Abstract
INTRODUCTION
This report is a meta-analysis of the human muscle architecture literature that analyzes the number of muscles, number of subjects, and muscle fiber length coefficient of variation (CV) by body region.
METHODS
Muscle fiber length data are used to make recommendations for dissection-based architectural study sample sizes.
RESULTS
An average of 9 ± 10 (mean ± SD) muscles and an average of 9 ± 5 subjects were reported in the 26 studies considered. Across all studies, average fiber length CV was highly variable (18% ± 5%). This shows that sample sizes required to achieve adequate power varies by anatomical region.
DISCUSSION
Studies involving muscle architecture should consider regional variability and effect size and determine sample size accordingly.
Keywords: muscle, architecture, sample size, fiber length variation, statistical power
INTRODUCTION
Muscle architectural studies are used to describe and predict skeletal muscle structure and function. Human muscle architecture has been investigated using a variety of methods including ultrasound, magnetic resonance imaging (MRI), computed tomography (CT), histology and dissection. While imaging methods have the advantage of being non-invasive and can be performed on living humans, dissection studies provide the gold-standard method of describing muscle architecture since fiber length at a known sarcomere length can be quantified.1 While large sample sizes are desirable in dissection studies, lack of access to cadavers, cost, and technically challenging methodology often limit the actual number of subjects reported per study. This can result in underpowered, inaccurate studies if variability is high between humans or between muscles of different anatomical areas. This can have significant clinical impact if these numbers are then used to create models that impact surgical decisions.
The purpose of this study was to identify all of the human muscle architecture studies in the literature, determine their sample sizes, quantify fiber length coefficient of variation (CV) and then recommend adequate sample size for such studies in human muscle.
METHODS
PubMed was used to define all human muscle architecture studies published from 1968 through July 2011. Once the studies were identified, they were separated by methodology into dissection studies, ultrasound, magnetic resonance imaging (MRI), biopsy/histology, and studies utilizing a combination of these methods. We focused on those studies that utilized dissection methods to define muscle architecture, because they contain accurate measurements of muscle fiber length and sarcomere length. We analyzed the number of different muscles measured per study within a subject and the number of samples per muscle (number of subjects used). We calculated the total number of samples (number of muscles multiplied by number of subjects) and calculated the within-muscle fiber length (CVm) for the ith region according to the equation:
where the subscript i refers to the particular region (e.g., forearm or leg; numbered 1–11), and m refers to the particular muscle within a region containing n muscles. N varies from 5 to 42 in this analysis and represents the number of times fiber length has been reported in the literature in a particular region. For example, the thigh has an n of 42, because the quadriceps and hamstring muscles are often studied. The arm (excluding shoulder and forearm which are separate regions) has only been reported 5 times. The average CV for all muscles was simply calculated as:
