INTRODUCTION
Older adults are the largest consumers of medication in the United States (US), largely due to an age-associated increase in chronic conditions.1 Because of this, multiple medication use (i.e., polypharmacy) is a common consequence of providing health care to older adults.2 Polypharmacy is of particular concern to older adults because they may have unique barriers and challenges to managing multi-drug regimens, including cognitive impairment, functional limitations, financial restraints, use of multiple health care providers, or transportation limitations, to name a few.2,3 In addition, one of the most significant potential consequences of polypharmacy could be its impact on medication adherence in the older adult.
Medication adherence can be defined as “the extent to which a person’s behavior (in this case, taking medication) corresponds with agreed recommendations from a health care provider.”4 Widely varying rates of nonadherence have been previously reported depending on the definition of nonadherence, the population studied, and the length of observation; yet, multiple reviews on medication adherence report that approximately 50% of older adults do not adhere to at least one of their chronic medications.4,5 Moreover, it is important to identify the specific type of nonadherence within the medication use process – nonfulfillment (or primary nonadherence), nonpersistence, or nonconforming.6 Primary nonadherence occurs when the provider prescribes a medication, but it is never filled by the patient. Nonpersistence is when the patient decides to stop taking a medication after starting it, without being advised by a health professional to do so. Furthermore, so-called ‘nonconforming’ nonadherence includes a variety of ways in which medications are not taken as prescribed (e.g., taking incorrect doses, skipping doses, or taking doses at incorrect times).3
Thus, it is clear that taking medication is a complex behavior that requires multiple successful steps on the part of patients (i.e., to fill, initiate, continue, and take the prescription as intended). Prescribers play important roles in the process as well, since they are ultimately responsible for prescribing the most appropriate medication and, along with other health care professionals, monitoring their use. Taken together, polypharmacy and medication adherence present a unique challenge for the older adult, their caregiver(s), and the healthcare team. In this review, we first discuss the various ways in which medication adherence is measured, and then we present a conceptual model illustrating the complex interplay between polypharmacy and medication (non)adherence in older adults, summarize key literature on the topic, highlight strategies for improvement, and conclude by describing areas of uncertainty and priorities for future research.
MEASUREMENT
One of the greatest challenges in the field of adherence research is the accurate measurement of this complex health behavior. Various methods have been described, including using self-reported surveys, pill counts, drug levels, physiologic measures (e.g., heart rate with β-blockers), pharmaceutical claims, electronic medication monitoring, and physician ordering in electronic health records. Each method has inherent limitations, and various iterations of each method have been used in previous studies, leading to substantial heterogeneity in the current evidence base.
It is commonly accepted that there is no ‘gold standard’ for measuring medication adherence. However, because medication adherence is a complex health behavior, it may be more prudent to focus on which specific aspects of medication adherence each measure is actually capturing. For example, pharmaceutical claims have been used frequently in recent years to study medication adherence across multiple conditions using large insurance-based data sources by evaluating medication refill patterns.7 This approach typically reports a proportion of days covered (PDC), a medication possession ratio (MPR), or a cumulative medication gap (CMG). That is, claims-based adherence studies are simply reporting on the rate of medication possession among patients and not the actual medication-taking itself. Moreover, these methods rely on at least two medication refills to be calculated, thus missing altogether the patient exhibiting primary nonadherence or nonpersistence after one refill.8 Of note, electronic health records that track physician ordering and patient refilling of chronic medications also offer an opportunity to capture these important and often undocumented cases of primary nonadherence or nonpersistence.
