Abstract
Stiff Person Syndrome (SPS) is a disabling autoimmune CNS disorder characterized by progressive muscle rigidity and gait impairment with superimposed painful spasms that involve axial and limb musculature, triggered by heightened sensitivity to external stimuli. Impaired synaptic GABAergic inhibition resulting from intrathecal B-cell-mediated clonal synthesis of autoantibodies against various presynaptic and synaptic proteins in the inhibitory neurons of the brain and spinal cord is believed to be an underlying pathogenic mechanism. SPS is most often idiopathic, but it can occur as a paraneoplastic condition. Despite evidence that anti-GAD and related autoantibodies impair GABA synthesis, the exact pathogenic mechanism of SPS is not fully elucidated. The strong association with several MHC-II alleles and improvement of symptoms with immune-modulating therapies support an autoimmune etiology of SPS. In this review, we discuss the clinical spectrum, neurophysiological mechanisms, and therapeutic options, including a rationale for agents that modulate B cell function in SPS.
Keywords: GABA, anti-GAD antibodies, stiff person syndrome, autoimmunity, paraneoplastic disorders
Introduction
Stiff Person Syndrome (SPS) was first described in 1956 as a new clinical entity by Moersch and Woltman in a series of 14 patients.1 It is a rare CNS disorder characterized by progressive rigidity of the truncal muscles, superimposed spasms, and an exquisite sensitivity to external stimuli.2–5,1,6 Co-contractions of agonist and antagonist muscles and continuous involuntary firing of motor units at rest are the clinical and electrophysiological hallmarks of the disease.7–9,1 SPS is commonly associated with high anti-glutamic acid decarboxylase (GAD) antibody titers and a variety of other organ-specific autoantibodies across a wide spectrum of clinical presentations.10–13 The antibodies are believed to cause primarily a functional blockade in SPS by targeting antigens expressed in neurons of the brain and spinal cord at synapses using the neurotransmitter gamma-aminobutyric acid (GABA). Although some autopsies have shown evidence of perivascular inflammation, and, in the rapidly progressive encephalomyelitis variant, structural damage in the CNS,16,8,17,18 autopsies of typical cases showed no inflammation and relatively little decrease in neuronal numbers.14,15 High titers of anti-GAD antibodies in the serum and CSF of SPS patients seem to be directed against conformational forms of GAD selectively expressed in GABAergic neurons19,20,2,13,21,22,11,12 and can cause a blockade of GABA synthesis.23 The acquired malfunction of the spinal and supra-segmental inhibitory networks utilizing GABA is hypothesized to be the mechanism underlying the excessive motor neuron firing in SPS.9,24,25,3,26,27
GAD is also a major autoantigen in Insulin-dependant diabetes mellitus (IDDM), which is often associated with SPS. Although anti-GAD antibodies are detected in up to 80% of newly diagnosed type I diabetes patients, the titers are usually 50- to 100-fold less than in SPS patients with or without IDDM.19,28,2,29 Approximately 70% of SPS patients with high-titer GAD antibody also have antibodies against a synaptic protein, GABA-receptor-associated protein (GABARAP), that is involved in the endocytosis, recycling and maintenance of synaptic vesicles and receptors.30 In a subgroup of SPS patients, proximal muscle stiffness is a paraneoplastic manifestation of breast, ovarian or small-cell lung carcinomas (SCLC), associated with antibodies against amphiphysin, 31–41 and gephyrin,42 two synaptic proteins. Paraneoplastic SPS with anti-amphiphysin antibodies is most commonly found in association with breast adenocarcinoma and SCLC.31,32,40,37,38,43–45 Of interest, anti-GAD antibody is conspicuously absent in these patients; in only one reported paraneoplastic SPS case with co-morbid renal carcinoma, anti-GAD, but not amphiphysin antibodies were present.46 Currently, there are no immunoassays or ‘gold-standard’ diagnostic electrophysiological tests that unambiguously distinguish SPS from patients with other neurological syndromes associated with anti-GAD antibodies or IDDM.47 Although anti-GAD and amphiphysin antibodies are presumed to be pathogenic in SPS, proof of their direct causative role is still lacking. We include in this review an update on immunological aspects and the current understanding of electrophysiological concepts in SPS as a continuum of the earlier review by Espay et al.48
Clinical features and course
SPS rigidity usually begins insidiously in the thoracolumbar paraspinal muscles in patients in their mid-to-late 30s, usually without antecedent infection or other triggering factors, and extends over time to involve proximal leg and abdominal wall muscles. As a result of the muscle rigidity, patients develop a stiff, robotic gait and hyperlordosis of the spine with ‘a board-like’ appearance. Muscle rigidity may fluctuate at first but gradually becomes fixed and impairs the ability to bend and walk independently. SPS patients can exhibit major fluctuations of stiffness and spasms during a week or even over the course of a day. In general, they experience more symptoms and falls during times of physical or emotional stress, cold weather, and intercurrent infections. Rigidity typically improves during sleep. Although muscle stiffness is the ‘sine qua non’ in SPS, not all patients experience prominent rigidity and muscle spasms initially, but they develop the classic symptoms over time. The increasing stiffness over time results in substantial progression of functional impairment, and, in general, most patients require increasing doses or addition of new symptomatic therapies in order to achieve the same level of function.49
The second set of pathognomonic symptoms is episodic spasms, which are sudden and sometimes painful. They are often precipitated by external stimuli and physical obstacles and may result in unprotected falls. Besides a heightened response to unexpected stimuli, SPS patients also suffer from marked anticipatory anxiety and task-specific phobias, and often from reactive depression as well.50,51 Much of the anxiety in SPS patients appears to be a realistic fear of falling, rather than an inherent psychiatric disorder. However, conditioned responses and acquired dysregulation of hippocampus and amygdala circuits may play a role in the neuropsychological manifestations of SPS.50,52 As SPS progresses, the majority of patients have an increasing frequency of falls, require assistance for walking and activities of daily living, and frequently lose their ability to work.
Several subsets of SPS with more-or-less distinct clinical phenomenology and disease course have been described: ‘Stiff-limb syndrome’;53–55 SPS associated with myoclonus (Jerking stiff man syndrome), presumably from predominant brainstem involvement;56,57,26 SPS associated with epilepsy and dystonia;57–59 or SPS with neurophthalmologic manifestations such as autoimmune retinopathy.60 Stiff person syndrome with progressive rigidity and encephalomyelitis is a much rarer form of SPS. It is characterized by a subacute encephalomyelitis that primarily affects the grey matter, resulting in widespread rigidity and rapid decline of cognitive capacities and typically leads to premature death.57,61,59,26 A cerebellar variant of SPS is characterized by prominent gait ataxia and dysmetria, as well as ocular findings consistent with cerebellar dysfunction without evidence of structural brain abnormalities.47,62–67
The diagnosis of SPS is established by clinical findings and exclusion of pyramidal and extrapyramidal disorders, with supportive evidence from electrophysiological findings on EMG studies and serological and CSF testing that show elevated anti-GAD antibodies. Conventional MR imaging studies of the nervous system are usually normal.68 Magnetic resonance spectroscopy has demonstrated a significant regional decrease in GABA levels in the motor cortex,3,68 providing supportive evidence of deficient GABAergic inhibition as a pathophysiological mechanism in SPS. Diseases that should be differentiated from SPS include myelopathies, dystonias and other extrapyramidal diseases, neurodegenerative disorders such as spinocerebellar degenerations, primary lateral sclerosis, neuromyotonia or ‘Isaacs syndrome’, as well as rare forms of chronic tetanus and psychogenic disorders. Magnetic resonance imaging studies of the brain and spine are useful to exclude certain structural disorders, such as myelopathies. Electromyography plays an important role in establishing a diagnosis of SPS by demonstrating the characteristic involuntary firing of motor units.
Up to 35% of SPS patients have coexistent Type I diabetes, which may precede the onset of SPS by months to years or, more commonly, develop soon after the onset of stiffness.2 Besides the relatively high prevalence of IDDM, there are several other organ-specific autoimmune diseases associated with SPS, including autoimmune thyroiditis, Graves’ disease, pernicious anemia, vitiligo and celiac disease. Anti-GAD antibodies are an excellent serological marker for SPS; in addition, various other antibodies such as anti-thyroid, anti-intrinsic factor, anti-nuclear, anti-RNP, anti-gliadin and others are frequently present in serum. These likely represent a dysregulated immune system targeting different organs, as it is also observed in myasthenia gravis and other autoimmune disorders.
Physiology of SPS
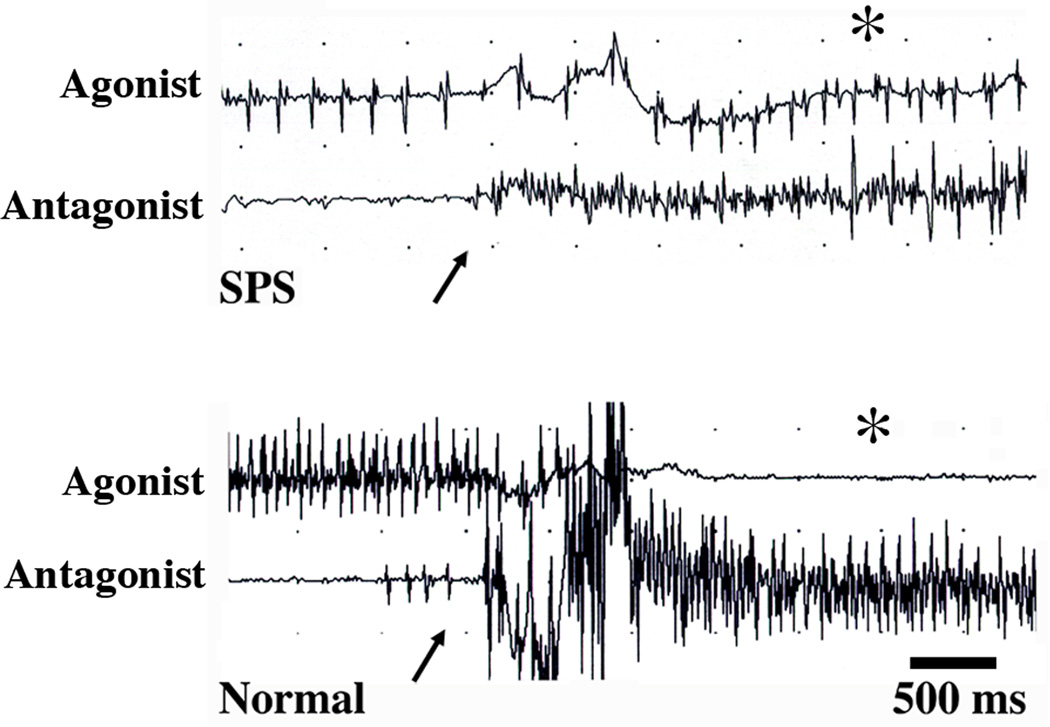
The muscle stiffness in SPS is produced by involuntary firing of motor neurons resembling a normal voluntary contraction in needle EMG recordings.7,1 The motor unit potentials (MUPs) have normal configurations and firing rates, and there are no findings suggestive of denervation. However, MUP firing continues when the SPS patient is at rest and during maneuvers, such as contraction of the antagonist muscle, which normally induce a reflex relaxation of the agonist muscle (Figure 1). Demonstrating failure of reciprocal inhibition by recording from antagonist muscle pairs can be helpful to support the diagnosis of SPS and to illustrate the involuntary nature of the contraction. In SPS, MUP firing at rest is particularly prominent in those muscles which exhibit clinical stiffness, typically the proximal leg and paraspinal muscles, and EMG recording from paraspinal muscles may be useful when limb muscle recordings are equivocal. Although the MUP activity is typically referred to as ‘continuous MUP firing’, the amount of activity observed in individual muscles fluctuates, and periods of relative relaxation can be appreciated in prolonged recordings made with surface EMG.69 Sleep, treatment with benzodiazepines or baclofen, and general anesthesia reduce MUP firing as well as the stiffness and spasms.70–72,7,9,69 Reduction of MUP firing and spasms by diazepam has been used as one of the clinical diagnostic criteria for SPS.4–6
Figure 1.
