost-to-follow-up, the use of inherently flawed outcome measures, a poor understanding of these limitations and the relative lack of clinical relevance of trial outcomes. Over 30 years later, these challenges in clinical research remain. However, in the field of ALS, new tools now allow clinical researchers to successfully plot a course through these once unnavigable waters. ALS clinical research, once typified by setbacks and obstacles, is now characterized by increasingly rapid basic and translational scientific discoveries, flourishing biomarker discovery projects, growing biorepositories, innovative clinical trial designs and an international and collaborative research community. These developments have led to a recent resurgence in the number of ALS clinical trials (Figure 1).
Figure 1.
Number of published amyotrophic lateral sclerosis trials by year.
Preclinical tools
Genetic discovery
Since the discovery of the gene, causative mutations in a host of other genes have been reported, including TARDBP [18], FUS/TLS [17,22], ALS2 [14], DCTN1 [13], ANG, VAPB [16], SPG11 [20], OPTN [19], FIG4 [21], CHMP2B, SETX [15], VCP [11], UBQLN22 [23] and C9ORF72 [60,61] (Table 2). New causative mutations are being described with increasing frequency owing to powerful new techniques such as whole exome capture. However, limitations in our knowledge of the genetics of ALS are still evident. The list of known causative genes is not exhaustive and the pathophysiology linking any given mutation to the ALS phenotype remains uncertain.
Table 2.
Causative or susceptibility genes for amyotrophic lateral sclerosis.
| Gene | Gene product | Normal function | Phenotype comments |
|---|---|---|---|
| Causative genes | |||
| SOD1 | Cu/Zn superoxide dismutase | Antioxidant | A4V mutation is rapidly progressive and most common in the USA |
| TARDBP | Transactive response DNA-binding protein 43 | DNA and RNA binding protein, associated with Drosha complex | |
| FUS/TLS | Fused in sarcoma/translated in liposarcoma protein | DNA and RNA binding protein, associated with Drosha complex | Described in some juvenile-onset cases |
| ALS2 | Alsin | Unknown | Juvenile onset, AR, also causative of PLS/HSP phenotype |
| DCTN1 | Dynactin subunit 1 | Intracellular transport (links dynein and kinesin to transport vesicles) | Lower motor neuron predominant |
| ANG | Angiogenin | Stimulates blood vessel growth, hydrolyzes tRNA decreasing mRNA translation | |
| VAPB | Vessicle associated membrane protein – associated proteins B and C | Membrane-bound protein, likely involved in vesicle transport | Described in a Portuguese population |
| SPG11 | Spatacsin | Unknown, possible role in vesicle trafficking | Juvenile onset |
| OPTN | Optineurin | Possible role in vesicle trafficking and apoptosis, role in innate immunity | |
| FIG4 | PI(3,5)P(2)5-phosphatase | Unknown, presumably affects signaling pathways | Upper motor neuron predominant, also causative of CMT IV |
| CHMP2B | Charged multivesicular body protein 2b | Component of ESCRT-III complex, involved in endosome invagination | Lower motor neuron predominant; associated with FTD in French population |
| VCP | Valosin-containing protein | Cell survival, cell division, DNA repair | Causative of IBMPFD |
| SETX | Senataxin | Probably a DNA helicase | Juvenile onset, slowly progressive |
| UBQLN2 | Ubiquilin-2 | Ubiquitin-like protein involved in chaperoning ubiquitinated proteins for degredation | Juvenile and adult, X-linked dominant, ALS ± dementia |
| C9ORF72 | C9ORF72 protein | Unknown | Due to a GGGGCC hesanucleotide repeat expandion; causative of ALS, FTD and ALS–FTD |
| Susceptibility gene | |||
| ATXN2 | Ataxin-2 | Unknown, appears to interact with endoplasmic reticulum | Causative of SCA-2 when PolyQ repeats are >34, risk factor for ALS if >23 and <34 |
| PON-1 | Paraoxonase | An arylesterase that detoxifies organophosphates | Possible susceptibility gene |
ALS: Amyotrophic lateral sclerosis; AR: Autosomal recessive; CMT IV: Charcot–Marie–Tooth disease Type 4; ESCRT-III: Endosomal sorting required for transport; FTD: Frontotemporal dementia; IBMPFD: Inclusion body myopathy associated with Paget’s disease of bone ± frontotemporal dementia; PLS/HSP: Primarily lateral sclerosis/hereditary spastic paraparesis; SCA-2: Spinocerebellar atrophy type 2.
If the field of ALS genetics is young, then epigenetic research in ALS is in its infancy. In 2010, intermediate-length polyglutamine repeat expansion in the ataxin-2 gene was shown to confer increased risk of ALS in patients with TARDMP mutations, thus making it the first disease-modifying gene characterized in ALS [62]. The search for genetic mutations that confer disease risk or susceptibility to an environmental toxin has also begun. Numerous studies of the paroxonase genes (lipid oxidation and organophosphate detoxification) have suggested a role of polymorphisms in ALS susceptibility [63–66], although others have failed to demonstrate this association [67–69], and still others have concluded that some polymorphisms in these genes represent causative ALS mutations [70].
Investigation into gene expression and the role of miRNA processing is similarly nascent, but is gaining interest. Both TDP-43- and FUS-encoded proteins associate with the Drosha complex, which is required to process primary miRNA transcripts [71]. The Dicer1 enzyme provides further miRNA processing, and Dicer1-deficient mice have been shown to develop spinal motor neuron degeneration, suggesting a role of miRNA in this process [72]. However, since miRNAs often mediate multiple RNA pathways, these results require further investigation. If the culprit miRNAs are identified, then preclinical and clinical research can focus on a means to alter the miRNA machinery.
Animal models
The number of animal models has grown alongside the number of ALS gene mutations. Current transgenic animals can be broadly categorized into models of protein misfolding, intermediate filament disorganization, microtubule transport disruption and a newer category of RNA processing disruption in the form of TDP-43 mice [73,74]. Thus far, however, no model has been as robust or widely applied for preclinical testing as the SOD1G93A model.
Preclinical stem cell models
The process for transforming human embryonic stem cells into motor neurons for study is well-established and numerous protocols now exist [75]. In vitro investigations have revealed a susceptibility of embryonic stem cell-derived motor neurons to factors secreted by cocultured glia from transgenic mice expressing SOD1 gene mutations, giving some insight into noncell autonomous factors in the disease [76].