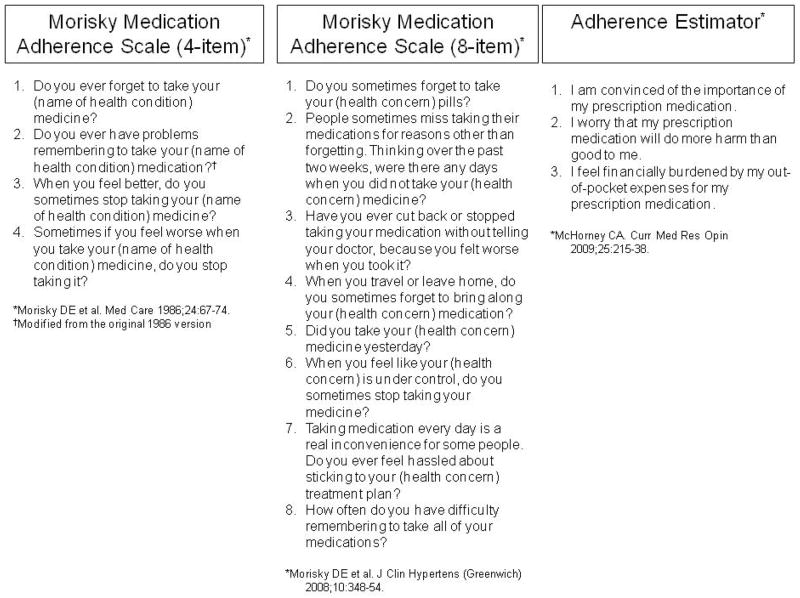
Self-report is another common method used to measure medication adherence in older adults. Several self-reported measures have been published (Figure 1), including the Morisky Medication Adherence Scales (MMAS; a 4-item and a newer 8-item measure), various cost-related measures, and the Adherence Estimator.9–12 The Adherence Estimator is a new 3-item measure that can be used to predict medication adherence in patients newly initiated on a chronic medication.11 Self-reported measures offer the convenience of low respondent burden and minimal cost, however, there is the potential for social desirability bias (i.e., patients may report what they think the clinician wants to hear and not their true medication-taking behavior). If prefaced appropriately in a non-accusatory manner, self-reported measures can help understand the reasons for nonadherence, which may better identify areas for immediate intervention compared to impersonal measures such as pharmacy claims.
Figure 1.
Finally, pill counts or electronic medication monitoring (e.g., medication event monitoring system [MEMS]) are common approaches used in clinical trials to provide objective and quantitative measures of medication adherence. MEMS devices are limited by their high cost, restricting their widespread use. Moreover, pill counts provide an estimate of medication adherence based on the number of pills in the vial (usually for a single medication) but do not ensure that the patient actually took the medication (e.g., in the case of a patient ‘pill dumping’ prior to an appointment).
When comparing the psychometric properties across the various methods for measurement of adherence, large amounts of heterogeneity are present in the literature. Grymonpre et al compared medication adherence calculated using pill count, self-report, and pharmacy claims data in a sample of community-dwelling older adults (≥65 years) and found that the pill count method underestimated medication adherence.13 A recent meta-analysis examining the correlation between MEMS and self-reported questionnaires reported that these two measurements tend to be at least moderately correlated.14 However, others have described the limitations of self-reported measures of medication adherence and called for attention to improving the development of such measures in future research.15 While recognizing the inherent limitations for each method, the most robust approach may be to use multiple measurements in order to capture a broader range of adherence information in older adults.
Each of the methods described above is commonly used in adherence research, but simple measures of adherence for use in the clinical setting are difficult to come by. Some clinical practices (as well as insurers and pharmacy benefit managers) have begun to provide claims-based measures of adherence to the clinician in an effort to identify non-adherent patients at the time of the visit. Others may be employing some of the self-reported measures in their clinic, although we are not aware of many examples of this approach currently in the literature. At the very least, all providers should be asking patients simple questions about any problems they may be having with their medications at each clinic visit.
CONCEPTUAL FRAMEWORK OF BARRIERS TO MEDICATION ADHERENCE
Extensive literature has been published on barriers to medication adherence in older adults.3,5,6,16 In turn, various conceptual models have been proposed to illustrate the complex relationship in older adults between patient, health-system, and provider factors and medication adherence.3,17,18 Yet, there is an apparent mismatch between these conceptual frameworks and the existing body of literature; for example, limited evidence is available assessing how the different barriers interact. Of importance to this review, polypharmacy is frequently studied as a potential barrier in adherence research, but it is unclear how polypharmacy affects (or is affected by) other barriers to either increase or decrease adherence.