Reciprocal inhibition between antagonist muscles. The upper pair of traces shows needle EMG recordings from a pair of antagonist muscles in a patient with SPS, with involuntary MUP firing in the agonist muscle (top trace). Volitional contraction of the antagonist muscle (arrow) does not silence the agonist MUP firing (asterisk). In contrast, in the lower pair of traces, contraction of the antagonist muscle (arrow) silences the voluntary contraction (asterisk) in a healthy control subject voluntarily contracting the agonist muscle.
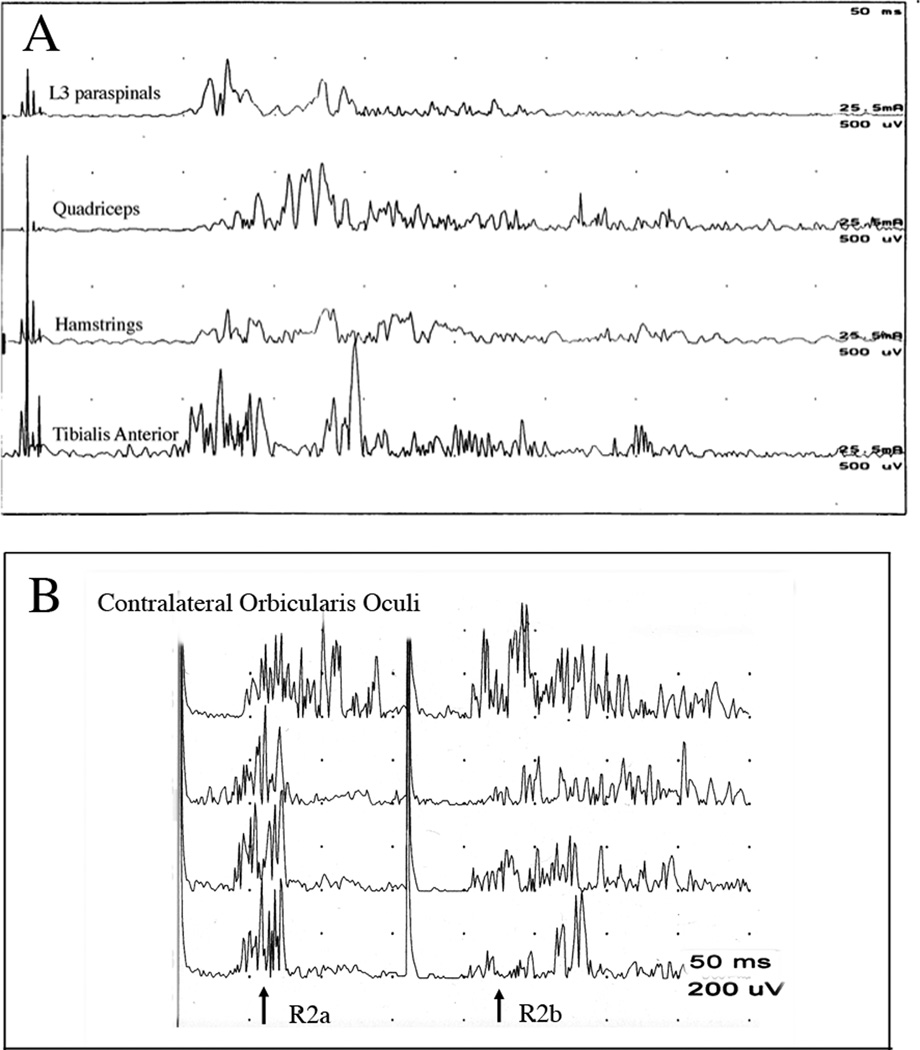
The spasms that occur in SPS can occur spontaneously or be triggered by external stimuli such as touch or loud sounds. Spasms typically begin abruptly, involve co-contraction of multiple muscles, are often bilateral, and may last for minutes or recur over several hours.7,26,69,9 Spasms can be strong enough to produce posturing of the limbs or spine and cause bone fractures.70,26 When spasms are elicited by cutaneous or acoustic stimuli, the timing and pattern of the initial muscle activation may resemble an exaggerated segmental or brainstem reflex, although there is abnormal spread of activity to additional muscles, particularly the clinically stiff muscles. However, following the normal reflex, a prolonged muscle activation with co-contraction of antagonist muscles typically occurs, and is clinically observed as a spasm.9,26 This excessive spread of reflexes and spasms occurs with stimulation of cutaneous nerves at non-noxious intensities, as shown for the leg flexor reflex in an example from one patient (Figure 2A) and for blink reflexes73 from another patient (Figure 2B). Demonstrating that stimulation of a cutaneous or mixed nerve produces EMG activity in distant limbs or paraspinal muscles can provide supportive evidence for the clinical impression of SPS. Acoustic startle responses are also abnormal in SPS, with spread to limb muscles and prominent spasms in leg or axial muscles where stiffness predominates.74,25 The disinhibition of startle responses and other brainstem reflexes in SPS is also seen in hereditary hyperekplexia, a disorder of glycinergic transmission, leading to the proposal that the excessive responsiveness to stimuli may reflect loss of inhibition at brainstem as well as spinal levels.25,73,75 The prolonged spasms following acousic stimuli that occur in SPS are not seen, however, in hereditary hyperekplexia.
Figure 2.
Increased reflex excitability in SPS patients. A. Flexor reflex of the leg. Stimulation of the sural nerve with 4 pulses elicits contraction of the two flexor muscles (tibialis anterior, hamstrings) and spreads abnormally to extensor (quadriceps) and paraspinal muscles. B. Hyperexcitability of the blink reflex in an SPS patient following paired stimulation of the contralateral supraorbital nerve at 16 mA with an interstimulus interval of 160 ms. Four stimulation trials are shown. The first stimulus of each pair elicits a response (R2a) with normal latency, although the first trial produced a prolonged response. The R2 response (R2b) to the second stimulus of the pair should normally be fully inhibited at this interval, but instead a robust and prolonged blink occurs.
The involuntary motor neuron firing observed in SPS is not a primary abnormality of the motor neuron or of the monosynaptic stretch reflex arc. The MUPs fire at normal rates, and volitional recruitment is normal – except for co-contraction of antagonists. There is notable absence of the doublets, multiplets or repetitive discharges that are commonly seen with peripheral nerve hyperexcitability syndromes such as Isaacs disease.76 Motor nerve conduction velocities, F-waves, T-waves, and H-reflexes are normal, as are the silent periods induced by mixed nerve stimulation and muscle stretch,77,7 which contrasts to findings in patients with tetanus.78 Despite the patient’s muscle rigidity, stretch reflexes are brisk, and untreated SPS patients may exhibit clonus, but without abnormal plantar responses. Following the discovery of anti-GAD antibodies in SPS patients, several studies investigated the actions of interneuron circuits believed to use GABA as a neurotransmitter, with an initial focus on the inhibitory spinal cord interneuron circuits. Several studies reported enhanced H-reflex recovery and reduced vibration-induced H-reflex inhibition, phenomena which are believed to be mediated by GABAergic interneurons that produce presynaptic inhibition of stretch reflex afferents.24,79,9 One study that examined additional spinal inhibitory reflexes found a complex pattern of disinhibition, with sparing of some presumptive GABAergic spinal reflex circuits, and impairment of some presumptive glycinergic inhibitory circuits.24 The authors speculated that these findings could result from previously unrecognized GABAergic contributions to presumed glycinergic reflexes, differential suscepibility of interneuron populations, or from impaired descending modulation of spinal inhibitory circuits by descending supraspinal systems.
Because the corticospinal system is known to modulate inhibitory spinal interneurons, Sandbrink and colleagues examined the excitability of the motor cortex in seven SPS patients using transcranial magnetic stimulation (TMS).27 A paired-pulse TMS paradigm with subthreshold conditioning stimulation was used to assess short intracortical inhibition (SICI) and intracortical facilitation (ICF). In this paradigm, a subthreshold conditioning stimulus is given that activates cortical interneurons without producing a motor evoked potential (MEP), followed by a second “test” stimulus at an intensity sufficient to produce a small MEP. At short interstimulus intervals, less than 5 ms, the MEP is inhibited, whereas at longer intervals, from 8–30 ms, the MEP is facilitated.80 Sandbrink and colleagues found that SPS patients had markedly increased ICF compared to healthy controls; conditioning TMS stimuli did not produce similar facilitation of H-reflexes, demonstrating that the facilitation was not due to increased motor neuron excitability. Short intracortical inhibition is thought to be mediated by cortical GABAergic interneurons, but the mechanism of ICF is not entirely clear. Drugs that enhance GABAergic transmission or block the glutamatergic NMDA receptor reduce ICF.81–83 Because ICF does not produce changes in spinal motor neuron excitability, as measured by its effects on H-reflexes,84 it has been inferred that the facilitation is generated by intracortical circuits. It should be noted, however, that a recent study in patients with implanted epidural electrodes failed to find an increase in the number or amplitude of descending volleys associated with the facilitated MEP, raising the question whether facilitation occurred through unidentified subcortical circuits, undetected dispersed descending volleys, or changes in the composition of corticospinal neurons firing in the volley.85
Sandbrink and colleagues also found that cortical silent periods following MEPs were shortened and that paired suprathreshold stimulation, which reflects cortical and spinal excitability, produced greater facilitation in SPS patients than healthy controls. SPS patients had normal thresholds for activating MEPs and normal central motor conduction times, providing evidence that interneurons, and not corticospinal neurons, were responsible for the increased excitability.27 In a larger study, Koerner and colleagues extended these findings to show that the magnitude of ICF was greater in untreated than treated SPS patients, that it was associated with high levels of anti-GAD antibody in the CSF, and that ICF was reduced by GABAergic medications.86 In one SPS patient who underwent physiological and serological testing before and throughout immunosuppressive treatment, treatment was associated with a concurrent decline in excessive ICF, serum anti-GAD antibody titers, and clinical symptoms.87 A reduction in intracortical inhibition would be consistent with magnetic resonance spectroscopy findings of reduced levels of GABA in the sensory-motor cortex.3,68 However, as GABAergic neurons are widespread in the brain and spinal cord, reduced inhibition at multiple levels in the neuraxis is likely to contribute to the excessive excitatory drive upon the motor neurons that produces muscle stiffness and spasm. The relative contributions from cortical, brainstem and spinal circuits to the generation of clinical symptoms are difficult to ascertain and could differ among individual patients.