Induced pluripotent stem (iPS) cells can be created from fibroblasts isolated from human skin biopsies [77], a technology that is being increasingly applied in the study of ALS. This approach to stem cell procurement eliminates some ethical debate surrounding the use of embryonic stem cells, but also allows for the derivation of stem-cells from ALS patients. iPS cells, like embryonic stem cells, can be differentiated into motor neurons for use as a tool in the laboratory [75,78]. iPS-derived motor neurons from patients with ALS are currently being created. As a preclinical disease model, they could be instrumental in both the understanding of ALS pathophysiology and high-throughput candidate drug screening that might complement, or supplant, mouse model screening.
Biomarkers & surrogate outcomes
A great deal of work has gone into developing novel surrogate outcome measures and biomarkers. Useful biomarkers might be found in biofluids (abnormal protein configuration or concentration, altered small molecule concentration or dysregulated RNA), imaging studies (MRI or MR spectroscopy), quantitative strength testing or new electrodiagnostic techniques.
Neuroimaging
Patients with ALS can show mild global brain atrophy and hyperintensity in the corticospinal tracts on T2-weighted MRI of the brain; however, this finding is neither sensitive nor specific enough to be useful diagnostically [79]. In fact, traditional MRI of the brain and spinal cord is currently only used in the diagnostic workup of ALS to exclude disease mimics. Newer MRI techniques, including diffusion tensor imaging, task-based functional MRI (fMRI), resting state–fMRI and magnetic resonance spectroscopy (MRS) are being increasingly studied in ALS. Previously published clinico-pathological studies had demonstrated the focal onset and spread of the disease in the CNS, thus, these studies did not reveal previously undiscovered dimensions of the disease [80,81]. However, because MRI technology is widely available, non-invasive and can be repeated, MRI-based biomarkers are attractive for use in clinical trials.
Diffusion tensor imaging is, perhaps, the best characterized novel MRI technique, and has the potential to facilitate diagnosis and disease monitoring; however, confirmatory studies are necessary before the technique can be reliably implemented [82,83]. Task-based fMRI has shown increased activation of the contralateral supplemental motor area, basal ganglia and cerebellum, but is not sensitive enough to confer diagnostic benefit [82]. Resting state fMRI use has been explored preliminarily in ALS, and in combination with tractography techniques such as diffusion tensor imaging, may identify changes in brain region connectivity in ALS [84]. Relaxation rate of the corticospinal tracts and deep grey matter have been used to show tract disruption and iron accumulation, suggesting a potential role of iron-induced oxidative damage [85]. Proton MRS has been used to show a reduction in cortical N-acetyl aspartate, a neuronal marker [82].
Biofluid & tissue biomarkers
Biofluid and tissue biomarker development in ALS has focused primarily on proteomics (the study of the protein complement in a cell or organism) [86–89], metabolomics (the study of the complement of small molecules present in a cell or organism reflecting the metabolic state of the organism) [90], and gene expression analysis (the study of the type and amount of RNA transcripts in a cell or organism) [91].
Biomarkers of ALS may be most effectively identified from the CSF because of its proximity to the motor neurons and limited protein content, which is well-characterized in healthy control subjects [92,93]. Cross-sectional analysis will help identify diagnostic biomarkers, but CSF markers of disease severity must be identified through serial sampling of biofluids and intra-subject analyses.
To this end, the Northeast ALS consortium has an ongoing study aimed at collecting, cataloging, and storing samples of serum, plasma, DNA and CSF. Serum and plasma are being collected serially and collection of serial CSF samples is just beginning.
Novel quantitative strength & electrodiagnostic biomarkers
Accurate test of limb isometric strength
The accurate test of limb isometric strength is a device under development to test 12 muscle groups in the limbs using a fixed, wireless load cell, which does not require the examiner to provide counterforce [94–96]. The accurate test of limb isometric strength testing generates force curves whose shape provides information about patient effort. The device remains in development, but confirmatory testing is planned and could provide a detailed functional outcome measure for clinical trials.
Electrical impedance myography
Electrical impedance myography is a technique for investigating the electrical properties of a localized area of muscle to evaluate the muscle tissue health. A high-frequency, low-intensity electrical stimulation is applied transdermally and recorded by a nearby electrode to derive muscle impedance, which changes as muscle health declines [97]. It is highly reproducible and requires little training to perform [98]. In the SOD1G93A rat model, electrical impedance myography phase slope correlated strongly with survival and motor unit number estimation [99].
Human trial designs
As the basic and preclinical science discoveries in ALS have improved, ALS clinical trialists have implemented an increasing number of innovations to find ways to maximize trial efficiency and power, investigate potential new therapies and implement new assessment tools.
The evolving understanding of ALS genetics and pathophysiology, and an improving preclinical research tool set has led to a growing pipeline of potential therapies for ALS. Some of these potential therapies come from repurposing an FDA-approved medication, while others are novel compounds. Traditionally prescribed rigorous, but dogmatic, testing follows three clinical phases: Phase I trials are safety trials. Phase II trials are somewhat larger and provide further safety information and dose-selection. Large Phase III trials evaluate efficacy. The stalwart performance of the three-phase paradigm is admirable but lacks efficiency. Between each phase, the trial must be halted, databases locked, data cleaned and analyzed and results presented and published. As opportunities to move promising compounds from the laboratory bench into clinical research emerge, clinical researchers have begun to apply novel trial designs to improve efficiency. Unique trends have emerged in each of the trial phases [100].
Early phase trials
Phase I trials evaluate the safety and tolerability of a study drug. Phase II trials extend safety testing and allow dose-ranging and selection. Most trial sponsors also include secondary outcomes aimed at evaluating efficacy in Phase II designs, but the trials lack statistical power to do so. This creates a conundrum for ALS clinical trialists – full evaluation of a promising preclinical drug requires Phase I–III testing, yet limited resources exist for testing many promising therapies. The development of new preclinical tools, biomarkers and surrogate outcome measures might reduce trial duration. Innovative designs can streamline trials, preserve safety and help trialists quickly evaluate more candidate drugs.
Phase I trials evaluate safety and provide information about human pharmacokinetics. Safety testing is usually accomplished by establishing the maximum tolerated dose (MTD) in humans, but when reactions are irreversible or cannot be monitored Phase I trials might instead test predesignated doses, define the drug half-life or assess for the minimum effective dose [101]. Phase I trials can be performed in healthy volunteers, but most Phase I trials in ALS are performed in patients. The ethical considerations are complex [102]. To improve efficiency, some ALS Phase I trials now allow subjects to enroll in multiple dosage cohorts. For some novel therapies, investigators or regulatory agencies choose to include a placebo group in Phase I trials, although there is no strong statistical argument for doing so.
