Abstract
Orange peel extract appears to exhibit beneficial effects on skin whitening, inflammation, UVB protection, as well as keratinocyte proliferation. In the present study, we determine whether topical hesperidin influences epidermal permeability barrier function and its underlying mechanisms. Hairless mice were treated topically with 2% hesperidin or 70% ethanol alone twice daily for 6 days. At the end of treatment, basal barrier function as well as transepidermal water loss (TEWL) was measured 2 and 4 hours post barrier disruption. Epidermal proliferation and differentiation were evaluated by immunohistochemical staining and Western blot analysis. Additionally, lamellar body density and secretion were assessed by electron microscopy. Although there were no significant differences in basal barrier function, in comparison to control animals, topical hesperidin significantly accelerated barrier recovery at both 2 and 4 hours after acute barrier abrogation. Enhanced barrier function in hesperidin-treated skin correlated with stimulation of both epidermal proliferation and differentiation, as well as enhanced lamellar body secretion. These results indicate that topical hesperidin enhances epidermal permeability barrier homeostasis at least in part due to stimulation of epidermal proliferation, differentiation, as well as lamellar body secretion.
Keywords: Hesperidin, Transepidermal Water Loss, Differentiation, Proliferation
Introduction
Orange peel has been used for centuries as an ingredient of Chinese herbal medicines (CHM) used for the treatment of respiratory and cardiovascular diseases. Hesperidin found in orange peel is a flavanone glycoside consisting of the flavone hesperitin bound to the disaccharide rutinose (Spring 2010 Volume 12, Issue 4, www.integrativeRD.org). The sugar group makes hesperidin more water-soluble than hesperitin, another compound in orange peel. Exogenous hesperidin has been shown to influence a wide variety of biological functions. For example, hesperidin induces apoptosis and suppresses proliferation in human cancer cells (1-5), inhibition of tumor development in various tissues including the skin (6-11). Moreover, hesperidin stimulates osteoblast differentiation and proliferation (12,13). Furthermore, in vivo studies demonstrated that hesperidin exhibits anti-inflammatory properties in acute inflammation models induced by phorbol ester and lipopolysaccharide (9-11). Additional studies have demonstrated that hesperidin exerts antibacterial, antiviral, as well as antifungal activities (14-18). Likewise, administration of hesperidin inhibits Salmonella typhimurium aroA-induced endotoxin shock (19). Many of these beneficial effects of hesperidin can be attributed to its antioxidant activity (20-22), but its effects on proliferation could be due to other action. For example, Nazari et al (23) and Ghorbani et al (24) reported that hesperidin upregulates peroxisome proliferator-activated (PPAR) γ mRNA and protein expression in vitro.
With regard to the effects of hesperidin on skin, it has been shown the whiten skin as result of the inhibition of tyrosinase (25-27). Prior studies have shown that exposure to oxidative stress can induce epidermal permeability barrier disruption (reviewed in 28), suggesting that the antioxidant activity of hesperidin could improve barrier function. Additionally, our previous studies have demonstrated that PPAR α and γ activation enhances barrier function in parallel with a stimulation of keratinocyte proliferation, differentiation and lipid synthesis (29). Yet, whether and how hesperidin improves barrier function remains largely unknown. Hence, we assessed here whether topical hesperidin influences epidermal permeability barrier function, and the responsible mechanisms in normal murine skin.
Materials and Methods
Materials
Since male mice housed in the same cage tend to fight each other, which causes psychological stress and skin damage, only female mice were used. 6-8 weeks old female hairless mice (hr/hr) were purchased from Charles River Laboratories (Wilmington, MA, USA) and fed mouse diet (Ralston-Purina Co., St Louis, MO, USA) and water ad libitum. Hesperidin powder was from P&G Corporation. Biotinylated anti-PCNA (Proliferating Cell Nuclear Antigen) antibody was from CalTag Laboratories (Burlingame, CA, USA). Affinity-purified, rabbit anti-mouse antibodies to loricrin, involucrin, and filaggrin were purchased from BabCo (Richmond, CA, USA).