Immunogenetics
Genetic risk for SPS and overlapping autoimmune diseases includes genes within the major histocompatibility complex (MHC) such as the human leukocyte antigens (HLA) DR and DQ alleles.88,89 In both idiopathic and paraneoplastic SPS, there is a strong association with several DQB1 and DRB1 MHC-II alleles. It appears that the HLA class II locus confers most of the shared susceptibility for these diseases; the DQB1* 0201 allele is present in approximately 70 % of patients with SPS,89 which is also a prevalent allele in IDDM without SPS and other autoimmune disorders. The DQB1*0602 allele seems to have a protective property, and it associated with a reduced occurrence of IDDM in SPS patients.89
Antibodies against components of inhibitory synapses
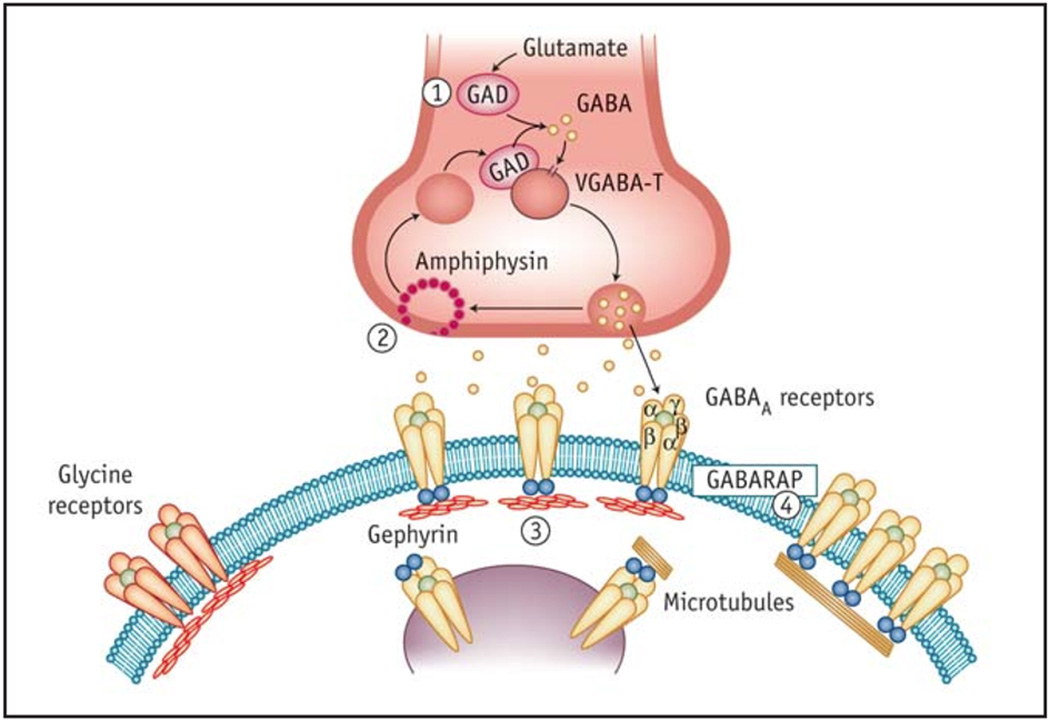
Circulating antibodies against several of the components of inhibitory synapses have been found in SPS (Figure 3)90,91 The best serological marker for SPS is an antibody directed against GAD, a protein that catalyzes the decarboxylation of L-glutamate to GABA and is widely expressed in presynaptic GABAergic terminals in the CNS. GAD is a cytoplasmic enzyme present in two isoforms that are encoded by genes on different chromosomes.92 These isoforms mostly differ in the amino-terminal region that accounts for their sub-cellular localization; GAD65 is attached to the surface of synaptic vesicles in GABAergic neurons or microvesicles in the pancreatic beta-cells, while GAD67 is a soluble form detectable only in the CNS.93,20 GABA is the main inhibitory neurotransmitter in the forebrain, whereas both GABA and glycine serve as inhibitory neurotransmitters in the brainstem and spinal cord94–96. GABARAP is a postsynaptic protein that stabilizes and modulates the conductance of GABA-A receptors in the postsynaptic membranes of GABAergic synapses.97,98 The protein gephyrin is found at both glycinergic and GABAergic synapses, where it plays a role in clustering glycine receptors and GABA-A receptors in the brain and the spinal cord.96
Figure 3.
Components of the inhibitory synapse recognized by known antibodies in Stiff Person Syndrome. The pre-synaptic glutamic acid decarboxylase (GAD) (1) is the rate-limiting enzyme in GABA synthesis; amphiphysin (2) is a cytosolic, pre-synaptic vesicle-associated protein responsible for endocytosis of vesicle plasma membranes following GABA release. The post-synaptic target antigens in SPS are gephyrin (3), and GABA-A-receptor-associated protein (GABARAP) (4). Gephyrin is a cytosolic, tubulin-binding protein involved in clustering the glycine and GABA-A receptors in the spinal cord and the brain. GABARAP is a linker protein between gephyrin and GABA-A receptors and promotes recycling and organization of the GABA receptors. The most common autoantigen in SPS is GAD, which is seen in 85 % of patients, followed by GABARAP, which is found in 65 % of patients. Amphiphysin is detected in 5 % of patients, while gephyrin has been seen only in one case. (From reference 91 with permission).
Anti-GAD antibodies were first reported by Solimena and colleagues in a patient afflicted by SPS, diabetes mellitus and epilepsy.12 Anti-GAD antibodies have also been found in the serum of patients affected by insulin-dependent diabetes mellitus (IDDM) without associated neurological disorders, but in much lower titers.28,11,47,99 Anti-GAD antibodies are also reported in 1% of the normal population and in 5% of patients with other neurological syndromes.100 However, the recognized GAD epitopes differ between patients with diabetes mellitus and those with SPS. In IDDM, the antibodies are found to recognize conformational epitopes, while in SPS they mostly recognize linear and denatured epitopes in the –NH2 terminal region of the GAD antigen.19,20,101,21,22 A recent study points toward the decarboxylase catalytic site as a particularly antigenic motif.102 Differences in epitope fidelity and specificity may explain the low incidence of stiff person syndrome in patients with diabetes mellitus (about 1 in 10,000 persons). 21,103
Anti-GAD antibodies have been measured in the serum and CSF using ELISA and more sensitive RIA methods.104,105 Anti-GAD65 antibodies are present in the serum of 80% of SPS patients, while antibodies against the GAD67 isoform occur in less than 50% of patients and at much lower levels.23,90 When GAD-titers were compared to the disease severity, as measured by the Stiffness Index and Heightened Sensitivity scores, no consistent correlation was found between the serum and CSF titers and the clinical fluctuations of the disease; the titers were high in some patients with mild disease and low in some others with severe disease as previously reported.13,52 In the CSF, antibodies against GAD65 are detected in 75% of patients at titers 50-fold lower than in the serum, but with a 10-fold higher rate of synthesis and binding avidity.52,47,90 It is suggested that this is due to intrathecal synthesis of GAD-specific IgG by clonally restricted B-cells within the CSF compartment and driven by local antigens. The different epitope specificity noted between paired serum and CSF specimens further suggests local stimulation of B cells within the confines of the blood-brain barrier.106 A potential role of infection in the loss of immune tolerance on the basis of molecular mimicry has also been implicated, especially since GAD65 is expressed in thymus and was also localized in antigen-presenting cells.107 In a patient who developed SPS following West Nile virus infection, a stretch of 12 amino acids homologous between the virus and GAD65 suggested that loss of tolerance after the infection may have been responsible for autoimmune SPS.108
The exact mechanism by which these autoantibodies interact with intracellular antigens in the brain parenchyma remains unknown, since GAD, gephyrin and amphiphysin are cytosolic and not readily recognized by the immune system. One hypothesis is that, during GABA exocytosis, GAD65 peptide fragments may be exposed on the neuronal surface and become the target of autoantibodies. It has been postulated that intrathecally produced immunoglobulins may target antigens expressed in the brain and spinal cord by recognizing different epitopes than those in the serum, and may exert a change of synaptic transmission at the neuronal level by blocking either function or synthesis of GAD. 85,101 Arguably, the lack of neurological symptoms in infants who acquire high GAD65 titers through passive transfer from mothers with SPS and failed experiments to induce SPS symptoms in mice using patients’ GAD sera suggest that these antibodies may not be pathogenic. 85, 115
Anti-GAD antibodies are also found in association with neurological conditions other than SPS, such as cerebellar ataxia, limbic encephalitis with myoclonus, temporal lobe epilepsy and others.109,63 GAD-associated cerebellar ataxia is often accompanied by polyendocrine autoimmunity including IDDM and is manifested by prominent cerebellar dysmetria, nystagmus and dysarthria.47 These patients may respond favorably to steroid treatment.110,111 Cerebellar symptoms in SPS patients with prominent cerebellar findings seem not to respond to immunotherapies,67 despite observations that their anti-GAD antibody titers and immunoreactivity are not significantly different from patients with cerebellar ataxia only.63,47 Drug-refractory temporal lobe epilepsy patients may also have high-titers of anti-GAD antibodies, which could be acting to lower the seizure threshold through decreased inhibition by hippocampal GABAergic neurons.112–114
Amphiphysin and SPS
In the paraneoplastic variant of SPS (5% of all SPS patients), there are anti-amphiphysin and anti-gephyrin antibodies (n = 1),42 most commonly found in association with breast adenocarcinoma and small-cell lung carcinoma.31,33,115,40,116,45 Amphiphysin is a widely expressed presynaptic protein that supports endocytosis by formation of dynamin rings around clathrin vesicles117,118 and regulates the density of receptors, particularly GABA-A, at the axon membrane.119 It is possible that antibodies to amphiphysin interfere with the expression of GABA-A receptors at synapses on the membranes of spinal and other motor neurons. Such a mechanism could be related to the signs and symptoms of SPS as shown in the experiments using passive transfer of amphiphysin-specific IgG from a patient with breast cancer and SPS into rats with induced blood-brain barrier leakage.120,121 These animals developed dose-dependent motor signs of SPS including typical EMG findings, and IgG binding was demonstrated in their CNS. These experiments support the hypothesis of a direct pathogenic role of amphiphysin antibodies as shown in other landmark passive transfer experiments in myasthenia gravis and Lambert-Eaton myasthenic syndrome.122–124 Also, clinical improvement correlates with lowering of amphiphysin antibody titers by plasmapheresis.125 Nevertheless, the induction of an autoimmune SPS by active immunization with GAD and amphiphysin antigens, as shown for nicotinic acetylcholine receptors in myasthenia gravis, has not been demonstrated.121 Furthermore, SPS is not transmissible by passive transfer of anti-GAD and amphiphysin antibodies in the setting of an intact blood-brain barrier, such as through maternal transfer of antibodies.126,127
Since anti-amphiphysin and gephyrin antibodies target the antigens expressed in tumor tissue as well as the CNS, this raises the possibility of cross-reactive binding of antibodies that leads to disruption of the functioning of GABAergic neurons. There appears to be a close link between amphiphysin and SPS associated with breast and lung cancer, since anti-amphiphysin antibodies are not typically present in SPS without cancer, or in cancer patients without SPS. GAD antibodies were notably absent in most previously described cases of amphiphysin antibody-positive paraneoplastic SPS; there has been only one case report with both anti-GAD and anti-amphiphysin antibodies in association with breast cancer.44 Cancer patients with paraneoplastic disorders are prone to develop a complex state of autoimmunity due to ectopic expression or over-expression of neuronal antigens. This can lead to simultaneous production of several autoantibodies, which may be specific for neuronal tissue and may or may not be clinically relevant.128 Enhanced expression of amphiphysin in breast cancer tissue and its potential role in the neoplastic transformation of normal cells through an impairment of growth regulatory mechanisms has also been described.38 The degree of molecular mimicry at the tumor site may be more important in the pathogenesis of immune-mediated manifestations rather than the actual titers of paraneoplastic antibodies. This hypothesis is supported by the observation that high titers of anti-neuronal antibodies directed against putative antigens of neuroectodermal tumors, such as SCLC, are less commonly associated with paraneoplastic SPS than with adenocarcinoma.115,116,128