Traditional designs use a fixed dose-escalation scheme to reach MTD. Once a predetermined number of patients has tolerated a given dose, the dose is increased for the next group. The continual reassessment model (CRM), applies mathematical modeling and incorporates real-time data to modify dose escalation [103,104]. If more toxicity is encountered, the dose escalation is slowed or stopped. This method has been shown to improve trial efficiency and MTD estimation accuracy [103,104]. However, CRM is statistically complicated and can lead to rapid dose escalation, which has hampered its use [105]. Most CRMs in current use include rules that modify the underlying model to prevent very rapid dosage escalation. Given the potential benefits, CRM designs hold promise for Phase I ALS trials.
Phase II designs fill numerous roles. For investigators they provide dose ranging to identify a dosage level for a Phase III trial. For patients, they may be seen as providing access to investigational therapy, for regulatory bodies they provide additional safety data prior to launching a Phase III trial, and finally, for sponsors, these trials may provide preliminary hope of efficacy. Both selection and futility designs aim to improve Phase II trial efficiency.
Futility trials are designed to abandon large-scale efficacy testing if a given threshold for benefit is not met. The design may lead investigators to erroneously designate treatments with small benefits as futile (type II error). In light of this, it is less frequently employed by pharmaceutical company sponsors. However, academic investigators have used futility designs to evaluate potential therapies whose efficacy has been suggested. One example is co-enzyme Q10 for ALS [106]. A preliminary dose-ranging stage was followed by a futility design to quickly assess the promise of a larger trial. Ultimately, the drug failed to surpass futility thresholds and further trials were abandoned [107].
Phase II selection design trials run multiple different drugs, or multiple drug/dosing combinations against one another with the goal of carrying the most promising one forward to a Phase III trial. Selection design trials eliminate placebo arms and test candidate drugs against one another. They do not assess whether the selected treatment is beneficial relative to placebo, but a secondary non-inferiority test against placebo or historical control can partially address this issue. However, without a true placebo control, data from selection design trials cannot be used to infer treatment benefit, only to select a candidate drug for further study.
The first selection design study in ALS compared minocycline 100 mg + creatine 10 g twice daily to celecoxib 400 mg + creatine 10 g twice daily for ALS and included a non-inferiority test against historical controls [108,109]. The trial demonstrated a more favorable response to celecoxib–creatine and non-inferiority to placebo using a pool of just 60 participants. As understanding of ALS grows and the number of candidate drugs for its treatment proliferates, the benefits of selection design trials become increasingly important and may grow in popularity.
Phase III trials
For a decade, Phase III trials have embraced well-established surrogate outcomes such as ALSFRS-R or VC in place of, or more commonly in addition to, tracheostomy-free survival. Surrogate outcomes, for the purposes of Phase III trials, must be easily measured, reliable, valid and clinically relevant. Trialists are eager to apply such surrogate outcomes in clinical trials when they become available. Collaboration among trial centers and multinational clinical trials are increasingly being used to speed up Phase III trial enrollment.
The ALSFRS-R, now a trusted surrogate outcome for trialists and regulatory bodies, can be a challenge for statistical analysis. Slopes of ALSFRS-R decline can be compared using linear mixed effects modeling, but this method does not take death into account. The development of the ALSFRS-R Joint Rank [110] has allowed for the simultaneous analysis of survival and ALSFRS-R decline in a combined end point. The Joint Rank statistic ranks study participants in each treatment group, first by survival and then by ALSFRS-R score. The Joint Rank can increase power relative to analysis of either ALSFRS-R or survival analysis alone in some circumstances, for example when mortality rates are high. It has preliminarily been accepted by oversight committees as an acceptable outcome measure for Phase III registration trials.
There is now a Center for Disease Control (CDC)-funded National ALS Registry, which could become a critical resource for trial recruitment and epidemiologic studies in ALS, but patient confidentiality concerns will have to be addressed first. Disease-specific networks, such as the Great Lakes ALS Group, WALS Study Group, the Canadian ALS Consortium, NEALS Consortium and European ALS Consortium have mobilized ALS clinical trialists, prepared centers to participate in multicenter trials, provided support for investigators designing multi-center trials and improved multicenter trial efficiency tremendously. Even more centralized networks using federated (centralized) institutional review board processes and standard contracts for all sites could provide additional efficiency. In 2010, dextromethorphan/quinidine was approved under the brand name Nuedexta® for the treatment of pseudobul-bar affect due to ALS or MS [111,112]. Clinical trials of the medication employed the use of ALS trial networks to perform these trials efficiently.
In addition to improving design to add efficiency to ALS clinical trials, trialists are now focusing on international opportunities to increase the number of potential centers. With proper site training, this approach has already met with significant success in trials and the trend is expanding.
The traditional placebo-controlled trial design continues to be critical for Phase III clinical trials, to enable determination of efficacy and safety. It is also the only type of Phase III trial currently accepted by regulatory agencies. Some trialists routinely use 2:1 randomization schemes to entice potential participants to enroll. Others use traditional 1:1 designs, but include an open-phase extension at the end of the trial to allow all participants an opportunity to receive the study drug and thus encourage enrollment.
Novel interventions
Gene therapies
Genetic discoveries in ALS have provided potential opportunities to explore gene therapy in humans in the form of antisense oligonucleotides (ASOs) and RNA-silencing (small interfering RNA). Both reduce gene expression in a targeted fashion through Watson–Crick base-pair binding to a specific mRNA and have shown promise in preclinical research [113]. Gene therapy using siRNA has shown mixed results in ALS [114,115], but is plagued by two major challenges; delivery and off-target effects. First, the short half-life and modest cellular uptake have led researchers to use various viral vectors to transport the siRNA to cells. However, viral vectors may themselves carry risk. Second, off-target effects are common, perhaps because siRNA disrupts cellular mechanisms used to process naturally occurring miRNA. ASOs do not cross the blood–brain barrier, so must be delivered to the CNS directly, but cause few side effects and, for now, appear more promising as a potential gene therapy for ALS. The relative lack of experience with gene therapy in humans has led oversight organizations to take a conservative approach in most cases, which has tended to slow clinical trial progress.
Cell therapies
The use of stem cells as a preclinical model has been addressed, but stem cell transplantation into the spinal cord or motor cortex might also be used as a therapy. Transplanted stem cells might provide neurotrophic support to motor neurons and slow ALS progression. Motor neuron replacement is a more complex and distant goal of stem cell therapy [116]. Other authors argue that cell-replacement therapy will not reverse the inflammatory changes or decrease in autophagy seen in ALS [117]. For now, the first Phase I trial of human embryonic stem cell transplantation in patients with ALS has begun (trial description in ‘Examples of innovative ongoing clinical trials’) and additional groups have begun to explore stem cell transplantation in ALS patients. In the near future, safety concerns will likely mean that meticulously designed and implemented trials with a strong focus on safety evaluation will take precedent over efficiency in the field of stem cell transplantation for ALS.