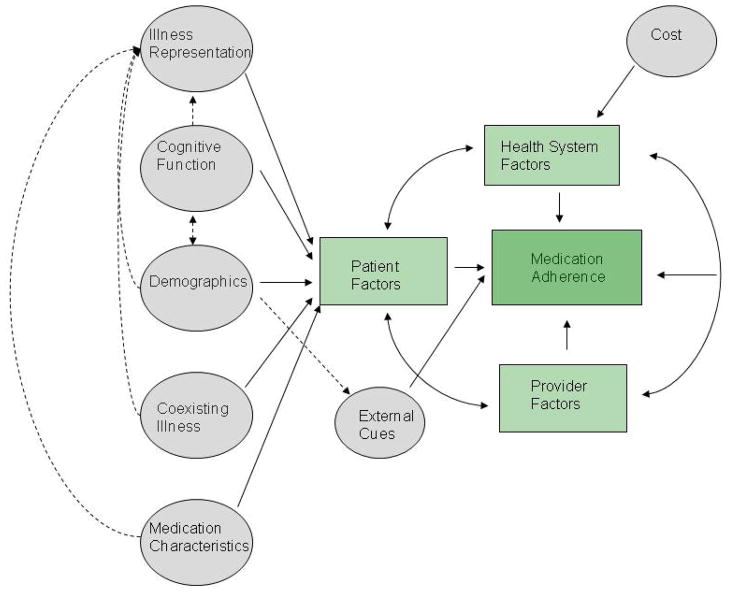
For the current review, we adapted a conceptual framework for medication adherence in older adults proposed by Gellad et al, (Figure 2) originally modeled from the framework proposed by Park and Jones.3,17 In the previous models, the complex interplay among patient, health-system, and provider factors are central to their explanatory value. Not only do those factors interact, but each individual patient-level factor also interacts with other patient factors. For example, the presence of multiple co-morbid conditions in a patient could affect the patient’s perceived need for certain medications – in this case, co-morbid conditions and perceived need for medications each affect adherence individually, but the interaction between the two may also affect adherence. In another example, cognitive function, another patient-level barrier to adherence, could affect the socioeconomic status of a patient (which in turn may be related to adherence), and it could also affect how patients perceive their need for medications.
Figure 2.
General Conceptual Model of Medication Adherence and the Interaction Between Patient Factors, from Gellad et al, 20093,17
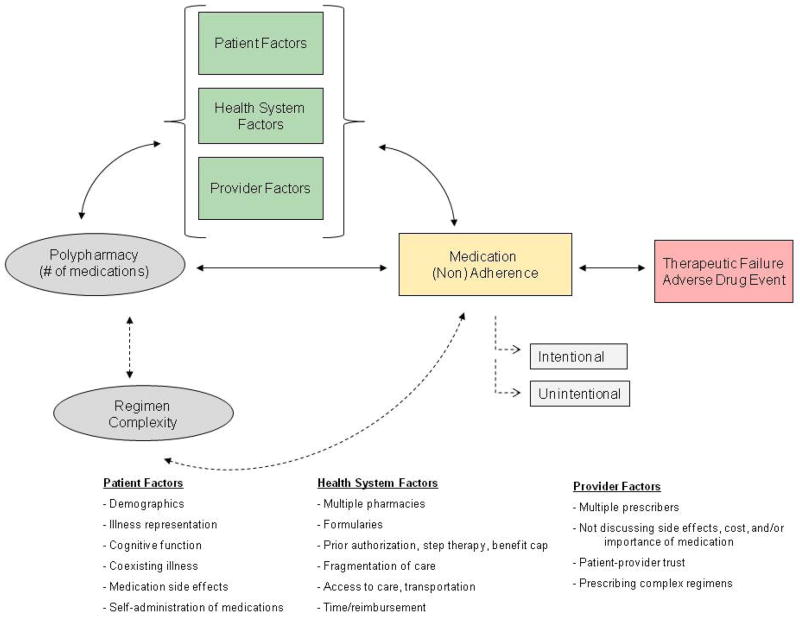
Our proposed model focuses on the potential pathways from one of the medication characteristics in the previous model – polypharmacy – to medication (non)adherence, potentially being mediated by various patient, health-system and/or provider factors (Figure 3). Polypharmacy can lead to medication nonadherence simply because of the greater number of medications that can be missed on a daily basis, yet polypharmacy can also lead to nonadherence through other mechanisms. Polypharmacy here is defined as the number of different medications a patient is prescribed and taking; this is distinct from the concept of regimen complexity, which encompasses the number of daily doses for a medication, the presence of non-oral routes of administration, and the need for specific dosing instructions (e.g., warfarin, bisphosphonates). Regimen complexity is closely correlated with polypharmacy and has previously been shown to be associated with lower medication adherence.19 An additional important distinction highlighted in the model is the differentiation between intentional and unintentional medication nonadherence, which are two very different medication-taking behaviors. Distal health outcomes, including therapeutic failures and adverse drug events, are also included since they are significant consequences and/or causes of medication nonadherence.20
Figure 3.