SPS patients who develop cancer cannot be distinguished from idiopathic cases on clinical or electrodiagnostic grounds. Different patterns of stiffness and phenotypes in cryptogenic and paraneoplastic SPS are likely to represent a clinical continuum with a similar underlying mechanism in which a disregulated immune system allows autoantibodies to target GABAergic pathways in the CNS.129–131 Nevertheless, prominent trunk muscle involvement together with a poor response to standard SPS therapy, as well as symptoms of primary tumor, should raise the possibility of a paraneoplastic SPS. A comprehensive screen is indicated to look for occult malignancy in the setting of unusual and progressive neurological syndromes such as SPS, with a high suspicion for commonly encountered breast and lung carcinoma. Although not specific, amphiphysin antibodies may be useful in pointing to an undiscovered cancer as the etiology of the neurological syndrome. In paraneoplastic SPS, cross-reactive binding of serum antibodies with malignant cells expressing neuronal antigens such as GAD and amphiphysin may be responsible for triggering the autoimmune response. Management of the primary tumor is central to neurological outcome in patients with paraneoplastic disease.132 When specific antibodies are identified and clinical suspicion is high, in addition to full body CT scans, Fluoro-2-deoxy-glucose (FDG)-positron emission tomography scanning is important to increase the sensitivity of tumor detection.133,132,134
Experimental studies of antibody pathogenesis
The proposed pathogenic role of anti-GAD antibodies in SPS was initially inferred from the immunostaining pattern against GABAergic neurons using SPS patient sera.12 Two mechanisms have been proposed to explain how anti-GAD and amphiphysin antibodies impair GABAergic neurotransmission: 1) inhibition of GABA synthesis and 2) interference with the exocytosis of vesicles containing GABA.23,65,120 Meinck and colleagues showed that anti-GAD antibodies inhibited the synthesis of GABA in extracts of rat cerebellum; inhibition occurred in a dose-dependent manner with IgG from the serum and cerebrospinal fluid of several patients with SPS with anti-GAD antibodies, but not from IDDM patients with anti-GAD antibodies or patients without anti-GAD antibodies.23 Such studies support the mechanism of impaired synthesis of GABA. However, patch clamp recordings from intact neurons in slices of rat cerebellum or hippocampus that were perfused with anti-GAD antibodies from patients with various CNS syndromes showed changes consistent with decreased presynaptic GABA release.65,135,113 The mechanism by which antibodies impair synaptic transmission has been studied in greater detail for anti-amphiphysin antibodies than for anti-GAD antibodies. Using calcium imaging to measure postsynaptic potentials in cultured embryonic motor neurons, anti-amphiphysin IgG from a patient with paraneoplastic SPS was shown to reduce GABA-induced calcium influx, consistent with reduced presynaptic release of GABA.136 Intrathecal administration of the purified antibodies from this same patient into a rat produced stiffness, muscle spasms, and reduced postactivation depression of H-reflexes,120 paralleling the clinical and electrophysiological findings in patients with SPS.24,7,77,26 It has also been shown that anti-amphiphysin antibodies were internalized by mouse hippocampal neurons and that synaptic activity produced progressive reduction of induced GABAergic postsynaptic currents.120 The antibodies co-localized with other presynaptic proteins associated with synaptic vesicles and vesicle recycling. The internalization of antibodies occurred to a greater extent in GABAergic terminals than in excitatory terminals, and it was proposed that the high rate of vesicle turnover in GABAergic terminals was a factor in the preferential internalization. To date, there is no evidence for similar internalization of anti-GAD antibodies, however.
Physiological studies have found that the functioning of some presumptive GABAergic inhibitory circuits of the brain and spinal cord are less affected than others.24 This may reflect the complexity of the networks at multiple levels that use GABA and are also modulated by GABAergic neurons. Other explanations for this heterogeneity might be differences in the antigenic determinants among GABAergic neurons or in the accessibility of antibodies to GAD to different terminal fields. Additionally, in some neurons or circuits, inhibitory transmitters such as glycine may be able to compensate for the loss of GABA, as some classes of spinal interneurons have been shown to contain both GABA and glycine in the same synaptic vesicle during development.137 In paraneoplastic SPS, GABAergic synapses appear more vulnerable than glutamatergic synapses to defective endocytosis induced by anti-amphiphysin antibodies. Whole-cell patch-clamp experiments on hippocampus granule cells have demonstrated a decrease in the amplitude of evoked inhibitory postsynaptic currents in vivo when the brain slices were treated with antibodies against amphiphysin.120
Therapeutic considerations in patients with SPS
Based on the presumed pathogenesis of SPS, the two main therapeutic approaches are: 1) GABA-enhancing drugs and 2) immunomodulating or immunosuppressant agents. As the reduced level of GABAergic tone appears to be responsible for muscle stiffness, medications that increase GABA activity alleviate SPS symptoms. Howard initially observed that the spasms dramatically improve with use of diazepam71 and this has been used to help confirm the clinical diagnosis of SPS, although not always reliably. At the onset of SPS symptoms and the time of establishing the appropriate diagnosis, diazepam or other benzodiazepines (GABAA agonists) are usually the first choice and the mainstay of therapy.70,71,138 Most patients respond favorably to diazepam, baclofen or similar drugs139–141 for some period of time, although they eventually require higher doses, which invariably cause drowsiness and other undesirable effects. Other, less commonly used approaches have included various muscle relaxants, botulinum toxin injections and some centrally acting agents. Botulinum toxin and intrathecal baclofen administration have been used sporadically but seem not to confer long-term benefit. They also have the potential for serious complications and are inconvenient to administer.142,143 Several reports have described substantial beneficial effect of immunotherapies such as prednisone, plasmapheresis144–146 and high-dose IVIg147–150 in the treatment of SPS. Intravenous immunoglobulin has been shown to be an efficacious and safe therapy for SPS patients in a controlled clinical trial,151 although not all the patients experienced a sustained benefit. Some patients are not able to tolerate intravenous immunoglobulin secondary to infusion-related headache, nausea and vomiting, as well as flu-like symptoms, rash, fatigue, or, less often, serious complications such as aseptic meningitis and stroke, which are rarely life-threatening.152,153 More recently, anti-B cell therapies using humanized monoclonal antibodies directed against CD20 + cells have been proposed as a rational approach to modulating autoreactive and clonally expanded B cells in the CNS in SPS.154 Several case reports have indicated that rituximab, a B-cell depleting monoclonal antibody, was well-tolerated and appeared to exert long-lasting clinical remissions,155–158 although circulating antibody titers did not decline.155,158 In a placebo-controlled trail, although muscle stiffness and spasms improved considerably in several treated patients, rituximab was found to be ineffective overall.159 It has been proposed that the immune response has rituximab-sensitive and -resistant components, with persistent antibody secretion, possibly from long-lived plasma and memory B cells.160
Concluding Remarks
The diagnosis of SPS requires a high degree of clinical suspicion in addition to diagnostic testing, with emphasis on specific serological markers such as anti-GAD, GABARAP and amphiphysin antibodies. Anti-GAD antibodies are produced intrathecally, presumably by B cells that have crossed the blood-brain barrier. 13,106,161 There is evidence that clonal expansion of B cells, either in situ or intrathecally, and circulating autoantibodies play a causative or contributory role in the pathophysiology of many neurological diseases that overlap with SPS, some of which are associated with GAD antibodies such as subacute cerebellar ataxia, drug-refractory temporal epilepsy, brainstem encephalitis, and various forms of organ-specific autoimmune diseases.47 The occurrence of multiple neurological symptoms and signs in SPS patients, as well as the association of coexisting nuclear and cytoplasmic autoantibodies, may reflect evolving immune responses to multiple CNS and other tissue-specific antigens similar to the phenomenon of ‘intermolecular epitope spreading’ described in the paraneoplastic setting.41
A criticism against the pathogenic role of anti-GAD65, GABARAP, amphiphysin and gephyrin antibodies has been that they recognize cytoplasmic antigens. One possible explanation for how antibodies come to recognize GAD and other intracellular antigens is that certain peptide fragments could be transiently expressed at the cell surface during exocytosis and are presented to T-cell receptors by the antigen-presenting cells. For example, T-cell mediated mechanisms are evident in patients with IDDM, where a Th-1 response is seen with upregulation of interleukin-1 and interferon-gamma, and generation of cytotoxic T cells against the GAD of the pancreatic beta cells. In patients with SPS, however, the very high anti-GAD titers may be consistent with a Th-2 response, in which relevant cytokines, such as interleukin- 4 and interleukin- 6, suppress a T-cell-mediated cytotoxicity.162,103 However, a recent study using a mouse model demonstrated that GAD65CD4 + response caused SPS-like encephalomyelitis by disrupting the function of GABAergic neurons.163 An active T-cell response, especially in the early stages of SPS, appears to play an important role in driving humoral autoimmune processes,164 but significant T-cell infiltration is rarely observed in the brain and spinal cord of SPS patients post-mortem.165 Additional supportive evidence for the humoral autoimmune process is the clinical response to immunomodulatory therapies.91 Further advances in understanding the neurobiology and pathophysiology of SPS through emerging B-cell and T-cell depleting therapies will likely provide additional insight into the complex immune pathways involved in this autoimmune disorder.