Experimental protocols and functional studies
All animal procedures were approved by the Animal Studies Subcommittee (IACUC) of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. Since hesperidin is not soluble in 100% ethanol, 70% ethanol was used as vehicle. Both flanks of mice were treated topically with 60 μl of 2% hesperidin or 70% ethanol twice daily for 6 days. Basal epidermal permeability barrier function was assessed by measuring transepidermal water loss (TEWL) using TM300 connected to MPA5 (C&K, Cologne, Germany). For barrier recovery, TEWL was measured using an electrolytic water analyzer (Meeco, Warrington, PA) at 0, 2 and 4 hours after tape stripping (10-fold increase in TEWL), and percent barrier recovery was calculated as described earlier (30).
Immunohistochemistry
Immunohistochemical staining for assessing changes in epidermal differentiation was performed as described earlier (31, 32). Briefly, 5 μm paraffin sections were incubated with the primary antibodies overnight at 4°C. After washes ×3, sections were incubated with the secondary antibody for 30 minutes. Staining was detected with ABC-peroxidase kit from Vector Lab, and sections were then counterstained with hematoxylin. For immunohistochemistry for proliferating cells, changes in overall morphology were visualized after hematoxylin and eosin staining of 5 μm paraffin-enabled sections, and proliferating cells were detected by proliferating cell nuclear antigen staining. Briefly, 5 μm paraffin sections were incubated with biotinylated monoclonal antibody against proliferating cell nuclear antigen (CalTag Laboratories, Burlingame, CA) overnight at 4°C, and staining was detected by the ABC-peroxidase method (Vector). Sections were examined with a Zeiss fluorescence microscope (Jena, Germany) and digital images were captured with AxioVision software (Carl Zeiss Vision, Munich, Germany).
Western blot analysis of Epidermal Differentiation Proteins
Mouse epidermis was isolated following 6 day treatment with 2% hesperidin or vehicle alone. Epidermis was chopped well with No. 22 surgical blade, followed by homogernization in RIPA (Radio-Immunoprecipitation Assay from Fisher scientific Pittsbergh, PA, USA) buffer with 19.5G needle for 20 times. Extract was resolved by electrophoresis on 4-12% Bis-Tris Gel (Invitrogen, Carlsbad, CA) according to manufacturer’s instruction. Resultant bands were blotted onto polyvinylidene fluoride membranes, and were subsequently probed with anti-murine β-actin (loading control from Abcam, Cambrige, MA) antibody, anti-Filaggrin, anti-Involucrin or anti-Loricrin (Lovus Biologicals, Littleton, CO), and detected using enhanced chemiluminescence (Thermo Fisher Sci., Rockford, IL). Results were presented as percentage of vehicle-treated control, setting vehicle-treated as 100%.
Electron Microscopy
Skin biopsies from both vehicle and hesperidin-treated mice were taken for electron microscopy (29). Briefly, samples were minced to <0.5 mm3, fixed in modified Karnovsky’s fixative overnight, and post-fixed in either 0.2% ruthenium tetroxide or 1% aqueous osmium tetroxide, containing 1.5% potassium ferrocyanide. After fixation, all samples were dehydrated in a graded ethanol series, and embedded in an Epon-epoxy mixture. Ultrathin sections were examined, with or without further contrasting with lead citrate, in a Zeiss 10A electron microscope (Carl Zeiss, Thornwood, NJ), operated at 60 kV.
Statistics
GraphPad Prism 4 software was used for all statistical analyses. An unpaired t-test with Welch’s correction was used for comparisons between two groups. Data are expressed as mean ± SEM.