Conceptual Model of the Effect of Polypharmacy onMedication Adherence
The following paragraphs on patient, health-system, and provider factors highlight potential mediators of the association between polypharmacy and medication nonadherence. These factors should not be considered in isolation; rather, they should be viewed as interacting with many other factors, and clinicians must evaluate each patient individually to assess their unique barriers as they change over time.
Patient Factors
Older adults may be influenced by various factors that could affect their medication-taking behavior, including demographic factors such as socioeconomic status and age, cognitive function, coexisting illness, illness representation, and medication side effects, to name a few. While these patient factors can affect medication adherence on their own, they can also both cause polypharmacy (e.g., coexisting illnesses leading to more medications prescribed) and mediate the relationship between polypharmacy and nonadherence (e.g., drug interactions and side effects are more likely when taking more medications). Patients’ representation of their own illness can potentially be affected by their pill burden, and that representation has previously been shown to affect adherence.17 In some cases the beliefs that patients have about the number of medications they are taking, rather than the actual number, may have a stronger association with their adherence. For example, in a study of women taking osteoporosis medications, agreeing with the statement that one was taking too many different medications was a more important predictor of very low adherence (MPR <20%) than the actual number of medications taken.21
Health-System Factors
The most common health-system barrier to medication adherence is cost.12,22 Cost is clearly more of a problem for those patients taking more medications, and thus polypharmacy may affect adherence through higher cost-sharing. In addition, polypharmacy could lead to filling at multiple pharmacies depending on the type of medication, the type of providers, and the patient’s insurance; older adults filling prescriptions at multiple pharmacies may face greater burden to adhering than those using a single pharmacy.23 Moreover, formulary substitutions, prior authorization, fragmentation of care, and ability to access care are all potential barriers to medication adherence for the older adult and are likely to be even more significant in those taking multiple medications.
Provider Factors
Providers may unknowingly contribute to medication nonadherence by not discussing potential medication side effects or the importance of the medication or simply by prescribing complex regimens (when more simplified options are available). Patients receiving specialized care are often required to visit multiple providers and subsequently encounter multiple prescribers who may not be in communication with each other, thus leading to unintended polypharmacy.
While we make note of the above barriers, the list is by no means completely inclusive. Perhaps the most important concept when it comes to identifying barriers to medication adherence and how polypharmacy may lead to problems with adherence is that each patient will be different, for each medication they take, and at different points in time. Simply put, understanding the potential barriers patients face does nothing more than prepare clinicians to better understand what the specific patient in front of them might be facing.
EVIDENCE
Having discussed the conceptual framework for understanding how polypharmacy may be associated with medication adherence, we now discuss the current available evidence on the topic. Numerous reviews have been published on medication adherence in older adults, and polypharmacy has been one of the commonly studied risk factors for nonadherence. Balkrishnan conducted a review from 1962 to 1997 examining predictors of medication adherence in the elderly and found mixed results for the association between the number of medications and adherence.16 We recently conducted a systematic review from 1998–2010 focusing on older adults in the United States and, similarly, found mixed results for the association between polypharmacy and medication adherence.6 This heterogeneity in the literature is consistent with other systematic reviews reporting on the relationship between polypharmacy and medication adherence.24
To build on the above reviews, we searched PubMed (through July 2011) and the reference lists of articles included in our prior review and found only 3 additional studies focusing on older adults examining polypharmacy and adherence. Thus, in total, we identified 9 articles for the purpose of this review (Table 1).23,25–32 Among these 9 articles, 6 reported a negative association between greater number of medications and medication adherence – that is, the presence of more medications was associated with worse adherence. Alternatively, 1 study reported a positive association between polypharmacy and medication adherence, and 2 studies found no significant association. Importantly, when only assessing those studies using a more rigorous design (i.e., excluding cross-sectional studies), 4 out of 5 studies reported that polypharmacy was associated with greater risk of nonadherence.
Table 1.