Acknowledgements
Dr. Floeter is supported by the Intramural Research Program of the National Institutes of Health, NINDS.
List of Abbreviations
- Anti-RNP
Anti-Ribonucleoprotein antibody
- CNS
Central Nervous System
- CSF
Cerebrospinal fluid
- DM1
Diabetes mellitus type I
- ELISA
Enzyme-linked immunosorbent assay
- EMG
Electromyography
- GABA
Gamma-aminobutyric acid
- GABARAPA
GABA-A- receptor associated protein
- GAD
Glutamic acid decarboxylase
- GAD65
Glutamic acid decarboxylase 65 kilodalton isoform
- GAD67
Glutamic acid decarboxylase 67 kilodalton isoform
- HLA
Histocompatibility leukocyte antigen
- ICF
Intracortical facilitation
- IDDM
Insulin-dependant diabetes mellitus
- IgG
Immunoglobulin class G
- IVIg
Intravenous immunoglobulin
- MEP
motor evoked potential
- mA
Miliamperes
- MUP
motor unit potential
- SICI
short intracortical inhibition
References
- 1.Moersch FP, Woltman HW. Progressive fluctuating muscular rigidity and spasm ("stiff-man" syndrome); report of a case and some observations in 13 other cases. Proc Staff Meet Mayo Clin. 1956;31(15):421–427. [PubMed] [Google Scholar]
- 2.Dalakas MC, Fujii M, Li M, McElroy B. The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology. 2000;55(10):1531–1535. doi: 10.1212/wnl.55.10.1531. [DOI] [PubMed] [Google Scholar]
- 3.Levy LM, Dalakas MC, Floeter MK. The stiff-person syndrome: an autoimmune disorder affecting neurotransmission of gamma-aminobutyric acid. Ann Intern Med. 1999;131(7):522–530. doi: 10.7326/0003-4819-131-7-199910050-00008. [DOI] [PubMed] [Google Scholar]
- 4.Lorish TR, Thorsteinsson G, Howard FM., Jr Stiff-man syndrome updated. Mayo Clin Proc. 1989;64(6):629–636. doi: 10.1016/s0025-6196(12)65339-7. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy KM. Stiff-man syndrome. Semin Neurol. 1991;11(3):197–205. doi: 10.1055/s-2008-1041222. [DOI] [PubMed] [Google Scholar]
- 6.Toro C, Jacobowitz DM, Hallett M. Stiff-man syndrome. Semin Neurol. 1994;14(2):154–158. doi: 10.1055/s-2008-1041073. [DOI] [PubMed] [Google Scholar]
- 7.Meinck HM, Ricker K, Conrad B. The stiff-man syndrome: new pathophysiological aspects from abnormal exteroceptive reflexes and the response to clomipramine, clonidine, and tizanidine. J Neurol Neurosurg Psychiatry. 1984;47(3):280–287. doi: 10.1136/jnnp.47.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meinck HM, Ricker K, Hulser PJ, Schmid E, Peiffer J, Solimena M. Stiff man syndrome: clinical and laboratory findings in eight patients. J Neurol. 1994;241(3):157–166. doi: 10.1007/BF00868343. [DOI] [PubMed] [Google Scholar]
- 9.Meinck HM, Ricker K, Hulser PJ, Solimena M. Stiff man syndrome: neurophysiological findings in eight patients. J Neurol. 1995;242(3):134–142. doi: 10.1007/BF00936885. [DOI] [PubMed] [Google Scholar]
- 10.Grimaldi LM, Martino G, Braghi S, Quattrini A, Furlan R, Bosi E, Comi G. Heterogeneity of autoantibodies in stiff-man syndrome. Ann Neurol. 1993;34(1):57–64. doi: 10.1002/ana.410340111. [DOI] [PubMed] [Google Scholar]
- 11.Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med. 1990;322(22):1555–1560. doi: 10.1056/NEJM199005313222202. [DOI] [PubMed] [Google Scholar]
- 12.Solimena M, Folli F, Denis-Donini S, Comi GC, Pozza G, De Camilli P, Vicari AM. Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. N Engl J Med. 1988;318(16):1012–1020. doi: 10.1056/NEJM198804213181602. [DOI] [PubMed] [Google Scholar]
- 13.Dalakas MC, Li M, Fujii M, Jacobowitz DM. Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies. Neurology. 2001;57(5):780–784. doi: 10.1212/wnl.57.5.780. [DOI] [PubMed] [Google Scholar]
- 14.Ishizawa K, Komori T, Okayama K, Qin X, Kaneko K, Sasaki S, Iwata M. Large motor neuron involvement in Stiff-man syndrome: a qualitative and quantitative study. Acta Neuropathol. 1999;97(1):63–70. doi: 10.1007/s004010050956. [DOI] [PubMed] [Google Scholar]
- 15.Warich-Kirches M, Von Bossanyi P, Treuheit T, Kirches E, Dietzmann K, Feistner H, Wittig H. Stiff-man syndrome: possible autoimmune etiology targeted against GABA-ergic cells. Clin Neuropathol. 1997;16(4):214–219. [PubMed] [Google Scholar]
- 16.Holmoy T, Skorstad G, Hestvik AL, Alvik KM, Vartdal F. Protective and detrimental immunity: lessons from stiff person syndrome and multiple sclerosis. Acta Neurol Scand Suppl. 2009;(189):22–26. doi: 10.1111/j.1600-0404.2009.01207.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson PD. The stiff-man syndrome and related disorders. Parkinsonism Relat Disord. 2001;8(2):147–153. doi: 10.1016/s1353-8020(01)00029-3. [DOI] [PubMed] [Google Scholar]
- 18.Warren JD, Scott G, Blumbergs PC, Thompson PD. Pathological evidence of encephalomyelitis in the stiff man syndrome with anti-GAD antibodies. J Clin Neurosci. 2002;9(3):328–329. doi: 10.1054/jocn.2001.1014. [DOI] [PubMed] [Google Scholar]
- 19.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 20.Butler MH, Solimena M, Dirkx R, Jr, Hayday A, De Camilli P. Identification of a dominant epitope of glutamic acid decarboxylase (GAD-65) recognized by autoantibodies in stiff-man syndrome. J Exp Med. 1993;178(6):2097–2106. doi: 10.1084/jem.178.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis TM, Atkinson MA. The clinical significance of an autoimmune response against glutamic acid decarboxylase. Nat Med. 1996;2(2):148–153. doi: 10.1038/nm0296-148. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Namchuk M, Bugawan T, Fu Q, Jaffe M, Shi Y, Aanstoot HJ, Turck CW, Erlich H, Lennon V, Baekkeskov S. Higher autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen GAD65, distinguish stiff-man syndrome from insulin-dependent diabetes mellitus. J Exp Med. 1994;180(2):595–606. doi: 10.1084/jem.180.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinkel K, Meinck HM, Jury KM, Karges W, Richter W. Inhibition of gamma-aminobutyric acid synthesis by glutamic acid decarboxylase autoantibodies in stiff-man syndrome. Ann Neurol. 1998;44(2):194–201. doi: 10.1002/ana.410440209. [DOI] [PubMed] [Google Scholar]
- 24.Floeter MK, Valls-Sole J, Toro C, Jacobowitz D, Hallett M. Physiologic studies of spinal inhibitory circuits in patients with stiff-person syndrome. Neurology. 1998;51(1):85–93. doi: 10.1212/wnl.51.1.85. [DOI] [PubMed] [Google Scholar]
- 25.Khasani S, Becker K, Meinck HM. Hyperekplexia and stiff-man syndrome: abnormal brainstem reflexes suggest a physiological relationship. J Neurol Neurosurg Psychiatry. 2004;75(9):1265–1269. doi: 10.1136/jnnp.2003.018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meinck HM, Thompson PD. Stiff man syndrome and related conditions. Mov Disord. 2002;17(5):853–866. doi: 10.1002/mds.10279. [DOI] [PubMed] [Google Scholar]
- 27.Sandbrink F, Syed NA, Fujii MD, Dalakas MC, Floeter MK. Motor cortex excitability in stiff-person syndrome. Brain. 2000;123(Pt 11):2231–2239. doi: 10.1093/brain/123.11.2231. [DOI] [PubMed] [Google Scholar]
- 28.Solimena M, Butler MH, De Camilli P. GAD, diabetes, and Stiff-Man syndrome: some progress and more questions. J Endocrinol Invest. 1994;17(7):509–520. doi: 10.1007/BF03347745. [DOI] [PubMed] [Google Scholar]
- 29.Manto MU, Laute MA, Aguera M, Rogemond V, Pandolfo M, Honnorat J. Effects of anti-glutamic acid decarboxylase antibodies associated with neurological diseases. Ann Neurol. 2007;61(6):544–551. doi: 10.1002/ana.21123. [DOI] [PubMed] [Google Scholar]
- 30.Raju R, Rakocevic G, Chen Z, Hoehn G, Semino-Mora C, Shi W, Olsen R, Dalakas MC. Autoimmunity to GABAA-receptor-associated protein in stiff-person syndrome. Brain. 2006;129(Pt 12):3270–3276. doi: 10.1093/brain/awl245. [DOI] [PubMed] [Google Scholar]
- 31.De Camilli P, Thomas A, Cofiell R, Folli F, Lichte B, Piccolo G, Meinck HM, Austoni M, Fassetta G, Bottazzo G, et al. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of Stiff-Man syndrome with breast cancer. J Exp Med. 1993;178(6):2219–2223. doi: 10.1084/jem.178.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dropcho EJ. Antiamphiphysin antibodies with small-cell lung carcinoma and paraneoplastic encephalomyelitis. Ann Neurol. 1996;39(5):659–667. doi: 10.1002/ana.410390516. [DOI] [PubMed] [Google Scholar]
- 33.Folli F. Stiff man syndrome, 40 years later. J Neurol Neurosurg Psychiatry. 1998;65(5):618. doi: 10.1136/jnnp.65.5.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto R, Li X, Winter S, Francke U, Kilimann MW. Primary structure of human amphiphysin, the dominant autoantigen of paraneoplastic stiff-man syndrome, and mapping of its gene (AMPH) to chromosome 7p13-p14. Hum Mol Genet. 1995;4(2):265–268. doi: 10.1093/hmg/4.2.265. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49(2):146–154. [PubMed] [Google Scholar]
- 36.Butler MH, David C, Ochoa GC, Freyberg Z, Daniell L, Grabs D, Cremona O, De Camilli P. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol. 1997;137(6):1355–1367. doi: 10.1083/jcb.137.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.David C, Solimena M, De Camilli P. Autoimmunity in stiff-Man syndrome with breast cancer is targeted to the C-terminal region of human amphiphysin, a protein similar to the yeast proteins, Rvs167 and Rvs161. FEBS Lett. 1994;351(1):73–79. doi: 10.1016/0014-5793(94)00826-4. [DOI] [PubMed] [Google Scholar]