Results
Topical Hesperidin Accelerates Epidermal Permeability Barrier Recovery in Normal Murine Skin
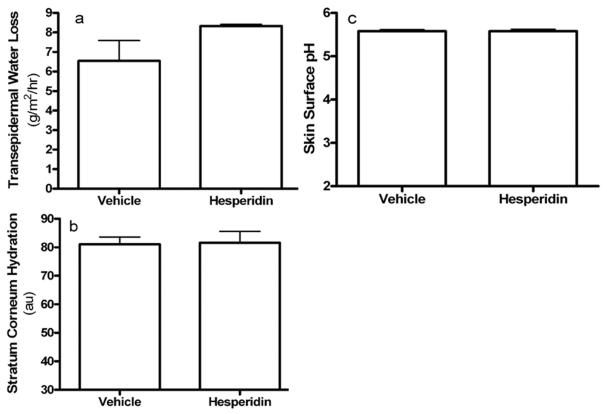
We first assessed whether the topical hesperidin improves epidermal permeability barrier function in normal murine skin. No changes in gross appearance were apparent after topical applications of 2% hesperidin or vehicle twice-daily for 6 days (not shown). There were no differences in baseline TEWL, surface pH, or stratum corneum (SC) hydration between hesperidin- and vehicle-treated groups (Fig. 1a-c). However, barrier recovery significantly accelerated in mice treated with hesperidin at both 2 and 4 hours following acute barrier disruption with repeated tape stripping (recovery rates at 2 hours, 11.21 ± 4.64 for vehicle-treated and 48.54 ± 4.56 for hesperidin-treated, p<0.0001; at 4 hours, 37.41 ± 5.17 for vehicle-treated and 60.90 ± 4.58 for hesperidin-treated, p<0.005. N=12 for all). These results indicate that topical hesperidin improves epidermal permeability barrier homeostasis.
Figure 1. Topical Hesperidin Accelerates Epidermal Permeability Barrier Recovery in Normal Mouse Skin.
Hairless mice were topically treated with 60 μl of 2% hesperidin in 70% ethanol or 70% ethanol alone twice daily for 6 days. Basal epidermal permeability barrier function, skin surface pH and stratum corneum (SC) hydration were assessed with a MPA5 (CK electronic GmbH, Cologne, Germany) connected to TM 300, pH905 and Corneometer 825. Two readings were taken from each mouse for basal TEWL, hydration, as well as pH. For barrier recovery, TEWL was measured at 0, 2 and 4 hours after tape stripping, which results in a 10-fold increase in TEWL, and percent barrier recovery rates were calculated as described earlier (30). Figure 1a is basal TEWL (n=10); 1b, SC hydration (n=10); 1c, skin surface pH (n=10).
Topical Hesperidin Stimulates Epidermal Proliferation and Differentiation in Normal Murine Skin
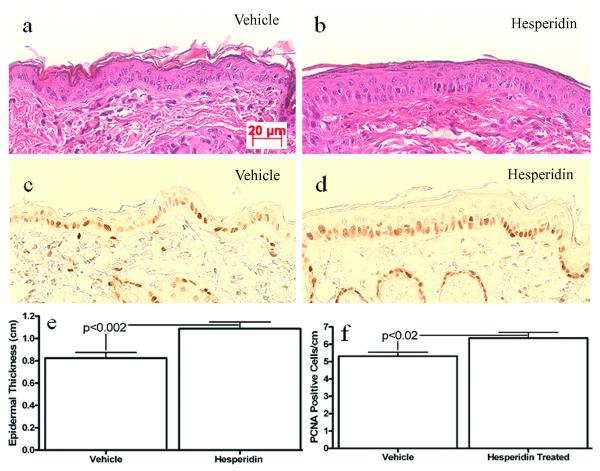
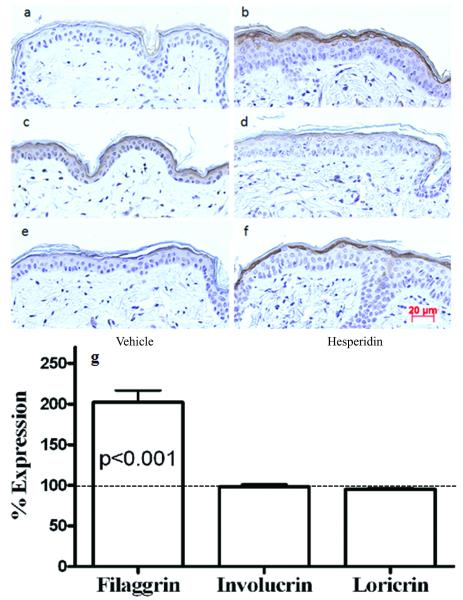
We next assessed the mechanistic bases for enhanced barrier function in hesperidin-treated skin. Since previous studies demonstrated that hesperidin stimulates osteoblasts differentiation and proliferation (12, 13), we first determined whether topical hesperidin influcences keratinocyte proliferation and differentiation. As seen in Figure 2, topical hesperidin induced a significant increase in