Studies Reporting on the Association between Polypharmacy and Medication Adherence in Older Adults in the United States, 1998–2011
| Citation | Study Design | Sample Description | Measure of Adherence | Polypharmacy Definition | Key Polypharmacy Findings |
|---|---|---|---|---|---|
| Chapman et al (2008) | Observational cohort | Adults aged ≥ 65 years who initiated treatment with both AH and LL therapy within a 90-day period | Medication possession ratio (cutoff 80%) | # of prescription medications | Adherence rate was decreased with taking more medications (AOR 0.43 for ≥ 6 medications vs 0–1 medication; 95% CI 0.36 to 0.50; P<0.001). |
| Choudhry et al (2011) | Observational cohort | Patients prescribed a statin or an ACE-inhibitor (2 separate cohorts) from a large pharmacy benefit manager | Proportion of days covered (also assessed therapeutic complexity) | # of prescription medications | Adherence was decreased with a greater number of medications in both cohorts (statin users: adjusted % change in adherence per additional medication, 0.89, P<0.001; ACE-inhibitor users: adjusted % change in adherence per additional medication, 0.69, P<0.001). |
| Gazmararian et al (2006) | Observational cohort | Adults aged ≥ 65 years with coronary heart disease, diabetes, hyper-lipidemia, and/or HTN | Cumulative medication gap less than 20% | # of oral prescription medications (≤ 3 vs >3) | Multivariate analysis showed that those who took more medications had a lower odds of having nonadherence compared to those taking less medication (AOR 0.77; 95%, CI 0.73 to 0.95) after controlling for health literacy, age, race, sex, and education. |
| Grant et al (2003) | Cross-sectional | Patients with diabetes (mean age 66 years) receiving primary care | Self-reported diabetes-related medication adherence | # of prescription medications | Adherence was not significantly associated with the number of diabetes- related medications. |
| Gray et al (2001) | Observational cohort | Adults aged ≥ 65 years receiving home health care following hospitalization for medical illness | Underadherence: at least one medication < 70%; Overadherence: at least one medication > 120% | # of prescription medications; Taking a drug ≥3 times/day | Multivariable analysis showed that underadherence was significantly associated with greater medication use (AOR 1.16, 95% CI 1.03 to 1.31) after controlling for demographic, health- related and medication-related covariates. |
| Ownby et al (2006) | Cross-sectional | Patients with memory disorders cared for at a memory disorders clinic | Caregivers’ reports of patients’ medication adherence | # of prescription medications | Adherence was not found to be associated with the number of medications. |
| Turner et al (2009) | Cross-sectional | Adults aged ≥ 70 years with HTN | Self-reported not missing any medication in the past 3 months | Antihypertensive regimen complexity ≥4 medications) | Adherence was significantly negatively 4 associated (less likely) with having ≥ 4 antihypertensive medications in regimen (AOR 0.23; 95% CI, 0.08 to 0.72). |
| Stoehr et al (2008) | Cross-sectional | Adults aged ≥ 65 years cared for in 7 private office practices | Global judgment by research nurses (ie, dichotomous outcome, yes/no) after a home visit | # of prescription medications (≥ 5 vs < 5); Dosing frequency(≥4 vs <4 times/day) | Adherence was negatively associated with a greater number of prescription medications (OR 0.45; 95% CI, 0.21 to 0.95; P=0.04). |
| van Bruggen et al (2009) | Cluster-RCT | Patients with diabetes (mean age ~67 years) receiving primary care (Netherlands) | Medication possession ratio (cutoff 80%) | # of prescription medications | Adherence to blood pressure lowering drugs was negatively associated with a greater number of prescription medications (AOR 0.84; 95% CI 0.78–91; P<0.0001), however, adherence was not significantly associated with the number of oral blood glucose or cholesterol lowering drugs. |
It is important to highlight that polypharmacy is often assessed along with multiple other covariates to determine their association with medication adherence in epidemiologic studies or during secondary analyses of previously completed trials. In other words, polypharmacy is rarely pre-specified as the primary independent variable for predicting medication adherence, making it difficult to determine the true association between polypharmacy and medication adherence.
Taken together, there appears to be an association between polypharmacy and poorer medication adherence. However, it is difficult to be certain from the literature because of the mixed results, the likely unmeasured confounding, limited study designs (e.g., cross sectional), small sample sizes, and limited generalizability. In addition, this uncertainty is surely a result of the complexity of measuring adherence – the interaction between polypharmacy and all the additional factors described above is impossible to capture in most studies, and the fact that adherence can vary for different medications within the same person at different times makes adherence an even more complicated behavior to quantify. While the effect of polypharmacy on medication adherence may not be completely clear in the literature, improving medication adherence to maximize the therapeutic benefit of pharmacotherapy remains a cornerstone of geriatric care. We discuss some strategies for improving adherence below.