- 38.Floyd S, Butler MH, Cremona O, David C, Freyberg Z, Zhang X, Solimena M, Tokunaga A, Ishizu H, Tsutsui K, De Camilli P. Expression of amphiphysin I, an autoantigen of paraneoplastic neurological syndromes, in breast cancer. Mol Med. 1998;4(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- 39.McCabe DJ, Turner NC, Chao D, Leff A, Gregson NA, Womersley HJ, Mak I, Perkin GD, Schapira AH. Paraneoplastic "stiff person syndrome" with metastatic adenocarcinoma and anti-Ri antibodies. Neurology. 2004;62(8):1402–1404. doi: 10.1212/01.wnl.0000123694.64121.d5. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen-Huu BK, Urban PP, Schreckenberger M, Dieterich M, Werhahn KJ. Antiamphiphysin-positive stiff-person syndrome associated with small cell lung cancer. Mov Disord. 2006;21(8):1285–1287. doi: 10.1002/mds.20910. [DOI] [PubMed] [Google Scholar]
- 41.Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56(5):715–719. doi: 10.1002/ana.20269. [DOI] [PubMed] [Google Scholar]
- 42.Butler MH, Hayashi A, Ohkoshi N, Villmann C, Becker CM, Feng G, De Camilli P, Solimena M. Autoimmunity to gephyrin in Stiff-Man syndrome. Neuron. 2000;26(2):307–312. doi: 10.1016/s0896-6273(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 43.Rojas-Marcos I, Rousseau A, Keime-Guibert F, Rene R, Cartalat-Carel S, Delattre JY, Graus F. Spectrum of paraneoplastic neurologic disorders in women with breast and gynecologic cancer. Medicine (Baltimore) 2003;82(3):216–223. doi: 10.1097/01.md.0000076004.64510.ce. [DOI] [PubMed] [Google Scholar]
- 44.Rosin L, DeCamilli P, Butler M, Solimena M, Schmitt HP, Morgenthaler N, Meinck HM. Stiff-man syndrome in a woman with breast cancer: an uncommon central nervous system paraneoplastic syndrome. Neurology. 1998;50(1):94–98. doi: 10.1212/wnl.50.1.94. [DOI] [PubMed] [Google Scholar]
- 45.Saiz A, Dalmau J, Butler MH, Chen Q, Delattre JY, De Camilli P, Graus F. Anti-amphiphysin I antibodies in patients with paraneoplastic neurological disorders associated with small cell lung carcinoma. J Neurol Neurosurg Psychiatry. 1999;66(2):214–217. doi: 10.1136/jnnp.66.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McHugh JC, Murray B, Renganathan R, Connolly S, Lynch T. GAD antibody positive paraneoplastic stiff person syndrome in a patient with renal cell carcinoma. Mov Disord. 2007;22(9):1343–1346. doi: 10.1002/mds.21374. [DOI] [PubMed] [Google Scholar]
- 47.Saiz A, Blanco Y, Sabater L, Gonzalez F, Bataller L, Casamitjana R, Ramio-Torrenta L, Graus F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(Pt 10):2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 48.Espay AJ, Chen R. Rigidity and spasms from autoimmune encephalomyelopathies: stiff-person syndrome. Muscle Nerve. 2006;34(6):677–690. doi: 10.1002/mus.20653. [DOI] [PubMed] [Google Scholar]
- 49.Vasconcelos OM, Dalakas MC. Stiff-person Syndrome. Curr Treat Options Neurol. 2003;5(1):79–90. doi: 10.1007/s11940-003-0024-x. [DOI] [PubMed] [Google Scholar]
- 50.Ameli R, Snow J, Rakocevic G, Dalakas MC. A neuropsychological assessment of phobias in patients with stiff person syndrome. Neurology. 2005;64(11):1961–1963. doi: 10.1212/01.WNL.0000163984.71993.FE. [DOI] [PubMed] [Google Scholar]
- 51.Henningsen P, Meinck HM. Specific phobia is a frequent non-motor feature in stiff man syndrome. J Neurol Neurosurg Psychiatry. 2003;74(4):462–465. doi: 10.1136/jnnp.74.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rakocevic G, Raju R, Dalakas MC. Anti-glutamic acid decarboxylase antibodies in the serum and cerebrospinal fluid of patients with stiff-person syndrome: correlation with clinical severity. Arch Neurol. 2004;61(6):902–904. doi: 10.1001/archneur.61.6.902. [DOI] [PubMed] [Google Scholar]
- 53.Barker RA, Revesz T, Thom M, Marsden CD, Brown P. Review of 23 patients affected by the stiff man syndrome: clinical subdivision into stiff trunk (man) syndrome, stiff limb syndrome, and progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry. 1998;65(5):633–640. doi: 10.1136/jnnp.65.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gurol ME, Ertas M, Hanagasi HA, Sahin HA, Gursoy G, Emre M. Stiff leg syndrome: case report. Mov Disord. 2001;16(6):1189–1193. doi: 10.1002/mds.1224. [DOI] [PubMed] [Google Scholar]
- 55.Saiz A, Graus F, Valldeoriola F, Valls-Sole J, Tolosa E. Stiff-leg syndrome: a focal form of stiff-man syndrome. Ann Neurol. 1998;43(3):400–403. doi: 10.1002/ana.410430322. [DOI] [PubMed] [Google Scholar]
- 56.Alberca R, Romero M, Chaparro J. Jerking stiff-man syndrome. J Neurol Neurosurg Psychiatry. 1982;45(12):1159–1160. doi: 10.1136/jnnp.45.12.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown P, Marsden CD. The stiff man and stiff man plus syndromes. J Neurol. 1999;246(8):648–652. doi: 10.1007/s004150050425. [DOI] [PubMed] [Google Scholar]
- 58.Nemni R, Braghi S, Natali-Sora MG, Lampasona V, Bonifacio E, Comi G, Canal N. Autoantibodies to glutamic acid decarboxylase in palatal myoclonus and epilepsy. Ann Neurol. 1994;36(4):665–667. doi: 10.1002/ana.410360419. [DOI] [PubMed] [Google Scholar]
- 59.Shaw PJ. Stiff-man syndrome and its variants. Lancet. 1999;353(9147):86–87. doi: 10.1016/S0140-6736(05)76151-1. [DOI] [PubMed] [Google Scholar]
- 60.Steffen H, Menger N, Richter W, Nolle B, Krastel H, Stayer C, Kolling GH, Wassle H, Meinck HM. Immune-mediated retinopathy in a patient with stiff-man syndrome. Graefes Arch Clin Exp Ophthalmol. 1999;237(3):212–219. doi: 10.1007/s004170050221. [DOI] [PubMed] [Google Scholar]
- 61.Fogan L. Progressive encephalomyelitis with rigidity responsive to plasmapheresis and immunosuppression. Ann Neurol. 1996;40(3):451–453. doi: 10.1002/ana.410400315. [DOI] [PubMed] [Google Scholar]
- 62.Giometto B, Miotto D, Faresin F, Argentiero V, Scaravilli T, Tavolato B. Anti-gabaergic neuron autoantibodies in a patient with stiff-man syndrome and ataxia. J Neurol Sci. 1996;143(1–2):57–59. doi: 10.1016/s0022-510x(96)00065-2. [DOI] [PubMed] [Google Scholar]
- 63.Honnorat J, Saiz A, Giometto B, Vincent A, Brieva L, de Andres C, Maestre J, Fabien N, Vighetto A, Casamitjana R, Thivolet C, Tavolato B, Antoine J, Trouillas P, Graus F. Cerebellar ataxia with anti-glutamic acid decarboxylase antibodies: study of 14 patients. Arch Neurol. 2001;58(2):225–230. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- 64.Abele M, Weller M, Mescheriakov S, Burk K, Dichgans J, Klockgether T. Cerebellar ataxia with glutamic acid decarboxylase autoantibodies. Neurology. 1999;52(4):857–859. doi: 10.1212/wnl.52.4.857. [DOI] [PubMed] [Google Scholar]
- 65.Ishida K, Mitoma H, Song SY, Uchihara T, Inaba A, Eguchi S, Kobayashi T, Mizusawa H. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol. 1999;46(2):263–267. [PubMed] [Google Scholar]
- 66.Kono S, Miyajima H, Sugimoto M, Suzuki Y, Takahashi Y, Hishida A. Stiff-person syndrome associated with cerebellar ataxia and high glutamic acid decarboxylase antibody titer. Intern Med. 2001;40(9):968–971. doi: 10.2169/internalmedicine.40.968. [DOI] [PubMed] [Google Scholar]
- 67.Rakocevic G, Raju R, Semino-Mora C, Dalakas MC. Stiff person syndrome with cerebellar disease and high-titer anti-GAD antibodies. Neurology. 2006;67(6):1068–1070. doi: 10.1212/01.wnl.0000237558.83349.d0. [DOI] [PubMed] [Google Scholar]
- 68.Levy LM, Levy-Reis I, Fujii M, Dalakas MC. Brain gamma-aminobutyric acid changes in stiff-person syndrome. Arch Neurol. 2005;62(6):970–974. doi: 10.1001/archneur.62.6.970. [DOI] [PubMed] [Google Scholar]
- 69.Armon C, McEvoy KM, Westmoreland BF, McManis PG. Clinical neurophysiologic studies in stiff-man syndrome: use of simultaneous video-electroencephalographic-surface electromyographic recording. Mayo Clin Proc. 1990;65(7):960–967. doi: 10.1016/s0025-6196(12)65157-x. [DOI] [PubMed] [Google Scholar]
- 70.Cohen L. Stiff-man syndrome. Two patients treated with diazepam. JAMA. 1966;195(3):222–224. doi: 10.1001/jama.195.3.222. [DOI] [PubMed] [Google Scholar]
- 71.Howard FM., Jr A new and effective drug in the treatment of the stiff-man syndrome: preliminary report. Proc Staff Meet Mayo Clin. 1963;38:203–212. [PubMed] [Google Scholar]
- 72.Meinck HM, Conrad B. Neuropharmacological investigations in the stiff-man syndrome. J Neurol. 1986;233(6):340–347. doi: 10.1007/BF00313920. [DOI] [PubMed] [Google Scholar]
- 73.Molloy FM, Dalakas MC, Floeter MK. Increased brainstem excitability in stiff-person syndrome. Neurology. 2002;59(3):449–451. doi: 10.1212/wnl.59.3.449. [DOI] [PubMed] [Google Scholar]
- 74.Matsumoto JY, Caviness JN, McEvoy KM. The acoustic startle reflex in stiff-man syndrome. Neurology. 1994;44(10):1952–1955. doi: 10.1212/wnl.44.10.1952. [DOI] [PubMed] [Google Scholar]
- 75.Berger C, Meinck HM. Head retraction reflex in stiff-man syndrome and related disorders. Mov Disord. 2003;18(8):906–911. doi: 10.1002/mds.10451. [DOI] [PubMed] [Google Scholar]
- 76.Maddison P. Neuromyotonia. Clin Neurophysiol. 2006;117(10):2118–2127. doi: 10.1016/j.clinph.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Correale J, Garcia Erro M, Kosac S, Masci M, Fernandez Liguori N, Sica RE. An electrophysiological investigation of the "stiff-man" syndrome. Electromyogr Clin Neurophysiol. 1988;28(4):215–221. [PubMed] [Google Scholar]
- 78.Poncelet AN. Blink reflexes and the silent period in tetanus. Muscle Nerve. 2000;23(9):1435–1438. doi: 10.1002/1097-4598(200009)23:9<1435::aid-mus17>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 79.Martinelli P, Nassetti S, Minardi C, Macri S, Ippoliti M. Electrophysiological evaluation of the stiff-man syndrome: further data. J Neurol. 1996;243(7):551–553. doi: 10.1007/BF00886879. [DOI] [PubMed] [Google Scholar]