epidermal hyperplasia (Figure 2a vs. b; figure 2e). Accordingly, PCNA-positive cells, indicative of proliferative activity, increased in the basal layer following topical hesperidin treatment (Figure 2c vs. d; figure 2f). Moreover, topical hesperidin induced a marked increase in epidermal immunostaining for filaggrin and loricrin expression in comparison with vehicle-treated skin (Figure 3a vs. b; e vs. f). Involucrin expression instead appeared to decrease slightly following hesperidin treatment (Figure 3c vs. d). To confirm the results obtained by immunohistochemistry, expression of epidermal differentiation proteins was quantitated by Western blot analysis. Elevation of epidermal filaggrin expression was evident in hesperidin-treated skin (Figure 3g), while involucrin again did not change, results that were consistent with the immunohistochemistry data. These results demonstrate that topical hesperidin stimulates epidermal proliferation, as well as expression of some epidermal differentiation proteins.
Figure 2. Topical Hesperidin Stimulates Epidermal Proliferation in Normal Murine Skin.
Skin samples were from mice treated with either vehicle alone (2a, c) or 2% hesperidin (2b, d) twice daily for 6 days. 5 μm sections were incubated with the primary antibodies (1:500 dilutions) overnight at 4°C. After washing, sections were treated with DAB for 20 sec. Sections were examined with a Zeiss microscope (Jena, Germany), and digital images were captured with AxioVision software (Carl Zeiss Vision, Munich, Germany). Figure 2a and b are H&E staining. Figure 2c and d are PCNA staining. Magnification bars represent 20 μm (a-d). Figure 2e represents epidermal thickness and f, PCNA positive cells. Epidermal thickness of the nucleated cell layer was measured on 1260 × H & E sections. The measurement was taken at every 2 cm points along the epidermis. The data are presented as the mean of all measured points (n=33 for both groups). The number of PCNA positive cells was counted on every 2 cm segment along the epidermis (n=32 for vehicle-treated and n=24 for hesperidin-treated group). The data are presented as the mean of all segments counted ± SEM. Unpaired two-tailed student t test with Welch’s correction was used to determine statistical differences. P<0.05 was considered as significant difference.
Figure 3. Changes in Epidermal Differentiation Protein Expression Induced by Topical Hesperidin.