Examples of innovative ongoing clinical trials
Never before have such a number of promising clinical trials for ALS been underway at the same time. Trials of drugs aimed at a number of putative pathologic processes are being tested. Each of these selected ongoing trials represents a step forward in ALS clinical trials by implementing a novel design concept or tool.
Safety & efficacy study of creatine & tamoxifen in volunteers with ALS: an example of selection design
This trial compared creatine (30 g/day) to two dosages of tamoxifen (40 mg and 80 mg twice daily). The selection design was used to rapidly select the most promising of two potential ALS therapies for further testing.
A pilot study published in 2010 investigated the effect of creatine on brain chemistry using MRS and revealed that at 5 and 10 g twice daily (doses tested in prior ALS trials [118]), no changes were seen on MRS [119]. At 15 g twice daily, the MRS revealed increased creatine (8%; p = 0.06) and decreased glutamate and glutamine levels (−17%; p = 0.039). This provided a small, unpublished study suggesting that tamoxifen can slow disease in a dose-dependent fashion.
Ceftriaxone for the treatment of ALS: an example of multistage sequential design
Progression of ALS may be due, at least in part, to glutamate excitotoxicity, and in preclinical trials ceftriaxone upregulated EAAT2, the glial glutamate transporter responsible for clearing glutamate from the synaptic cleft [120,121]. On these strengths, a clinical trial of ceftriaxone for the treatment of ALS was designed and is ongoing. In order to hasten the study, while preserving its power to detect efficacy and monitor safety, a multistage, nonstop, sequential design was adopted. The total study length from first enrolled subject was planned to be approximately 53 months. In stages 1 and 2, 60 subjects were to be enrolled, with 8 months of enrollment for stage 1 followed by 20 weeks of follow-up in stage 2. These initial 60 subjects would continue into stage 3. Stage 3 would include an additional 540 subjects, for a total of 600 subjects at up to 50 sites in the USA and Canada [201].
Safety, tolerability & activity study of ISIS SOD1Rx to treat FALS caused by SOD1 gene mutations: an example of gene therapy for ALS
As noted above, the mechanism by which mutant SOD1 injures motor neurons is unknown, but preclinical models have demonstrated a dose–response relationship of mutant SOD1 protein to disease severity [122]. Thus, decreasing levels of the mutant protein with a drug such as ISIS 333611 (Isis Pharmaceuticals) may be therapeutic. ISIS 333611 is a modified ASO to SOD1. Currently, a Phase I trial to examine the safety and tolerability of a single 12 h intrathecal infusion of ISIS 333611 is underway. The trial utilizes a dose-escalation design with four dosage level cohorts, each consisting of eight subjects, two of whom receive placebo. This trial is making important inroads in designing trials to test gene therapy in ALS. Given the rarity of the patient population, study subjects have been permitted to participate in more than one dosecohort, providing that they meet eligibility criteria at the time of re-enrollment and that at least five half-lives of the study drug have passed, to ensure complete washout. This design feature offers increased trial efficiency in a study that draws from a small patient population [202].
Study of NP001 in subjects with ALS: an example of immune modulation for the treatment of ALS
NP001 (Neuraltus Pharmaceuticals) is a small-molecule drug aimed at altering macrophage activation patterns with the goal of reducing inflammatory activation in the CNS postulated to perpetuate the disease. A single-dose, Phase I trial of NP001 was conducted in 32 ALS participants in 2010, and a Phase II trial is now underway. Randomization is 1:1:1 placebo, low-dose and high-dose study drug. Study drug is administered in monthly infusion cycles. The trial of NP001 marks an important investigation into the role of neuroinflammation in ALS and the ability to alter this phenomenon to impact disease course. This type of innovative approach to the treatment of a well-described disease is too seldom investigated, given the significant investment required for a clinical trial [203].
Human spinal cord-derived neural stem cell transplantation for the treatment of ALS: an example of stem cell therapy for ALS
The first human trial of stem cell therapy in the USA is underway (Neuralstem Inc.). It is an open-label Phase I trial of human spinal stem cells (HSSC) engineered from the spinal cord of a single fetus and injected into the spinal cord using a proprietary device. The primary outcome of the trial is safety and tolerability. Five cohorts will be enrolled. The first has been completed and consisted of six participants with advanced ALS who were nonambulatory. Three received unilateral lumbar cord injections and the next three bilateral injections. The following cohorts will have three ambulatory participants each and will involve injections in the lumbar spine (cohorts 2 and 3), cervical spine (cohort 4), and cervical and lumbar spine (cohort 5). Investigators enrolled participants with late-stage disease first and performed lumbar injections before cervical to minimize risk [204].
Future perspective
The landscape of clinical research in ALS is now flush with clinical trials. Preclinical tools that are just coming into existence, such as iPS-derived motor neurons, new transgenic animals and novel genetic sequencing tools, have the potential to facilitate high-throughput screening of candidate drugs. Once identified, imaging and/or biofluid biomarkers could become indispensible tools in clinical research.
New and innovative study designs will continue to increase efficiency of clinical trials in ALS. Trials of lithium, dexpramipexole and ceftriaxone for ALS incorporate nonstandard designs and are leading a wave of novel trial designs that will continue to evolve and improve clinical trial efficiency. The creation of clinical trial networks will be extended to national and international networks with central IRB, contracts and study coordination resources to reduce study startup time. Finally, clinical trialists will incorporate telemedicine and create forums for better patient communication. Social networking resources may become important tools for trial publicity, including subject recruitment and retention.
Executive summary.
-
▪
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease primarily characterized by loss of motor neurons and progressive weakness.
-
▪
Survival averages 3–5 years after symptom onset, although there is a wide variability.
-
▪
Approximately 10% of ALS is familial (FALS) and the known complement of causative genes is expanding.
-
▪
Riluzole, the only US FDA-approved disease-modifying agent, has a modest effect.
-
▪
Dextromethorphan/quinidine (Neudexta®) has been approved for pseudobulbar affect, this is the first FDA-approved treatment specifically for an ALS symptom.
-
▪
Many theories of pathogenesis exist, although no unifying theory has been proven.
A brief history of clinical research in ALS
-
▪
In 1991, linkage analysis localized some FALS mutations to the long arm of chromosome 21, and shortly after mutations in the SOD1 gene encoding Cu/Zn superoxide dismutase were implicated.
-
▪
The SOD1 G93A transgenic mouse has become the most commonly used preclinical model.
-
▪
The El Escorial Criteria and ALS functional rating scale were developed by trialists in the 1990s.
Challenges in ALS clinical trials
-
▪
Disease rarity and heterogeneity, lack of knowledge of pathogenesis and absence of biomarkers are challenges for ALS clinical trialists.