- 80.Ziemann U. Intracortical inhibition and facilitation in the conventional paired TMS paradigm. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:127–136. [PubMed] [Google Scholar]
- 81.Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51(5):1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- 82.Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115(8):1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109(3):467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- 84.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96(4):1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- 86.Koerner C, Wieland B, Richter W, Meinck HM. Stiff-person syndromes: motor cortex hyperexcitability correlates with anti-GAD autoimmunity. Neurology. 2004;62(8):1357–1362. doi: 10.1212/01.wnl.0000120543.65812.33. [DOI] [PubMed] [Google Scholar]
- 87.Rossi S, Ulivelli M, Malentacchi M, Greco G, Bartalini S, Borgogni P, Giannini F. Effects of immunotherapy on motor cortex excitability in Stiff Person Syndrome. J Neurol. 2010;257(2):281–285. doi: 10.1007/s00415-009-5331-z. [DOI] [PubMed] [Google Scholar]
- 88.Golden B, Levin L, Ban Y, Concepcion E, Greenberg DA, Tomer Y. Genetic analysis of families with autoimmune diabetes and thyroiditis: evidence for common and unique genes. J Clin Endocrinol Metab. 2005;90(8):4904–4911. doi: 10.1210/jc.2004-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pugliese A, Solimena M, Awdeh ZL, Alper CA, Bugawan T, Erlich HA, De Camilli P, Eisenbarth GS. Association of HLA-DQB1*0201 with stiff-man syndrome. J Clin Endocrinol Metab. 1993;77(6):1550–1553. doi: 10.1210/jcem.77.6.8263140. [DOI] [PubMed] [Google Scholar]
- 90.Alexopoulos H, Dalakas MC. A critical update on the immunopathogenesis of Stiff Person Syndrome. Eur J Clin Invest. 2010;40(11):1018–1025. doi: 10.1111/j.1365-2362.2010.02340.x. [DOI] [PubMed] [Google Scholar]
- 91.Dalakas MC. Stiff person syndrome: advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol. 2009;11(2):102–110. doi: 10.1007/s11940-009-0013-9. [DOI] [PubMed] [Google Scholar]
- 92.Edelhoff S, Grubin CE, Karlsen AE, Alder DA, Foster D, Disteche CM, Lernmark A. Mapping of glutamic acid decarboxylase (GAD) genes. Genomics. 1993;17(1):93–97. doi: 10.1006/geno.1993.1288. [DOI] [PubMed] [Google Scholar]
- 93.Solimena M, Aggujaro D, Muntzel C, Dirkx R, Butler M, De Camilli P, Hayday A. Association of GAD-65, but not of GAD-67, with the Golgi complex of transfected Chinese hamster ovary cells mediated by the N-terminal region. Proc Natl Acad Sci U S A. 1993;90(7):3073–3077. doi: 10.1073/pnas.90.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alvarez FJ, Dewey DE, Harrington DA, Fyffe RE. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379(1):150–170. [PubMed] [Google Scholar]
- 95.Baer K, Waldvogel HJ, Faull RL, Rees MI. Localization of glycine receptors in the human forebrain, brainstem, and cervical spinal cord: an immunohistochemical review. Front Mol Neurosci. 2009;2:25. doi: 10.3389/neuro.02.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Waldvogel HJ, Baer K, Eady E, Allen KL, Gilbert RT, Mohler H, Rees MI, Nicholson LF, Faull RL. Differential localization of gamma-aminobutyric acid type A and glycine receptor subunits and gephyrin in the human pons, medulla oblongata and uppermost cervical segment of the spinal cord: an immunohistochemical study. J Comp Neurol. 2010;518(3):305–328. doi: 10.1002/cne.22212. [DOI] [PubMed] [Google Scholar]
- 97.Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100(2):279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- 98.Everitt AB, Luu T, Cromer B, Tierney ML, Birnir B, Olsen RW, Gage PW. Conductance of recombinant GABA (A) channels is increased in cells co-expressing GABA(A) receptor-associated protein. J Biol Chem. 2004;279(21):21701–21706. doi: 10.1074/jbc.M312806200. [DOI] [PubMed] [Google Scholar]
- 99.Solimena M, De Camilli P. Autoimmunity to glutamic acid decarboxylase (GAD) in Stiff-Man syndrome and insulin-dependent diabetes mellitus. Trends Neurosci. 1991;14(10):452–457. doi: 10.1016/0166-2236(91)90044-u. [DOI] [PubMed] [Google Scholar]
- 100.Meinck HM, Faber L, Morgenthaler N, Seissler J, Maile S, Butler M, Solimena M, DeCamilli P, Scherbaum WA. Antibodies against glutamic acid decarboxylase: prevalence in neurological diseases. J Neurol Neurosurg Psychiatry. 2001;71(1):100–103. doi: 10.1136/jnnp.71.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daw K, Ujihara N, Atkinson M, Powers AC. Glutamic acid decarboxylase autoantibodies in stiff-man syndrome and insulin-dependent diabetes mellitus exhibit similarities and differences in epitope recognition. J Immunol. 1996;156(2):818–825. [PubMed] [Google Scholar]
- 102.Burbelo PD, Groot S, Dalakas MC, Iadarola MJ. High definition profiling of autoantibodies to glutamic acid decarboxylases GAD65/GAD67 in stiff-person syndrome. Biochem Biophys Res Commun. 2008;366(1):1–7. doi: 10.1016/j.bbrc.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lohmann T, Londei M, Hawa M, Leslie RD. Humoral and cellular autoimmune responses in stiff person syndrome. Ann N Y Acad Sci. 2003;998:215–222. doi: 10.1196/annals.1254.024. [DOI] [PubMed] [Google Scholar]
- 104.Schmidli RS, Colman PG, Bonifacio E. Disease sensitivity and specificity of 52 assays for glutamic acid decarboxylase antibodies. The Second International GADAB Workshop. Diabetes. 1995;44(6):636–640. doi: 10.2337/diab.44.6.636. [DOI] [PubMed] [Google Scholar]
- 105.Vianello M, Keir G, Giometto B, Betterle C, Tavolato B, Thompson EJ. Antigenic differences between neurological and diabetic patients with anti-glutamic acid decarboxylase antibodies. Eur J Neurol. 2005;12(4):294–299. doi: 10.1111/j.1468-1331.2004.00933.x. [DOI] [PubMed] [Google Scholar]
- 106.Raju R, Foote J, Banga JP, Hall TR, Padoa CJ, Dalakas MC, Ortqvist E, Hampe CS. Analysis of GAD65 autoantibodies in Stiff-Person syndrome patients. J Immunol. 2005;175(11):7755–7762. doi: 10.4049/jimmunol.175.11.7755. [DOI] [PubMed] [Google Scholar]
- 107.Pugliese A, Brown D, Garza D, Murchison D, Zeller M, Redondo MJ, Diez J, Eisenbarth GS, Patel DD, Ricordi C. Self-antigen-presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. J Clin Invest. 2001;107(5):555–564. doi: 10.1172/JCI10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hassin-Baer S, Kirson ED, Shulman L, Buchman AS, Bin H, Hindiyeh M, Markevich L, Mendelson E. Stiff-person syndrome following West Nile fever. Arch Neurol. 2004;61(6):938–941. doi: 10.1001/archneur.61.6.938. [DOI] [PubMed] [Google Scholar]
- 109.Giometto B, Nicolao P, Macucci M, Tavolato B, Foxon R, Bottazzo GF. Temporal-lobe epilepsy associated with glutamic-acid-decarboxylase autoantibodies. Lancet. 1998;352(9126):457. doi: 10.1016/s0140-6736(05)79192-3. [DOI] [PubMed] [Google Scholar]
- 110.Kim JY, Chung EJ, Kim JH, Jung KY, Lee WY. Response to steroid treatment in anti-glutamic acid decarboxylase antibody-associated cerebellar ataxia, stiff person syndrome and polyendocrinopathy. Mov Disord. 2006;21(12):2263–2264. doi: 10.1002/mds.21041. [DOI] [PubMed] [Google Scholar]
- 111.Vulliemoz S, Vanini G, Truffert A, Chizzolini C, Seeck M. Epilepsy and cerebellar ataxia associated with anti-glutamic acid decarboxylase antibodies. J Neurol Neurosurg Psychiatry. 2007;78(2):187–189. doi: 10.1136/jnnp.2006.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peltola J, Kulmala P, Isojarvi J, Saiz A, Latvala K, Palmio J, Savola K, Knip M, Keranen T, Graus F. Autoantibodies to glutamic acid decarboxylase in patients with therapy-resistant epilepsy. Neurology. 2000;55(1):46–50. doi: 10.1212/wnl.55.1.46. [DOI] [PubMed] [Google Scholar]
- 113.Vianello M, Bisson G, Dal Maschio M, Vassanelli S, Girardi S, Mucignat C, Fountzoulas K, Giometto B. Increased spontaneous activity of a network of hippocampal neurons in culture caused by suppression of inhibitory potentials mediated by anti-gad antibodies. Autoimmunity. 2008;41(1):66–73. doi: 10.1080/08916930701619565. [DOI] [PubMed] [Google Scholar]
- 114.Vianello M, Giometto B, Vassanelli S, Canato M, Betterle C, Mucignat C. Peculiar labeling of cultured hippocampal neurons by different sera harboring anti-glutamic acid decarboxylase autoantibodies (GAD-Ab) Exp Neurol. 2006;202(2):514–518. doi: 10.1016/j.expneurol.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 115.Antoine JC, Absi L, Honnorat J, Boulesteix JM, de Brouker T, Vial C, Butler M, De Camilli P, Michel D. Antiamphiphysin antibodies are associated with various paraneoplastic neurological syndromes and tumors. Arch Neurol. 1999;56(2):172–177. doi: 10.1001/archneur.56.2.172. [DOI] [PubMed] [Google Scholar]
- 116.Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, Kim KK, Kilimann MW, Lennon VA. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58(1):96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- 117.Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1(1):33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 118.Wigge P, McMahon HT. The amphiphysin family of proteins and their role in endocytosis at the synapse. Trends Neurosci. 1998;21(8):339–344. doi: 10.1016/s0166-2236(98)01264-8. [DOI] [PubMed] [Google Scholar]