5μm paraffin sections were incubated with primary rabbit anti-mouse antibodies (Covance/BabCo. Berkely, CA) at the dilutions of 1:2000 for filaggrin, 1:1000 for involucrin, and 1:500 for loricrin, overnight at 4°C. After washing with 10mM citrate buffer, sections were incubated with goat anti-rabbit antibody (1:400) for 30 min at room temperature, followed by ABC-peroxidase (Vector, Burlingame, CA) reaction. For PCNA staining, sections were incubated with biotinylated monoclonal anti-PCNA antibody (CalTag Laboratories, Burlingame, CA) for 2 hours at room temperature. The sections were visualized with a Zeiss (Axioplan 2) microscope (Jena, Germany). Digital images were captured with AxioVision software 2.05 (Carl Zeiss Vision, Munich, Germany). Fig. 3a and b are filaggrin staining; c and d are involucrin staining; e and f are loricrin staining. Fig. 3a, c, e are vehicle-treated samples and b, d, f are hesperidin-treated samples. Magnifications are the same for all figures. Magnification bars represent 20 μm. For Western blot analysis, mouse epidermis was isolated following 6 day treatment with 2% hesperidin or vehicle alone. Epidermal proteins were fractionated by electrophoresis followed by transferring onto polyvinylidene fluoride membranes. The proteins on the membrane were probed with respective antibody. The corresponding bands were detected by enhanced chemiluminescence (Thermo Fisher Sci., Rockford, IL), and quantitated by scanning densitometry. Results were presented as percentage of vehicle-treated control, setting vehicle-treated as 100% (dotted line in Fig 3g). An unpaired t-test with Welch’s correction was used to determine the significance. (n=5 for all groups).
Lamellar body formation and secretion are crucial for epidermal permeability barrier homeostasis (33). To further elucidate the underlying mechanism for improved permeability barrier function induced by hesperidin, we next assessed lamellar body formation and secretion following hesperidin treatment. Although there were no differences in lamellar body density or secretion between hesperidin and vehicle treatment at baseline (supplemental figure 1a, b), lamellar body secretion accelerated in hesperidin-treated skin following barrier disruption (supplemental figure 1c-e, arrows in figure d, e). These results suggest that the hesperidin-induced acceleration of lamellar body secretion contributes to enhanced permeability barrier homeostasis.
Discussion
In the present studies, we demonstrate here that topical hesperidin treatment accelerates epidermal permeability barrier homeostasis and stimulates epidermal proliferation and differentiation in normal murine skin. Although all of the exact underlying mechanisms for improvements are not clear, these improvements could be partially attributed to hesperidin antioxidant properties (34-36), which were not assessed here. Pertinently, other antioxidant, such as vitamin E, elevates transglutaminase-1 expression and increases peroxisome proliferator-activated receptor (PPAR) activity and expression in keratinocyte cultures (37). Both PPARs and transglutaminase-1 are crucial for epidermal permeability barrier homeostasis (29, 38). Deletion or inactivation of antioxidation enzymes, such as NAD(P)H:quinone oxidoreductase 1, reduces the expression of keratinocyte differentiation marker and epidermal thickness (39). More recent study suggests that a disrupted antioxidant system contributes to the pathogenesis of Hailey–Hailey disease, which also is accompanied by differentiation and barrier abnormalities (40, 41). Here, we show a dramatic increase in filaggrin expression following hesperidin treatment. The significance of filaggrin in epidermal permeability barrier homeostasis is now well appreciated (42, 43). Therefore, the improved barrier function could be due, at least in part, to upregulated epidermal differentiation resulting via the antioxidant effect of hesperidin.
It is notable that the expression of filaggrin and loricrin increases, while involucrin expression declines following hesperidin treatment. This differential regulation of epidermal differentiation could represent that the keratinocytes in different layers response differently to hesperidin. While the expression of filaggrin and loricrin mainly localizes to the stratum granulosum and stratum corneum (44, 45), involucrin is an early differentiation marker, which is mainly expressed in the stratum spinosum (46). Moreover, the mRNA expression for involucrin differs from filaggrin mRNA expression in rat hair bulb cultures, in which calcium downregulates involucrin mRNA expression, while stimulating filaggrin mRNA expression (47). Likewise, retinoic acid regulates loricrin, but not involucrin expression in vitro (44). Together, the present study supports the concept that topical hesperidin preferentially up-regulates later differentiation markers in murine epidermis. Although upregulation of PPAR γ occurs following hesperidin treatment (23,24), increased differentiation is likely not due to activation of PPAR, because PPAR γ activators increase expression of filaggrin, involucrin and loricrin without stimulation of proliferation (29, 48). The discrepancy in involucrin and loricrin expression between immunohistochemistry (Figure 3) and Western blotting (Figure 4) could be attributed to different methods.