We then used this information to determine sample sizes (number of subjects) for an independent samples t-test with α=0.05 and power (1-β)=0.80 using the equation:2
where CVi = ith region coefficient of variation and δ= percent expected treatment effect.
RESULTS
We examined the 163 human muscle architecture studies that used a variety of methods. Of these 163 studies, 26 used dissection methods, 63 used ultrasound, 4 used MRI, 18 used a combination of ultrasound and MRI and 5 used biopsy/histology methods. An additional 47 studies were modeling studies, descriptive anatomical studies, or diffusion tensor imaging studies. Others reported fiber length data duplicated from a previous study or did not report mean and standard deviation, thus making it impossible to calculate coefficient of variation; these studies were not included in the analysis. We distilled the 163 studies down to 26 usable dissection studies.3–28
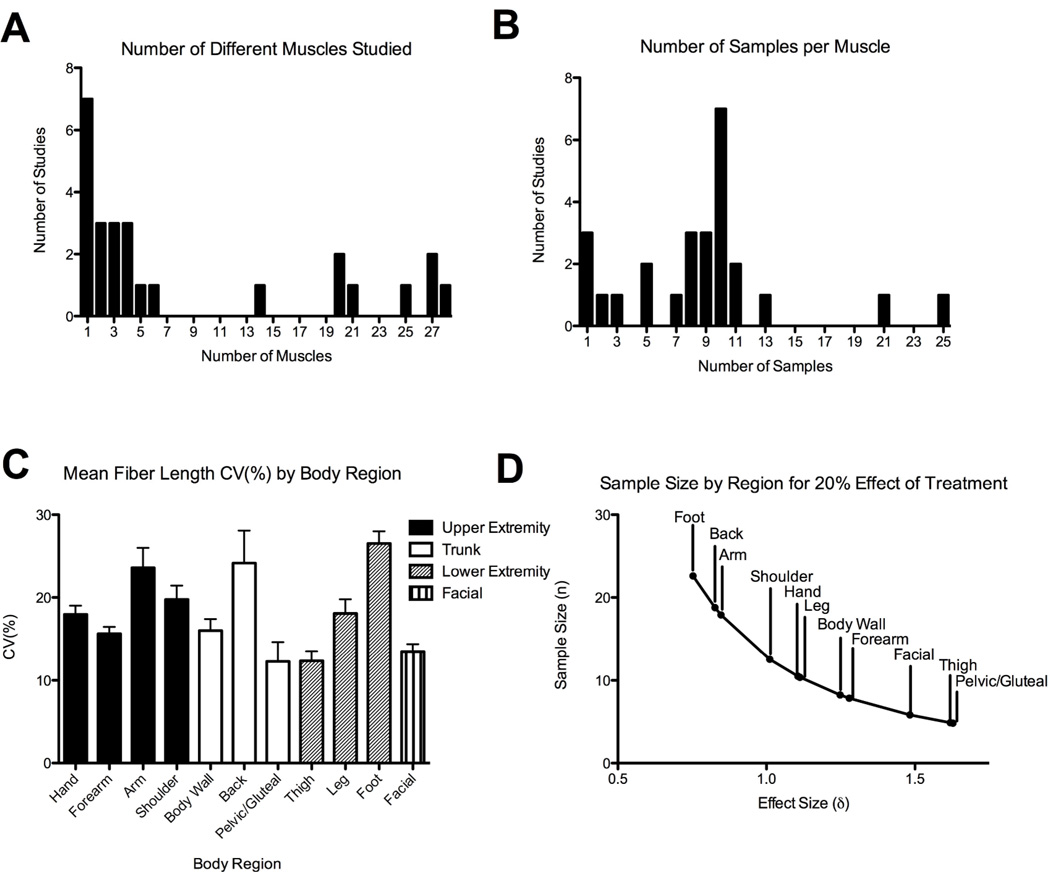
The average number of muscles measured per dissection study was 9 ± 10 (mean ± SD) with a range of 1–28 muscles measured per study (Fig. 1A). The average number of subjects per dissection study was 9 ± 5 with a range of 1–25 subjects per study (Fig. 1B). The average total number of samples (number of muscles×number of subjects per study) was highly variable, 71 ± 122 (range 1–567). The average fiber length coefficient of variation () by region was 18 ± 5% (range of 12–27%, Fig. 1C). Using these data in a one way ANOVA, if a treatment was expected to change fiber length by 10%, in the muscles of the thigh (CV = 12.35%), a sample size of 22 subjects per group would be required for a power of 0.80 and α=0.05 to determine a difference. Interestingly, given the same 10% treatment effect in the foot (CV = 26.54%), sample size increases to 102 subjects per group since foot fiber length CV is so much greater (this is primarily due to small average fiber lengths in the foot). If the size of the treatment effect is increased to 20%, the corresponding sample sizes decrease to 5 per group in the thigh and 23 per group in the foot (Fig. 1D). Regardless of the effect size, these data demonstrate anatomical variation in fiber length CVi and point to the need for anatomical region-specific experimental design.
Figure 1.
A) Histogram representation of the number of different muscles throughout the body that were measured per study B) Histogram representation of the samples studied per muscle. This typically corresponds to the number of cadaveric specimens C) Mean coefficient of variation (CVi; see text for details of calculation) grouped by body region; Error bars in this graph indicate SEM. D) Plot of effect size and sample size by anatomical region estimating a 20% treatment effect.
DISCUSSION
Here we show that significant and systematic muscle fiber length variability exists by anatomical region, which leads to variable sample sizes required to perform adequately powered experiments. The CV is important to consider when designing studies that use muscle architecture parameters. We showed that sample sizes can vary from 5 to more than 100 depending upon the expected treatment effect and the region of the body that is being studied. Indeed, no studies have ever reported sample sizes of greater than 100 subjects, which would be considered a major undertaking.