STRATEGIES FOR IMPROVING ADHERENCE
Several interventions have been trialed to find effective solutions for this ongoing public health problem in older adults, with minimal success to-date.5,33,34 In general, multi-faceted interventions have been shown to have the most impact in elders (who are often receiving polypharmacy) to enhance medication adherence, making it difficult to disentangle the effective from the ineffective components.5 There is evidence from clinical trials of the effectiveness of decreasing regimen complexity in improving adherence33, and some evidence from well-conducted natural experiments of lowering cost-sharing to increase adherence (although these are not specific to older patients).35 There is also evidence that pharmacist interventions can improve medication adherence and related healthcare utilization, yet the effect of these interventions commonly dissipates upon discontinuation and their cost-effectiveness is unclear.36,37
So what can we do to improve medication adherence in older adults in light of limited evidence available? The following are some key strategies that are rooted in the literature:2, 38–40
Promote rational, conservative prescribing – a necessary but not sufficient step alone.38 One of the most important strategies for improving adherence is to first make sure patients are receiving the most appropriate medications. Evaluate the medication regimen to ensure that it is appropriate, including discontinuing and/or dose-adjusting any unnecessary medications/doses, decreasing regimen complexity, and minimizing cost-sharing. Begin new medications only after assuring oneself and the patient/caregiver that the benefits outweigh the risks.
Avoid the “prescribing cascade.”40 If a patient develops new symptoms after starting a medication (e.g., pedal edema after starting a calcium channel blocker), try changing the medication before adding another one to treat the symptoms.
-
Incorporate the measurement of medication adherence and individual-level risk factors for nonadherence into clinical practice.
With the uptake of health information technology, detecting medication adherence problems (at least problems with refilling medications and/or experiencing ADEs) may be more easily incorporated into the clinical encounter. Other screening tests may be available to detect different aspects of medication adherence (e.g., taking medications incorrectly). Always solicit information from the patient/caregiver, and incorporate a multidisciplinary approach when possible (using nurses and pharmacists) to monitor medication adherence over time.
Evaluate for potential barriers in the older adult. Knowing some of the more common barriers to adherence in older adults, ask about them during the clinical encounter, including (but not limited to): financial restraints (cost-sharing), use of multiple pharmacies/prescribers, complex medication regimens, cognitive impairment, dexterity limitations, swallowing difficulty, and hearing/visual impairment.
Take into account the patient and caregivers’ underlying beliefs about medication use. Attention should be given to understanding patient beliefs about the necessity of their medications. Only then can patient-specific medication adherence recommendations be made and medication regimen monitoring plans developed and implemented.
LESSONS FOR FUTURE RESEARCH
Moving forward, there are clear gaps in the literature that deserve attention, due to the considerable impact of medication nonadherence in a rapidly aging population. Based on an evaluation of published research and our own clinical experience, we offer some suggestions for future work in order to advance the study of medication adherence in older adults. First, the most evident cause of the heterogeneity in the literature is the lack of standardization of the measurement of medication adherence across studies, preventing pooling of results to better describe the effectiveness of interventions. While we acknowledge that no one measure can capture the entire universe of medication adherence behavior, consistent use of reliable and valid instruments across studies and over time in large cohorts of older adults would allow for more systematic results and implications. Many researchers are now using the standard definitions of adherence and persistence as outlined by Cramer et al.41 Along those lines, there is a great need to study cohorts of older adults’ medication-taking behavior over time in order to study all aspects of medication nonadherence within the same population (i.e., primary nonadherence, nonpersistence, and nonconforming).
Second, future studies should report on any differential impact of interventions on intentional vs. unintentional medication nonadherence. Interventions found to be effective for unintentional nonadherence (e.g., using electronic reminder devices) will likely not be appropriate for older adults showing intentional nonadherence who may have knowingly stopped a medication. Such patients may benefit from an intervention that allows for a timely line of communication with a healthcare professional in order to address their key barrier(s).
Third, researchers should take seriously the call to develop larger, more rigorous, clinical trials testing interventions to improve adherence as well as distal outcomes (e.g., ADEs and therapeutic failures).5 The Institute of Medicine included interventions to improve adherence as one of the top 100 priorities for comparative effectiveness research.42 Importantly, cost-effectiveness studies should be incorporated into studies of adherence interventions in order to make coherent policy arguments. Comparative effectiveness studies are needed comparing interventions of different lengths of time, and of different “intensities” (e.g., single-faceted vs multi-faceted interventions).