Immunohistochemistry preferably depicts localized changes in expression, while Western blots show the expression in whole epidermis since total epidermal protein was used for analyzing expression. Therefore, Western blots may not reveal localized increases in expression, especially in hyperplastic epidermis such as occur in hesperidin-treated skin. Yet, hesperidin also stimulated epidermal proliferation by yet unknown mechanisms. Hence, how hesperidin influences epidermal proliferation and differentiation remain to be determined.
Taken together, the present study shows that topical hesperidin stimulates epidermal proliferation, differentiation, and lipid secretion, which together should account for improved epidermal permeability barrier homeostasis. In agreement with previous findings (49, 50), present work further confirms that measuring skin physiological parameters such as TEWL could be a valuable approach to evaluate the influence of naturally occurring and synthetic compounds on cutaneous function. Since hesperidin exhibits beneficial effects in inflammation, improving epidermal permeability barrier function with marked upregulation of filaggrin expression, it could be useful in treating some skin disorders characterized by cutaneous inflammation and abnormal barrier function, such as filaggrin-deficient atopic dermatitis.
Supplementary Material
Supplemental Figure 1. Hesperidin enhances LB secretion in mouse skin after tape stripping. Supplemental Fig. 1a and b are basal lamellar body density (red arrows) and secretion following vehicle (a) or hesperidin (b) treatment. Supplemental Fig. 1c and d represent lamellar secretion (yellow arrows) at 2 hours after tape-stripping of vehicle and hesperidin-treated skin, respectively. Supplemental Fig. 1e is higher maginification of hesperidin-treated skin after tape-stripping (yellow arrows). Magnification bars are indicated in the figures.
Acknowledgements
This work was supported by P&G Beauty & Grooming Scientific Foundation and National Institutes of Health grants AR19098.
Footnotes
Conflicts: All authors have no conflicts of interest.
Author Contributions
Maihua Hou: Interpreted data and wrote draft
Mona Man: Assess barrier function, statistical analyses, and prepared samples for morphology
Wenyan Man & Melanie Hupe: H&E and immunohistochemical staining
Debra Crumrine: Ultrastructural studies
Kyungho Park: Western blotting
Wenyuan Zhu and Peter M Elias: Critically reviewed data and manuscript; approved final version;
Mao-Qiang Man: Designed experiment, made graphs and figures for publication, and approved final version
References
- 1.Park HJ, Kim MJ, Ha E, Chung JH. Phytomedicine. 2008;15:147–51. doi: 10.1016/j.phymed.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 2.Lee CJ, Wilson L, Jordan MA, Nguyen V, Tang J, Smiyun G. Phytother Res. 2010;24:S15, 9. doi: 10.1002/ptr.2856. [DOI] [PubMed] [Google Scholar]
- 3.Jin YR, Han XH, Zhang YH, et al. J Cell Biochem. 2008;104:1–14. doi: 10.1002/jcb.21592. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Q, Hirose Y, Yoshimi N, et al. J Cancer Res Clin Oncol. 2002;128:539–46. doi: 10.1007/s00432-002-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Nutr Cancer. 1996;26:167–81. doi: 10.1080/01635589609514473. [DOI] [PubMed] [Google Scholar]
- 6.Koyuncu H, Berkarda B, Baykut F, et al. Anticancer Res. 1999;19:3237–41. [PubMed] [Google Scholar]