Currently, mathematical models are often implemented using data that come from studies with few samples or models that do not properly scale to account for variability between subjects or variability between body regions and muscles. The way in which any of the architectural parameters scale with body size is unknown.29 Therefore, using these models to define surgical methods, rehabilitation strategies, or motor control strategies should be considered with caution.
There has been a marked increase in the number of fiber length studies published that use ultrasound, since the seminal paper by Fukunaga and colleagues.30 However, it must be emphasized that none of these studies measure sarcomere length, and thus it is not clear whether long fiber lengths reported, for example, represent short fibers containing stretched sarcomeres or whether the fibers are actually long, composed of a high number of serial sarcomeres. We advocate that studies use gold-standard dissection methodology, when possible, with relatively large sample sizes (≥10) to define human muscle architecture. Further, studies using other methods for measuring muscle architecture, such as imaging, should consider body region variability as well as any treatment effect and calculate the number of subjects needed accordingly. We use 10% and 20% treatment effect/effect size of fiber length as an example in this manuscript, but other variables of interest may have larger or smaller effect sizes. If the variable of interest in a study is a muscle architecture parameter other than fiber length, we encourage investigators to perform a similar analysis to that presented here using literature values to ensure that studies have adequate sample sizes and power.
Acknowledgements
This work was supported in part by NSMRC R24 HD650837 and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Senior Research Career Scientist Award.
Abbreviations
- CT
computed tomography
- CV
coefficient of variation
- MRI
magnetic resonance imaging
References
- 1.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.van Belle G. Statistical Rules of Thumb. Hoboken, New Jersey: John Wiley & Sons Inc; 2008. 33 pp. [Google Scholar]
- 3.Roh MS, Wang VM, April EW, Pollock RG, Bigliani LU, Flatow EL. Anterior and posterior musculotendinous anatomy of the supraspinatus. J Shoulder Elbow Surg. 2000;9:436–440. doi: 10.1067/mse.2000.108387. [DOI] [PubMed] [Google Scholar]
- 4.Ward SR, Kim CW, Eng CM, Gottschalk LJ, 4th, Tomiya A, Garfin SR, Lieber RL. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Joint Surg Am. 2009;91:176–185. doi: 10.2106/JBJS.G.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Infantolino BW, Challis JH. Architectural properties of the first dorsal interosseous muscle. J Anat. 2010;216:463–469. doi: 10.1111/j.1469-7580.2009.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieber RL, Jacobson MD, Fazeli BM, Abrams RA, Botte MJ. Architecture of selected muscles of the arm and forearm: anatomy and implications for tendon transfer. J Hand Surg. 1992;17:787–798. doi: 10.1016/0363-5023(92)90444-t. [DOI] [PubMed] [Google Scholar]
- 7.Lieber RL, Fazeli BM, Botte MJ. Architecture of selected wrist flexor and extensor muscles. J Hand Surg. 1990;15:244–250. doi: 10.1016/0363-5023(90)90103-x. [DOI] [PubMed] [Google Scholar]
- 8.Van Eijden TM, Korfage JA, Brugman P. Architecture of the human jaw-closing and jaw-opening muscles. Anat Rec. 1997;248:464–474. doi: 10.1002/(sici)1097-0185(199707)248:3<464::aid-ar20>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Delp SL, Suryanarayanan S, Murray WM, Uhlir J, Triolo RJ. Architecture of the rectus abdominis, quadratus lumborum, and erector spinae. J Biomech. 2001;34:371–375. doi: 10.1016/s0021-9290(00)00202-5. [DOI] [PubMed] [Google Scholar]
- 10.Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res. 2009;467:1074–1082. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi Y. Comparative analysis of muscle architecture in primate arm and forearm. Anat Histol Embryol. 2010;39:93–106. doi: 10.1111/j.1439-0264.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JS, Hsu AW, Vasavada AN. Morphology, architecture, and biomechanics of human cervical multifidus. Spine. 2005;30:E86–E91. doi: 10.1097/01.brs.0000153700.97830.02. [DOI] [PubMed] [Google Scholar]