Finally, before attempting to improve medication adherence, it is critical to ensure that the medication regimen is the most appropriate one for the individual patient. Future trials could assess a stepwise approach to enhancing medication use by first determining any changes to improve medication appropriateness. Only after this has been done should interventions to improve medication adherence be trialed and implemented in order to avoid adherence to a suboptimal regimen. One intervention that will completely prevent nonadherence, and limit polypharmacy, is to stop a medication that is no longer clinically indicated or consistent with the patient’s goals of care.43
CONCLUSION
Despite the fact that medication adherence has been extensively described in the literature over the past several decades, a quote by Becker and Maiman from over 35 years ago best captures the current state of our understanding: “Patient compliance [sic adherence] has become the best documented, but least understood, health behavior.”44 Future research is greatly needed to identify and translate safe and effective interventions into routine clinical practice to improve adherence. Only then can we begin to make significant improvements to the medication use process and, in turn, the health of older adults.
SYNOPSIS.
Polypharmacy and medication adherence present a unique challenge for the older adult, their caregiver(s), and the healthcare team. In examining the current published literature, there appears to be an association between polypharmacy and poorer medication adherence in older adults. However, the heterogeneity of how adherence is defined in the literature, along with limited study designs, generally small sample sizes, and potential for unmeasured confounding limits the certainty of this conclusion. Nonetheless, improving medication adherence to maximize the therapeutic benefit of pharmacotherapy remains a cornerstone of geriatric care. We discuss strategies for limiting polypharmacy and improving adherence and suggest ideas for future research focused on identifying and translating safe and effective interventions to improve adherence into routine clinical practice.
Acknowledgments
Dr. Marcum is supported by a National Institute on Aging grant (P30AG024827) and Dr. Gellad is supported by a VA Career Development Award (09-207).
The authors would like to thank Joseph T. Hanlon, PharmD, MS for reviewing an earlier draft of this manuscript.
Footnotes
Disclosure: Dr. Gellad has received honorarium from Vindico Medical Education for preparation of a continuing medical education (CME) activity focused on improving medication adherence.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–78. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345–51. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Gellad WF, Grenard J, McGlynn EA. A review of barriers to medication adherence: a framework for driving policy options. RAND Corporation; 2009. [Google Scholar]
- 4.World Health Organization (WHO) Adherence to Long-Term Therapies: Evidence for Action. 2003. [Google Scholar]
- 5.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9:11–23. doi: 10.1016/j.amjopharm.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–74. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 8.Raebel MA, Carroll NM, Ellis JL, et al. Importance of including early nonadherence in estimations of medication adherence. Ann Pharmacother. 2011;45:1053–60. doi: 10.1345/aph.1Q146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Morisky DE, Ang A, Kroussel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.McHorney CA. The Adherence Estimator: a brief, proximal screener for patient propensity to adhere to prescription medications for chronic disease. Curr Med Res Opin. 2009;25:215–38. doi: 10.1185/03007990802619425. [DOI] [PubMed] [Google Scholar]
- 12.Soumerai SB, Pierre-Jacques M, Zhang F, et al. Cost-related medication nonadherence among elderly and disabled medicare beneficiaries: a national survey 1 year before the medicare drug benefit. Arch Intern Med. 2006;166:1829–35. doi: 10.1001/archinte.166.17.1829. [DOI] [PubMed] [Google Scholar]
- 13.Grymonpre RE, Didur CD, Montgomery PR, et al. Pill count, self-report, and pharmacy claims data to measure medication adherence in the elderly. Ann Pharmacother. 1998;32:749–54. doi: 10.1345/aph.17423. [DOI] [PubMed] [Google Scholar]
- 14.Shi L, Liu J, Fonesca V, et al. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life Outcomes. 2010;8:99. doi: 10.1186/1477-7525-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voils CI, Hoyle RH, Thorpe CT, et al. Improving the measurement of self-reported medication nonadherence. J Clin Epidemiol. 2011;64:250–4. doi: 10.1016/j.jclinepi.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998;20:764–71. doi: 10.1016/s0149-2918(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 17.Park DC, Jones TR. Medication adherence and aging. In: Fisk AD, Rogers WA, editors. Handbook of Human Factors and the Older Adult. San Diego (CA): Academic Press, Inc; 1997. pp. 257–87. [Google Scholar]