- 7.Berkarda B, Koyuncu H, Soybir G, Baykut F. Res Exp Med (Berl) 1998;198:93–9. doi: 10.1007/s004330050093. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Kohno H, Murakami M, et al. Int J Cancer. 2000;88:146–50. [PubMed] [Google Scholar]
- 9.Li R, Li J, Cai L, Hu CM, Zhang L. J Pharm Pharmacol. 2008;60:221–8. doi: 10.1211/jpp.60.2.0011. [DOI] [PubMed] [Google Scholar]
- 10.Kaur G, Tirkey N, Chopra K. Toxicology. 2006;226:152–60. doi: 10.1016/j.tox.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Tanabe S. Int J Mol Med. 2006;17:511–5. [PubMed] [Google Scholar]
- 12.Trzeciakiewicz A, Habauzit V, Mercier S, et al. J Nutr Biochem. 2010;21:424–31. doi: 10.1016/j.jnutbio.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Bae MS, Ko SY, Kim SW. Int J Oral Biol. 2006;31:119–25. [Google Scholar]
- 14.Aranganathan S, Selvam JP, Nalini N. J Pharm Pharmacol. 2008;60:1385–92. doi: 10.1211/jpp/60.10.0015. [DOI] [PubMed] [Google Scholar]
- 15.Cvetnić Z, Vladimir-Knezević S. Acta Pharm. 2004;54:243–50. [PubMed] [Google Scholar]
- 16.Panasiak W, Wleklik M, Oraczewska A, Luczak M. Acta Microbiol Pol. 1989;38:185–8. [PubMed] [Google Scholar]
- 17.Mandalari G, Bennett RN, Bisignano G, et al. J Appl Microbiol. 2007;103:2056–64. doi: 10.1111/j.1365-2672.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- 18.Cvetnić Z, Vladimir-Knezević S. Acta Pharm. 2004;54:243–50. [PubMed] [Google Scholar]
- 19.Kawaguchi K, Kikuchi S, Hasunuma R, Maruyama H, Yoshikawa T, Kumazawa Y. Biol Pharm Bull. 2004;27:679–83. doi: 10.1248/bpb.27.679. [DOI] [PubMed] [Google Scholar]
- 20.Kaur G, Tirkey N, Bharrhan S, Chanana V, Rishi P, Chopra K. Clin Exp Immunol. 2006;145:313–21. doi: 10.1111/j.1365-2249.2006.03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirkey N, Pilkhwal S, Kuhad A, Chopra K. BMC Pharmacol. 2005;5:2. doi: 10.1186/1471-2210-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pradeep K, Park SH, Ko KC. Eur J Pharmacol. 2008;587:273–80. doi: 10.1016/j.ejphar.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Nazari M, Ghorbani A, Hekmat-Doost A, Jeddi-Tehrani M, Zand H. Eur J Pharmacol. 2011;650:526–33. doi: 10.1016/j.ejphar.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 24.Ghorbani A, Nazari M, Jeddi-Tehrani M, Zand H. Eur J Nutr. 2011 Mar 29; doi: 10.1007/s00394-011-0187-2. DOI 10.1007/s00394-011-0187-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Gao J. J Invest Dermatol Symp Proc. 2008;13:20–4. doi: 10.1038/jidsymp.2008.8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Lu Y, Tao L, Tao X, Su X, Wei D. J Enzyme Inhib Med Chem. 2007;22:83–90. doi: 10.1080/14756360600953876. [DOI] [PubMed] [Google Scholar]
- 27.Tsai YH, Lee KF, Huang YB, Huang CT, Wu PC. Int J Pharm. 2010;388:257–62. doi: 10.1016/j.ijpharm.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Thiele JJ. Skin Pharmacol Appl Skin Physiol. 2001;14(Suppl 1):87–91. doi: 10.1159/000056395. [DOI] [PubMed] [Google Scholar]
- 29.Mao-Qiang M, Fowler AJ, Schmuth M, et al. J Invest Dermatol. 2004;123:305–12. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 30.Man M, Hupe M, Mackenzie D, et al. Exp Dermatol. 2011;20:285–8. doi: 10.1111/j.1600-0625.2010.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demerjian M, Man MQ, Choi EH, et al. Exp Dermatol. 2006;15:154–60. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 32.Man MQ, Shi Y, Man M, et al. Exp Dermatol. 2008;17:681–7. doi: 10.1111/j.1600-0625.2007.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feingold KR, Schmuth M, Elias PM. J Invest Dermatol. 2007;127:1574–6. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- 34.Al-Ashaal HA, El-Sheltawy ST. Pharm Biol. 2011;49:276–82. doi: 10.3109/13880209.2010.509734. [DOI] [PubMed] [Google Scholar]