Recent innovations & new directions in ALS clinical research
-
▪
Preclinical tools, such as stem cell disease models, new genetic discovery tools and new animal models, are improving our understanding of the disease, identifying new drug targets and will improve trial efficacy by identifying promising candidate drugs for human trials.
-
▪
Biomarker discovery efforts have been stepped up in an effort to identify reliable markers of diagnosis and progression for use in clinical trials.
-
▪
Early phase trials may begin to employ Bayesian dose-escalation schemes and efficient designs such as futility and selection designs to hasten early drug development.
-
▪
Phase III trials are building on traditional designs with novel statistical analysis, use of trial networks and the use of international multicenter trial designs to reach meaningful conclusions quickly.
-
▪
Novel gene and stem cell therapies for the treatment of ALS hold tremendous promise and are beginning to be tested in clinical trials.
Future perspective
-
▪
Improvement of preclinical tools, expansion of genetic knowledge, introduction of biomarkers, development of gene and cell therapy techniques, and clinical trial design innovation will create opportunities to better investigate potential therapies for ALS.
-
▪
The eventual introduction of new disease-modifying therapies for ALS will dramatically alter the research landscape in the field of motor neuron disease by energizing the research community, increasing prevalence of the disease, increasing our understanding of pathophysiology, and creating a new set of ethical considerations for patients and researchers.
-
▪
Patient-centered research will facilitate larger contributions from research participants and patient communities, an often overlooked resource.
Acknowledgments
J Berry is supported by an NIH training grant (T32NS048005) and Muscular Dystrophy Association Clinical Research Training Grant. M Cudkowicz has worked as a consultant for Shire, Trophos, Cytokinetics and GlaxoSmithKline. She has received industry-sponsored grants from Neuralstem Inc., ISIS Pharmaceuticals Inc., Knopp Biosciences and Biogen Idec. She has also received funding from NIH grants: UO1 NS049640, UO1 NS052592, RC1 NS069395 and RC1 NS068179.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Worms PM. The epidemiology of motor neuron diseases. a review of recent studies. J. Neurol. Sci. 2001;191(1–2):3–9. doi: 10.1016/s0022-510x(01)00630-x. [DOI] [PubMed] [Google Scholar]
- 2.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118(Pt 3):707–719. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- 3.Brooks BR, Miller RG, Swash M, et al. El Escorial revisited. revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 4.Traynor BJ, Codd MB, Corr B, et al. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria. A population-based study. Arch. Neurol. 2000;57(8):1171–1176. doi: 10.1001/archneur.57.8.1171. [DOI] [PubMed] [Google Scholar]
- 5.De Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008;119(3):497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- 6.Zoccolella S, Beghi E, Palagano G, et al. Signs and symptoms at diagnosis of amyotrophic lateral sclerosis: a population-based study in southern Italy. Eur. J. Neurol. 2006;13(7):789–792. doi: 10.1111/j.1468-1331.2006.01384.x. [DOI] [PubMed] [Google Scholar]
- 7.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddique T, Figlewicz DA, Pericak-Vance MA, et al. Linkage of a gene causing familial amyotrophic lateral sclerosis to chromosome 21 and evidence of genetic-locus heterogeneity. N. Engl. J. Med. 1991;324(20):1381–1384. doi: 10.1056/NEJM199105163242001. [DOI] [PubMed] [Google Scholar]
- 9.Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364(6435):362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- 10.Cudkowicz ME, Mckenna-Yasek D, Sapp PE, et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann. Neurol. 1997;41(2):210–221. doi: 10.1002/ana.410410212. [DOI] [PubMed] [Google Scholar]
- 11. Johnson JO, Mandrioli J, Benatar M, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68(5):857–864. doi: 10.1016/j.neuron.2010.11.036. ▪▪ This study is the first to use whole exome capture to identify a novel gene in amyotrophic lateral sclerosis (ALS). Exome sequencing is a more efficient way to identify causative genes and may lead to more rapid gene discovery.
- 12.Rosen D, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 13.Puls I, Jonnakuty C, Lamonte B, et al. Mutant dynactin in motor neuron disease. Nat. Genet. 2003;33(4):455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Hentati A, Deng H, et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat. Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Bennet C, Huynh H, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis. (ALS4) Am. J. Hum. Genet. 2004;74:1128–1135. doi: 10.1086/421054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura A, Mitne-Neto M, Silva H, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vance C, Rogelj B, Hortobagyi T, et al. Mutations, in, FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Deerlin VM, Leverenz JB, Bekris LM, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7(5):409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruyama H, Morino H, Ito H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465(7295):223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 20.Orlacchio A, Babalini C, Borreca A, et al. SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain. 2010;133(Pt 2):591–598. doi: 10.1093/brain/awp325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow CY, Landers JE, Bergren SK, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 2009;84(1):85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 23.Deng HX, Chen W, Hong ST, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477(7363):211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 25.Deng HX, Hentati A, Tainer JA, et al. Amyotrophic lateral sclerosis and structural defects in Cu/Zn superoxide dismutase. Science. 1993;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 26.Bracco F, Scarpa M, Rigo A, Battistin L. Determination of superoxide dismutase activity by the polarographic method of catalytic currents in the cerebrospinal fluid of aging brain and neurologic degenerative diseases. Proc. Soc. Exp. Biol. Med. 1991;196(1):36–41. doi: 10.3181/00379727-196-43160. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki Y, Ikeda K, Kinoshita M. Decreased cerebrospinal-fluid superoxide dismutase in amyotrophic lateral sclerosis. Lancet. 1993;342(8879):1118. doi: 10.1016/0140-6736(93)92104-2. [DOI] [PubMed] [Google Scholar]
- 28.Wong PC, Pardo CA, Borchelt DR, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14(6):1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 29.Reaume AG, Elliott JL, Hoffman EK, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13(1):43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 30.Cudkowicz ME, Warren L, Francis JW, et al. Intrathecal administration of recombinant human superoxide dismutase 1 in amyotrophic lateral sclerosis: a preliminary safety and pharmacokinetic study. Neurology. 1997;49(1):213–222. doi: 10.1212/wnl.49.1.213. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson PA, Graffmo KS, Andersen PM, et al. Disulphide-reduced superoxide dismutasedismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129(Pt 2):451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 32.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994;330(9):585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 33.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 34.Gurney ME, Fleck TJ, Himes CS, Hall ED. Riluzole preserves motor function in a transgenic model of familial amyotrophic lateral sclerosis. Neurology. 1998;50(1):62–66. doi: 10.1212/wnl.50.1.62. [DOI] [PubMed] [Google Scholar]
- 35.Turner MR, Parton MJ, Leigh PN. Clinical trials in ALS: an overview. Semin. Neurol. 2001;21(2):167–175. doi: 10.1055/s-2001-15262. [DOI] [PubMed] [Google Scholar]
- 36.The Amyotrophic Lateral Sclerosis Functional Rating Scale. Assessment of activities of daily living in patients with amyotrophic lateral sclerosis. The ALS CNTF treatment study (ACTS) Phase I-II Study Group. Arch. Neurol. 1996;53(2):141–147. [PubMed] [Google Scholar]
- 37.Fornai F, Longone P, Cafaro L, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA. 2008;105(6):2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aggarwal SP, Zinman L, Simpson E, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(5):481–488. doi: 10.1016/S1474-4422(10)70068-5. ▪▪ The two Phase II trials of lithium for the treatment of ALS [38,40] both used novel trial designs to investigate the effect of lithium in the setting of a promising Phase I trial, patient demand for the drug, and the possibility of off-label prescription.