- 18.Krousel-Wood M, Thomas S, Muntner P, Morisky D. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004;19:357–62. doi: 10.1097/01.hco.0000126978.03828.9e. [DOI] [PubMed] [Google Scholar]
- 19.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser RM, Schmader KE, Pieper CF, et al. Therapeutic failure-related hospitalisations in the frail elderly. Drugs Aging. 2006;23:579–86. doi: 10.2165/00002512-200623070-00004. [DOI] [PubMed] [Google Scholar]
- 21.Solomon DH, Brookhart MA, Tsao P, et al. Predictors of very low adherence with medications for osteoporosis: towards development of a clinical prediction rule. Osteoporosis Int. 2011;22:1737–43. doi: 10.1007/s00198-010-1381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madden JM, Graves AJ, Zhang F, et al. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299:1922–8. doi: 10.1001/jama.299.16.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171:814–22. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 24.Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303–12. doi: 10.1345/aph.1D252. [DOI] [PubMed] [Google Scholar]
- 25.Chapman RH, Petrilla AA, Benner JS, et al. Predictors of adherence to concomitant antihypertensive and lipid-lowering medications in older adults: a retrospective, cohort study. Drugs Aging. 2008;25:885–92. doi: 10.2165/00002512-200825100-00008. [DOI] [PubMed] [Google Scholar]
- 26.Gazmararian JA, Kripalani S, Miller MJ, et al. Factors associated with medication refill adherence in cardiovascular-related diseases: a focus on health literacy. J Gen Intern Med. 2006;21:1215–21. doi: 10.1111/j.1525-1497.2006.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant RW, Devita NG, Singer DE, et al. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26:1408–12. doi: 10.2337/diacare.26.5.1408. [DOI] [PubMed] [Google Scholar]
- 28.Gray SL, Mahoney JE, Blough DK. Medication adherence in elderly patients receiving home health services following hospital discharge. Ann Pharmacother. 2001;35:539–45. doi: 10.1345/aph.10295. [DOI] [PubMed] [Google Scholar]
- 29.Ownby RL, Hertzog C, Crocco E, et al. Factors related to medication adherence in memory disorder clinic patients. Aging Ment Health. 2006;10:378–85. doi: 10.1080/13607860500410011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner BJ, Hollenbeak C, Weiner MG, et al. Barriers to adherence and hypertension control in a racially diverse representative sample of elderly primary care patients. Pharmacepidemiol Drug Saf. 2009;18:672–81. doi: 10.1002/pds.1766. [DOI] [PubMed] [Google Scholar]
- 31.Stoehr GP, Lu SY, Lavery L, et al. Factors associated with adherence to medication regimens in older primary care patients: the Steel Valley Seniors Survey. Am J Geriatr Pharmacother. 2008;6:255–63. doi: 10.1016/j.amjopharm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Bruggen R, Gorter K, Stolk RP, et al. Refill adherence and polypharmacy among patients with type 2 diabetes in general practice. Pharmacoepidemiol Drug Saf. 2009;18:983–91. doi: 10.1002/pds.1810. [DOI] [PubMed] [Google Scholar]
- 33.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–50. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Mahtani KR, Heneghan CJ, Glasziou PP, et al. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Databse Syst Rev. 2011;9:CD005025. doi: 10.1002/14651858.CD005025.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Maciejewski ML, Farley JF, Parker J, et al. Copayment reductions generate greater medication adherence in targeted patients. Health Aff (Millwood) 2010;29:2002–8. doi: 10.1377/hlthaff.2010.0571. [DOI] [PubMed] [Google Scholar]
- 36.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–71. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 37.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146:714–25. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Schiff GD, Galanter WL, Duhig J, et al. Principles of conservative prescribing. Arch Intern Med. 2011;171:1433–40. doi: 10.1001/archinternmed.2011.256. [DOI] [PubMed] [Google Scholar]
- 39.Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304:1592–601. doi: 10.1001/jama.2010.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooney D, Pascuzzi K. Polypharmacy in the elderly: focus on drug interactions and adherence in hypertension. Clin Geriatr Med. 2009;25:221–33. doi: 10.1016/j.cger.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine (IOM) Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 43.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648–54. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 44.Becker MH, Maiman LA. Sociobehavioral determinants of compliance with health and medical care recommendations. Med Care. 1975;13:10–24. doi: 10.1097/00005650-197501000-00002. [DOI] [PubMed] [Google Scholar]