- 35.Jain M, Parmar HS. Inflamm Res. 2011;60:483–91. doi: 10.1007/s00011-010-0295-0. [DOI] [PubMed] [Google Scholar]
- 36.El-Sayed el-SM, Abo-Salem OM, Abd-Ellah MF, Abd-Alla GM. J Biochem Mol Toxicol. 2008;22:268–73. doi: 10.1002/jbt.20237. [DOI] [PubMed] [Google Scholar]
- 37.De Pascale MC, Bassi AM, Patrone V, Villacorta L, Azzi A, Zingg JM. Arch Biochem Biophys. 2006;447:97–106. doi: 10.1016/j.abb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Kuramoto N, Takizawa T, Takizawa T, et al. J Clin Invest. 2002;109:243–50. doi: 10.1172/JCI13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patrick BA, Gong X, Jaiswal AK. Oncogene. 2011;30:1098–107. doi: 10.1038/onc.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Manca S, Magrelli A, Cialfi S, et al. Exp Dermatol. 2011 Aug 6; doi: 10.1111/j.1600-0625.2011.01359.x. doi: 10.1111/j.1600-0625.2011.01359.x. [DOI] [PubMed] [Google Scholar]
- 41.Aberg KM, Racz E, Behne MJ, Mauro TM. J Invest Dermatol. 2007;127:1973–9. doi: 10.1038/sj.jid.5700785. [DOI] [PubMed] [Google Scholar]
- 42.Scharschmidt TC, Man MQ, Hatano Y, et al. J Allergy Clin Immunol. 2009;124:496–506. doi: 10.1016/j.jaci.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelova-Fischer I, Mannheimer AC, Hinder A, et al. Exp Dermatol. 2011;20:351–6. doi: 10.1111/j.1600-0625.2011.01259.x. [DOI] [PubMed] [Google Scholar]
- 44.Magnaldo T, Bernerd F, Asselineau D, Darmon M. Differentiation. 1992;49:39–46. doi: 10.1111/j.1432-0436.1992.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z, Hansmann B, Meyer-Hoffert U, Gläser R, Schröder JM. PLoS One. 2009;4:e5227. doi: 10.1371/journal.pone.0005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks-Schlegel S, Green H. J Cell Biol. 1981;90:732–7. doi: 10.1083/jcb.90.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niderla-Bielińska J, Moskalewski S. Folia Histochem Cytobiol. 2011;49:335–43. doi: 10.5603/fhc.2011.0046. [DOI] [PubMed] [Google Scholar]
- 48.Kömüves LG, Hanley K, Lefebvre AM, et al. J Invest Dermatol. 2000;115:353–60. doi: 10.1046/j.1523-1747.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- 49.Oudshoorn MH, Rissmann R, van der Coelen D, Hennink WE, Ponec M, Bouwstra JA. Exp Dermatol. 2009;18:178–84. doi: 10.1111/j.1600-0625.2008.00780.x. [DOI] [PubMed] [Google Scholar]
- 50.Aschoff R, Schwanebeck U, Bräutigam M, Meurer M. Exp Dermatol. 2009;18:24, 9. doi: 10.1111/j.1600-0625.2008.00756.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Hesperidin enhances LB secretion in mouse skin after tape stripping. Supplemental Fig. 1a and b are basal lamellar body density (red arrows) and secretion following vehicle (a) or hesperidin (b) treatment. Supplemental Fig. 1c and d represent lamellar secretion (yellow arrows) at 2 hours after tape-stripping of vehicle and hesperidin-treated skin, respectively. Supplemental Fig. 1e is higher maginification of hesperidin-treated skin after tape-stripping (yellow arrows). Magnification bars are indicated in the figures.