- 39.Miller RG, Moore DH, Forshew DA, et al. Phase II screening trial of lithium carbonate in amyotrophic lateral sclerosis: examining a more efficient trial design. Neurology. 2011;77(10):973–979. doi: 10.1212/WNL.0b013e31822dc7a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a Phase III randomised trial. Lancet Neurol. 2007;6(12):1045–1053. doi: 10.1016/S1474-4422(07)70270-3. ▪▪ The two Phase II trials of lithium for the treatment of ALS [38,40] both used novel trial designs to investigate the effect of lithium in the setting of a promising Phase I trial, patient demand for the drug, and the possibility of off-label prescription.
- 41.Traynor BJ, Alexander M, Corr B, Frost E, Hardiman O. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996–2000. J. Neurol. Neurosurg. Psychiatry. 2003;74(9):1258–1261. doi: 10.1136/jnnp.74.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Den Berg JP, Kalmijn S, Lindeman E, et al. Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology. 2005;65(8):1264–1267. doi: 10.1212/01.wnl.0000180717.29273.12. [DOI] [PubMed] [Google Scholar]
- 43.Chio A, Bottacchi E, Buffa C, Mutani R, Mora G. Positive effects of tertiary centres for amyotrophic lateral sclerosis on outcome and use of hospital facilities. J. Neurol. Neurosurg. Psychiatry. 2006;77(8):948–950. doi: 10.1136/jnnp.2005.083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez De Rivera FJ, Oreja Guevara C, Sanz Gallego I, et al. Outcome of patients with amyotrophic lateral sclerosis attending a multidisciplinary care unit. Neurologia. 2011;26(8):455–460. doi: 10.1016/j.nrl.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344(22):1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 46.Turner MR, Parton MJ, Shaw CE, Leigh PN, Al-Chalabi A. Prolonged survival in motor neuron disease: a descriptive study of the King’s database 1990–2002. J. Neurol. Neurosurg. Psychiatry. 2003;74(7):995–997. doi: 10.1136/jnnp.74.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wijesekera LC, Mathers S, Talman P, et al. Natural history and clinical features of the flail arm and flail leg ALS variants. Neurology. 2009;72(12):1087–1094. doi: 10.1212/01.wnl.0000345041.83406.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zoccolella S, Beghi E, Palagano G, et al. Predictors of long survival in amyotrophic lateral sclerosis: a population-based study. J. Neurol. Sci. 2008;268(1–2):28–32. doi: 10.1016/j.jns.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Ratovitski T, Corson LB, Strain J, et al. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Hum. Mol. Genet. 1999;8(8):1451–1460. doi: 10.1093/hmg/8.8.1451. [DOI] [PubMed] [Google Scholar]
- 50.Biomarkers Definitions Working Group. Biomarkers and surrogate end points. preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 51. Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8(1):94–109. doi: 10.1016/S1474-4422(08)70293-X. ▪▪ Contains a comprehensive review of candidate biofluid and imaging biomarkers in ALS.
- 52.Traynor BJ, Zhang H, Shefner JM, Schoenfeld D, Cudkowicz ME. Functional outcome measures as clinical trial end points in ALS. Neurology. 2004;63(10):1933–1935. doi: 10.1212/01.wnl.0000144345.49510.4e. [DOI] [PubMed] [Google Scholar]
- 53.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J. Neurol. Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 54.Kasarskis EJ, Dempsey-Hall L, Thompson MM, Luu LC, Mendiondo M, Kryscio R. Rating the severity of ALS by caregivers over the telephone using the ALSFRS-R. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2005;6(1):50–54. doi: 10.1080/14660820510027107. [DOI] [PubMed] [Google Scholar]
- 55.Montes J, Levy G, Albert S, et al. Development and evaluation of a self-administered version of the ALSFRS-R. Neurology. 2006;67(7):1294–1296. doi: 10.1212/01.wnl.0000238505.22066.fc. [DOI] [PubMed] [Google Scholar]
- 56.Beck M, Giess R, Wurffel W, Magnus T, Ochs G, Toyka KV. Comparison of maximal voluntary isometric contraction and Drachman’s hand-held dynamometry in evaluating patients with amyotrophic lateral sclerosis. Muscle Nerve. 1999;22(9):1265–1270. doi: 10.1002/(sici)1097-4598(199909)22:9<1265::aid-mus15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 57.Similowski T, Attali V, Bensimon G, et al. Diaphragmatic dysfunction and dyspnoea in amyotrophic lateral sclerosis. Eur. Respir. J. 2000;15(2):332–337. doi: 10.1034/j.1399-3003.2000.15b19.x. [DOI] [PubMed] [Google Scholar]
- 58.The European Agency for the Evaluation of Medicinal Products. Points to Consider on Clinical Investigation of Medicinal Products for the Treatment of Amyotrophic Lateral Sclerosis (ALS). CPMP/EWP/565/98, 19. 2000 Oct [Google Scholar]
- 59.Lasagna L. Problems in publication of clinical trial methodology. Clin. Pharmacol. Ther. 1979;25(5 Pt 2):751–753. doi: 10.1002/cpt1979255part2751. [DOI] [PubMed] [Google Scholar]
- 60.Dejesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Explanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-Linked FTD and ALS. Neuron. 2011 doi: 10.1016/j.neuron.2011.09.011. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion of C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011 doi: 10.1016/j.neuron.2011.09.010. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elden AC, Kim HJ, Hart MP, et al. AtaxinAtaxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466(7310):1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diekstra FP, Beleza-Meireles A, Leigh NP, et al. Interaction between PON1 and population density in amyotrophic lateral sclerosis. Neuroreport. 2009;20(2):186–190. doi: 10.1097/WNR.0b013e32831af220. [DOI] [PubMed] [Google Scholar]
- 64.Greenberg DA, Stewart WC, Rowland LP. Paraoxonase genes and susceptibility to ALS. Neurology. 2009;73(1):11–12. doi: 10.1212/WNL.0b013e3181aa2a39. [DOI] [PubMed] [Google Scholar]
- 65.Landers JE, Shi L, Cho TJ, et al. A common haplotype within the PON1 promoter region is associated with sporadic ALS. Amyotroph. Lateral Scler. 2008;9(5):306–314. doi: 10.1080/17482960802233177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valdmanis PN, Kabashi E, Dyck A, et al. Association of paraoxonase gene cluster polymorphisms with ALS in France, Quebec, and Sweden. Neurology. 2008;71(7):514–520. doi: 10.1212/01.wnl.0000324997.21272.0c. [DOI] [PubMed] [Google Scholar]