- 13.Wickiewicz TL, Roy RR, Powell PL, Edgerton VR. Muscle architecture of the human lower limb. Clin Orthop Relat Res. 1983;179:275–283. [PubMed] [Google Scholar]
- 14.Kellis E, Galanis N, Natsis K, Kapetanos G. Muscle architecture variations along the human semitendinosus and biceps femoris (long head) length. J Electromyogr Kinesiol. 2010;20:1237–1243. doi: 10.1016/j.jelekin.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Friederich JA, Brand RA. Muscle fiber architecture in the human lower limb. J Biomech. 1990;23:91–95. doi: 10.1016/0021-9290(90)90373-b. [DOI] [PubMed] [Google Scholar]
- 16.Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns KA, Fithian DC, Friden J, Lieber RL. Rotator cuff muscle architecture: implications for glenohumeral stability. Clin Orthop Relat Res. 2006;448:157–163. doi: 10.1097/01.blo.0000194680.94882.d3. [DOI] [PubMed] [Google Scholar]
- 17.Murray WM, Buchanan TS, Delp SL. The isometric functional capacity of muscles that cross the elbow. J Biomech. 2000;33:943–952. doi: 10.1016/s0021-9290(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 18.Becker I, Baxter GD, Woodley SJ. The vastus lateralis muscle: an anatomical investigation. Clin Anat. 2010;23:575–585. doi: 10.1002/ca.20974. [DOI] [PubMed] [Google Scholar]
- 19.Langenderfer JE, Patthanacharoenphon C, Carpenter JE, Hughes RE. Variability in isometric force and moment generating capacity of glenohumeral external rotator muscles. Clin Biomech. 2006;21:701–709. doi: 10.1016/j.clinbiomech.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovering RM, Anderson LD. Architecture and fiber type of the pyramidalis muscle. Anat Sci Int. 2008;83:294–297. doi: 10.1111/j.1447-073X.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kura H, Luo ZP, Kitaoka HB, An KN. Quantitative analysis of the intrinsic muscles of the foot. Anat Rec. 1997;249:143–151. doi: 10.1002/(SICI)1097-0185(199709)249:1<143::AID-AR17>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson MD, Raab R, Fazeli BM, Abrams RA, Botte MJ, Lieber RL. Architectural design of the human intrinsic hand muscles. J Hand Surg Am. 1992;17:804–809. doi: 10.1016/0363-5023(92)90446-v. [DOI] [PubMed] [Google Scholar]
- 23.Regev GJ, Kim CW, Tomiya A, Lee YP, Ghofrani H, Garfin SR, Lieber RL, Ward SR. Psoas Muscle Architectural Design, In Vivo Sarcomere Length Range, and Passive Tensile Properties Support its Role as a Lumbar Spine Stabilizer. Spine. 2011 doi: 10.1097/BRS.0b013e31821847b3. [DOI] [PubMed] [Google Scholar]
- 24.Janda S, van der Helm FCT, de Blok SB. Measuring morphological parameters of the pelvic floor for finite element modelling purposes. J Biomech. 2003;36:749–757. doi: 10.1016/s0021-9290(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 25.Friden J, Lieber RL. Quantitative Evaluation of the Posterior Deltoid to Triceps TendonTransfer Based on Muscle Architectural Properties. J Hand Surg. 2001;26A:147–155. doi: 10.1053/jhsu.2001.20161. [DOI] [PubMed] [Google Scholar]
- 26.Brown SH, Ward SR, Cook MS, Lieber RL. Architectural Analysis of Human Abdominal Wall Muscles Implications for Mechanical Function. Spine. 2011;36:355–362. doi: 10.1097/BRS.0b013e3181d12ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friden J, Lovering RM, Lieber RL. Fiber Length Variability Within the Flexor Carpi Ulnaris and Flexor Carpi Radialis Muscles: Implications for Surgical Tendon Transfer. J Hand Surg. 2004;29A:909–914. doi: 10.1016/j.jhsa.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Abrams GD, Ward SR, Friden J, Lieber RL. Pronator Teres Is an Appropriate Donor Muscle for Restoration of Wrist and Thumb Extension. J Hand Surg. 2005;30A:1068–1073. doi: 10.1016/j.jhsa.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Eng CM, Smallwood LJ, Rainiero MP, Lahey M, Ward SR, Lieber RL. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol. 2008;211:2336–2345. doi: 10.1242/jeb.017640. [DOI] [PubMed] [Google Scholar]
- 30.Ikai M, Fukunaga T. Calculation of muscle strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int Z Angew Physiol. 1968;26:26–32. doi: 10.1007/BF00696087. [DOI] [PubMed] [Google Scholar]