- 67.Ricci C, Battistini S, Cozzi L, et al. Lack of association of PON polymorphisms with sporadic ALS in an Italian population. Neurobiol. Aging. 2011;32(3):552, e557–e513. doi: 10.1016/j.neurobiolaging.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 68.Wills AM, Cronin S, Slowik A, et al. A large-scale international meta-analysis of paraoxonase gene polymorphisms in sporadic ALS. Neurology. 2009;73(1):16–24. doi: 10.1212/WNL.0b013e3181a18674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wills AM, Landers JE, Zhang H, et al. Paraoxonase 1 (PON1) organophosphate hydrolysis is not reduced in ALS. Neurology. 2008;70(12):929–934. doi: 10.1212/01.wnl.0000305956.37931.dd. [DOI] [PubMed] [Google Scholar]
- 70.Ticozzi N, Leclerc AL, Keagle PJ, et al. Paraoxonase gene mutations in amyotrophic lateral sclerosis. Ann. Neurol. 2010;68(1):102–107. doi: 10.1002/ana.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gregory RI, Yan KP, Amuthan G, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432(7014):235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 72.Haramati S, Chapnik E, Sztainberg Y, et al. miRNA malfunction causes spinal motor neuron disease. Proc. Natl. Acad. Sci. USA. 2010;107(29):13111–13116. doi: 10.1073/pnas.1006151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Julien JP, Kriz J. Transgenic mouse models of amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2006;1762(11–12):1013–1024. doi: 10.1016/j.bbadis.2006.03.006. ▪ Provides an overview of the existing mouse models, of, ALS, their relationship to putative disease mechanisms and benefits and drawbacks of each model.
- 74.Swarup V, Julien JP. ALS pathogenesis: recent insights from genetics and mouse models. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(2):363–369. doi: 10.1016/j.pnpbp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Nizzardo M, Simone C, Falcone M, et al. Human motor neuron generation from embryonic stem cells and induced pluripotent stem cells. Cell Mol. Life Sci. 2010;67(22):3837–3847. doi: 10.1007/s00018-010-0463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3(6):637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 78.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 79.Filippi M, Agosta F, Abrahams S, et al. EFNS guidelines on the use of neuroimaging in the management of motor neuron diseases. Eur. J. Neurol. 2010;17(4):e526–e520. doi: 10.1111/j.1468-1331.2010.02951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ravits J, Laurie P, Fan Y, Moore DH. Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007;68(19):1576–1582. doi: 10.1212/01.wnl.0000261045.57095.56. [DOI] [PubMed] [Google Scholar]
- 81.Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68(19):1571–1575. doi: 10.1212/01.wnl.0000260965.20021.47. [DOI] [PubMed] [Google Scholar]
- 82.Agosta F, Chio A, Cosottini M, et al. The present and the future of neuroimaging in amyotrophic lateral sclerosis. AJNR. Am. J Neuroradiol. 2010;31(10):1769–1777. doi: 10.3174/ajnr.A2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner MR, Modo M. Advances in the application of MRI to amyotrophic lateral sclerosis. Expert Opin Med. Diagn. 2010;4(6):483–496. doi: 10.1517/17530059.2010.536836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, Munte TF. Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp. Neurol. 2009;217(1):147–153. doi: 10.1016/j.expneurol.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 85.Langkammer C, Enzinger C, Quasthoff S, et al. Mapping of iron deposition in conjunction with assessment of nerve fiber tract integrity in amyotrophic lateral sclerosis. J. Magn. Reson. Imaging. 2010;31(6):1339–1345. doi: 10.1002/jmri.22185. [DOI] [PubMed] [Google Scholar]
- 86.Pasinetti GM, Ungar LH, Lange DJ, et al. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66(8):1218–1222. doi: 10.1212/01.wnl.0000203129.82104.07. [DOI] [PubMed] [Google Scholar]
- 87.Bowser R, Lacomis D. Applying proteomics to the diagnosis and treatment of ALS and related diseases. Muscle Nerve. 2009;40(5):753–762. doi: 10.1002/mus.21488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brettschneider J, Lehmensiek V, Mogel H, et al. Proteome analysis reveals candidate markers of disease progression in amyotrophic lateral sclerosis (ALS) Neurosci. Lett. 2010;468(1):23–27. doi: 10.1016/j.neulet.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 89.Ranganathan S, Williams E, Ganchev P, et al. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J. Neurochem. 2005;95(5):1461–1471. doi: 10.1111/j.1471-4159.2005.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rozen S, Cudkowicz ME, Bogdanov M, et al. Metabolomic analysis and signatures in motor neuron disease. Metabolomics. 2005;1(2):101–108. doi: 10.1007/s11306-005-4810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henkel JS, Beers DR, Wen S, Bowser R, Appel SH. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology. 2009;72(18):1614–1616. doi: 10.1212/WNL.0b013e3181a41228. [DOI] [PubMed] [Google Scholar]
- 92.Sussmuth SD, Brettschneider J, Ludolph AC, Tumani H. Biochemical markers in CSF of ALS patients. Curr. Med. Chem. 2008;15(18):1788–1801. doi: 10.2174/092986708785133031. [DOI] [PubMed] [Google Scholar]
- 93.Schutzer SE, Liu T, Natelson BH, et al. Establishing the proteome of normal human cerebrospinal fluid. PLoS One. 2010;5(6):e10980. doi: 10.1371/journal.pone.0010980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andres PL, Munsat TL, Thornell BJ, Cudkowicz ME. A new device to measure disease progression in ALS clinical trials. Amyotroph. Lateral Scler. 2007;8(S1):138. [Google Scholar]
- 95.Andres PL. Minimizing sample size requirements in ALS clinical trials. Neurology. 2008;70(S1) [Google Scholar]
- 96.Andres PL, Skerry L, Cudkowicz M. Validation of a new device to measure disease progression in ALS. Amyotroph. Lateral Scler. 2010;11(S1):23. [Google Scholar]
- 97. Rutkove SB. Electrical impedance myography: background, current state, and future directions. Muscle Nerve. 2009;40(6):936–946. doi: 10.1002/mus.21362. ▪ Provides an overview of electrical impedence myography, a promising electrodiagnostic biomarker that might be used as an end point for ALS clinical trials in the future.
- 98.Ahad MA, Rutkove SB. Electrical impedance myography at 50kHz in the rat: technique, reproducibility, and the effects of sciatic injury and recovery. Clin. Neurophysiol. 2009;120(8):1534–1538. doi: 10.1016/j.clinph.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang LL, Spieker AJ, Li J, Rutkove SB. Electrical impedance myography for monitoring motor neuron loss in the SOD1 G93A amyotrophic lateral sclerosis rat. Clin. Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.04.021. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schoenfeld DA, Cudkowicz M. Design of Phase II ALS clinical trials. Amyotroph. Lateral Scler. 2008;9(1):16–23. doi: 10.1080/17482960701875896. [DOI] [PubMed] [Google Scholar]
- 101.Resche-Rigon M, Zohar S, Chevret S. Adaptive designs for dose-finding in non-cancer Phase II trials: influence of early unexpected outcomes. Clin. Trials. 2008;5(6):595–606. doi: 10.1177/1740774508098788. [DOI] [PubMed] [Google Scholar]
- 102.Anderson JA, Kimmelman J. Extending clinical equipoise to Phase I trials involving patients: unresolved problems. Kennedy Inst. Ethics J. 2010;20(1):75–98. doi: 10.1353/ken.0.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sulmasy DP, Astrow AB, He MK, et al. The culture of faith and hope: patients’ justifications for their high estimations of expected therapeutic benefit when enrolling in early phase oncology trials. Cancer. 2010;116(15):3702–3711. doi: 10.1002/cncr.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Iasonos A, Wilton AS, Riedel ER, Seshan VE, Spriggs DR. A comprehensive comparison of the continual reassessment method to the standard 3 + 3 dose escalation scheme in Phase I dose-finding studies. Clin. Trials. 2008;5(5):465–477. doi: 10.1177/1740774508096474. ▪▪ Provides a useful review of traditional and Bayesian methods for Phase I dose-escalation trials.
- 105.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for Phase I clinical trials in cancer. Biometrics. 1990;46(1):33–48. [PubMed] [Google Scholar]
- 106.Levy G, Kaufmann P, Buchsbaum R, et al. A two-stage design for a Phase II clinical trial of coenzyme Q10 in ALS. Neurology. 2006;66(5):660–663. doi: 10.1212/01.wnl.0000201182.60750.66. [DOI] [PubMed] [Google Scholar]
- 107. Kaufmann P, Thompson JL, Levy G, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify Phase III. Ann. Neurol. 2009;66(2):235–244. doi: 10.1002/ana.21743. ▪ This Phase II trial of Coenzyme Q uses a futility design to evaluate the promise of coenzyme Q for further study.
- 108.Cheung YK, Gordon PH, Levin B. Selecting promising ALS therapies in clinical trials. Neurology. 2006;67(10):1748–1751. doi: 10.1212/01.wnl.0000244464.73221.13. [DOI] [PubMed] [Google Scholar]
- 109.Gordon PH, Cheung YK, Levin B, et al. A novel, efficient, randomized selection trial comparing combinations of drug therapy for ALS. Amyotroph. Lateral Scler. 2008;9(4):212–222. doi: 10.1080/17482960802195632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat. Med. 1999;18(11):1341–1354. doi: 10.1002/(sici)1097-0258(19990615)18:11<1341::aid-sim129>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 111.Pioro EP, Brooks BR, Cummings J, et al. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann. Neurol. 2010;68(5):693–702. doi: 10.1002/ana.22093. [DOI] [PubMed] [Google Scholar]
- 112.Brooks BR, Thisted RA, Appel SH, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology. 2004;63(8):1364–1370. doi: 10.1212/01.wnl.0000142042.50528.2f. [DOI] [PubMed] [Google Scholar]
- 113.Miller TM, Smith RA, Kordasiewicz H, Kaspar BK. Gene-targeted therapies for the central nervous system. Arch. Neurol. 2008;65(4):447–451. doi: 10.1001/archneur.65.4.nnr70007. [DOI] [PubMed] [Google Scholar]
- 114.Miller TM, Kaspar BK, Kops GJ, et al. Virus-delivered small RNA silencing sustains strength in amyotrophic lateral sclerosis. Ann. Neurol. 2005;57(5):773–776. doi: 10.1002/ana.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Towne C, Raoul C, Schneider BL, Aebischer P. Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol. Ther. 2008;16(6):1018–1025. doi: 10.1038/mt.2008.73. [DOI] [PubMed] [Google Scholar]
- 116.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders – time for clinical translation? J. Clin. Invest. 2010;120(1):29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fornai F, Meininger V, Silani V. Future therapeutical strategies dictated by preclinical evidence in ALS. Arch. Ital. Biol. 2011;149(1):169–174. doi: 10.4449/aib.v149i1.1269. [DOI] [PubMed] [Google Scholar]
- 118.Pastula DM, Moore DH, Bedlack RS. Creatine for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2010;(6) doi: 10.1002/14651858.CD005225.pub2. CD005225. [DOI] [PubMed] [Google Scholar]
- 119. Atassi N, Ratai EM, Greenblatt DJ, et al. A Phase, I, pharmacokinetic, dosage escalation study of creatine monohydrate in subjects with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010;11(6):508–513. doi: 10.3109/17482961003797130. ▪ The first study to use a pharmacodynamic CNS imaging biomarker in a Phase I study for ALS.
- 120.Heemskerk J. Screening existing drugs for neurodegeneration: The National Institute of Neurologic Disorders and Stroke (NINDS) model. Retina. 2005;25(8 Suppl.):S56–S57. doi: 10.1097/00006982-200512001-00025. [DOI] [PubMed] [Google Scholar]
- 121.Rothstein JD, Patel S, Regan MR, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 122.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Ann. Rev. Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
Websites
- 201.ClinicalTrials.gov Identifier: NCT00349622. www.clinicaltrials.gov/ct2/show/NCT00349622?term=NCT00349622&no_unk=Y&rank=1.
- 202.ClinicalTrials.gov Identifier: NCT01041222. www.clinicaltrials.gov/ct2/show/NCT01041222?term=NCT01041222&rank=1.
- 203.ClinicalTrials.gov Identifier: NCT01281631. www.clinicaltrials.gov/ct2/show/NCT01281631?term=NCT01281631&rank=1.
- 204.ClinicalTrials.gov Identifier: NCT01348451. www.clinicaltrials.gov/ct2/results?term=NCT01348451.