- 119.Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20(21):7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geis C, Weishaupt A, Hallermann S, Grunewald B, Wessig C, Wultsch T, Reif A, Byts N, Beck M, Jablonka S, Boettger MK, Uceyler N, Fouquet W, Gerlach M, Meinck HM, Siren AL, Sigrist SJ, Toyka KV, Heckmann M, Sommer C. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain. 2010;133(11):3166–3180. doi: 10.1093/brain/awq253. [DOI] [PubMed] [Google Scholar]
- 121.Sommer C, Weishaupt A, Brinkhoff J, Biko L, Wessig C, Gold R, Toyka KV. Paraneoplastic stiff-person syndrome: passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet. 2005;365(9468):1406–1411. doi: 10.1016/S0140-6736(05)66376-3. [DOI] [PubMed] [Google Scholar]
- 122.Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci U S A. 1983;80(24):7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lang B, Newsom-Davis J, Prior C, Wray D. Antibodies to motor nerve terminals: an electrophysiological study of a human myasthenic syndrome transferred to mouse. J Physiol. 1983;344:335–345. doi: 10.1113/jphysiol.1983.sp014943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Toyka KV, Drachman DB, Griffin DE, Pestronk A, Winkelstein JA, Fishbeck KH, Kao I. Myasthenia gravis. Study of humoral immune mechanisms by passive transfer to mice. N Engl J Med. 1977;296(3):125–131. doi: 10.1056/NEJM197701202960301. [DOI] [PubMed] [Google Scholar]
- 125.Wessig C, Klein R, Schneider MF, Toyka KV, Naumann M, Sommer C. Neuropathology and binding studies in anti-amphiphysin-associated stiff-person syndrome. Neurology. 2003;61(2):195–198. doi: 10.1212/01.wnl.0000073143.53337.dd. [DOI] [PubMed] [Google Scholar]
- 126.Burns TM, Phillips LH, 2nd, Jones HR. Stiff person syndrome does not always occur with maternal passive transfer of GAD65 antibodies. Neurology. 2005;64(2):399–400. doi: 10.1212/wnl.64.2.399-a. author reply 399–400. [DOI] [PubMed] [Google Scholar]
- 127.Nemni R, Caniatti LM, Gironi M, Bazzigaluppi E, De Grandis D. Stiff person syndrome does not always occur with maternal passive transfer of GAD65 antibodies. Neurology. 2004;62(11):2101–2102. doi: 10.1212/01.wnl.0000127446.61806.2f. [DOI] [PubMed] [Google Scholar]
- 128.Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol. 2002;1(5):294–305. doi: 10.1016/s1474-4422(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 129.Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257(4):509–517. doi: 10.1007/s00415-009-5431-9. [DOI] [PubMed] [Google Scholar]
- 130.McKeon A, Pittock SJ, Lennon VA. Stiff-person syndrome with amphiphysin antibodies: distinctive features of a rare disease. Neurology. 2009;73(24):2132. doi: 10.1212/WNL.0b013e3181bd6a72. author reply 2133. [DOI] [PubMed] [Google Scholar]
- 131.Murinson BB, Guarnaccia JB. Stiff-person syndrome with amphiphysin antibodies: distinctive features of a rare disease. Neurology. 2008;71(24):1955–1958. doi: 10.1212/01.wnl.0000327342.58936.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toothaker TB, Rubin M. Paraneoplastic neurological syndromes: a review. Neurologist. 2009;15(1):21–33. doi: 10.1097/NRL.0b013e3181870aa2. [DOI] [PubMed] [Google Scholar]
- 133.Linke R, Schroeder M, Helmberger T, Voltz R. Antibody-positive paraneoplastic neurologic syndromes: value of CT and PET for tumor diagnosis. Neurology. 2004;63(2):282–286. doi: 10.1212/01.wnl.0000129983.06983.4e. [DOI] [PubMed] [Google Scholar]
- 134.Younes-Mhenni S, Janier MF, Cinotti L, Antoine JC, Tronc F, Cottin V, Ternamian PJ, Trouillas P, Honnorat J. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain. 2004;127(Pt 10):2331–2338. doi: 10.1093/brain/awh247. [DOI] [PubMed] [Google Scholar]
- 135.Takenoshita H, Shizuka-Ikeda M, Mitoma H, Song S, Harigaya Y, Igeta Y, Yaguchi M, Ishida K, Shoji M, Tanaka M, Mizusawa H, Okamoto K. Presynaptic inhibition of cerebellar GABAergic transmission by glutamate decarboxylase autoantibodies in progressive cerebellar ataxia. J Neurol Neurosurg Psychiatry. 2001;70(3):386–389. doi: 10.1136/jnnp.70.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Geis C, Beck M, Jablonka S, Weishaupt A, Toyka KV, Sendtner M, Sommer C. Stiff person syndrome associated anti-amphiphysin antibodies reduce GABA associated [Ca(2+)]i rise in embryonic motoneurons. Neurobiol Dis. 2009;36(1):191–199. doi: 10.1016/j.nbd.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 137.Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281(5375):419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 138.Westblom U. Stiff-man syndrome and clonazepam. JAMA. 1977;237(18):1930. [PubMed] [Google Scholar]
- 139.Miller F, Korsvik H. Baclofen in the treatment of stiff-man syndrome. Ann Neurol. 1981;9(5):511–512. doi: 10.1002/ana.410090516. [DOI] [PubMed] [Google Scholar]
- 140.Olafson RA, Mulder DW, Howard FM. "Stiff-man" syndrome: a review of the literature, report of three additional cases and discussion of pathophysiology and therapy. Mayo Clin Proc. 1964;39:131–144. [PubMed] [Google Scholar]
- 141.Vermeij FH, van Doorn PA, Busch HF. Improvement of stiff-man syndrome with vigabatrin. Lancet. 1996;348(9027):612. doi: 10.1016/S0140-6736(05)64825-8. [DOI] [PubMed] [Google Scholar]
- 142.Silbert PL, Matsumoto JY, McManis PG, Stolp-Smith KA, Elliott BA, McEvoy KM. Intrathecal baclofen therapy in stiff-man syndrome: a double-blind, placebo-controlled trial. Neurology. 1995;45(10):1893–1897. doi: 10.1212/wnl.45.10.1893. [DOI] [PubMed] [Google Scholar]
- 143.Seitz RJ, Blank B, Kiwit JC, Benecke R. Stiff-person syndrome with anti-glutamic acid decarboxylase autoantibodies: complete remission of symptoms after intrathecal baclofen administration. J Neurol. 1995;242(10):618–622. doi: 10.1007/BF00866910. [DOI] [PubMed] [Google Scholar]
- 144.Brashear HR, Phillips LH., 2nd Autoantibodies to GABAergic neurons and response to plasmapheresis in stiff-man syndrome. Neurology. 1991;41(10):1588–1592. doi: 10.1212/wnl.41.10.1588. [DOI] [PubMed] [Google Scholar]
- 145.Harding AE, Thompson PD, Kocen RS, Batchelor JR, Davey N, Marsden CD. Plasma exchange and immunosuppression in the stiff man syndrome. Lancet. 1989;2(8668):915. doi: 10.1016/s0140-6736(89)91568-7. [DOI] [PubMed] [Google Scholar]
- 146.Vicari AM, Folli F, Pozza G, Comi GC, Comola M, Canal N, Besana C, Borri A, Tresoldi M, Solimena M, et al. Plasmapheresis in the treatment of stiff-man syndrome. N Engl J Med. 1989;320(22):1499. doi: 10.1056/nejm198906013202220. [DOI] [PubMed] [Google Scholar]
- 147.Dalakas MC. The role of IVIg in the treatment of patients with stiff person syndrome and other neurological diseases associated with anti-GAD antibodies. J Neurol. 2005;252(Suppl 1):I19–I25. doi: 10.1007/s00415-005-1105-4. [DOI] [PubMed] [Google Scholar]
- 148.Gerschlager W, Brown P. Effect of treatment with intravenous immunoglobulin on quality of life in patients with stiff-person syndrome. Mov Disord. 2002;17(3):590–593. doi: 10.1002/mds.10043. [DOI] [PubMed] [Google Scholar]
- 149.Karlson EW, Sudarsky L, Ruderman E, Pierson S, Scott M, Helfgott SM. Treatment of stiff-man syndrome with intravenous immune globulin. Arthritis Rheum. 1994;37(6):915–918. doi: 10.1002/art.1780370621. [DOI] [PubMed] [Google Scholar]
- 150.Khanlou H, Eiger G. Long-term remission of refractory stiff-man syndrome after treatment with intravenous immunoglobulin. Mayo Clin Proc. 1999;74(12):1231–1232. doi: 10.4065/74.12.1231. [DOI] [PubMed] [Google Scholar]
- 151.Dalakas MC, Fujii M, Li M, Lutfi B, Kyhos J, McElroy B. High-dose intravenous immune globulin for stiff-person syndrome. N Engl J Med. 2001;345(26):1870–1876. doi: 10.1056/NEJMoa01167. [DOI] [PubMed] [Google Scholar]
- 152.Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence-based indications and safety profile. Pharmacol Ther. 2004;102(3):177–193. doi: 10.1016/j.pharmthera.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 153.Dalakas MC, Clark WM. Strokes, thromboembolic events, and IVIg: rare incidents blemish an excellent safety record. Neurology. 2003;60(11):1736–1737. doi: 10.1212/01.wnl.0000074394.15882.83. [DOI] [PubMed] [Google Scholar]
- 154.Dalakas MC. Invited article: inhibition of B cell functions: implications for neurology. Neurology. 2008;70(23):2252–2260. doi: 10.1212/01.wnl.0000313840.27060.bf. [DOI] [PubMed] [Google Scholar]
- 155.Bacorro EA, Tehrani R. Stiff-person syndrome: persistent elevation of glutamic acid decarboxylase antibodies despite successful treatment with rituximab. J Clin Rheumatol. 2010;16(5):237–239. doi: 10.1097/RHU.0b013e3181e931fa. [DOI] [PubMed] [Google Scholar]
- 156.Baker MR, Das M, Isaacs J, Fawcett PR, Bates D. Treatment of stiff person syndrome with rituximab. J Neurol Neurosurg Psychiatry. 2005;76(7):999–1001. doi: 10.1136/jnnp.2004.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dupond JL, Essalmi L, Gil H, Meaux-Ruault N, Hafsaoui C. Rituximab treatment of stiff-person syndrome in a patient with thymoma, diabetes mellitus and autoimmune thyroiditis. J Clin Neurosci. 2010;17(3):389–391. doi: 10.1016/j.jocn.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 158.Katoh N, Matsuda M, Ishii W, Morita H, Ikeda S. Successful treatment with rituximab in a patient with stiff-person syndrome complicated by dysthyroid ophthalmopathy. Intern Med. 2010;49(3):237–241. doi: 10.2169/internalmedicine.49.2821. [DOI] [PubMed] [Google Scholar]
- 159.Dalakas M. Ann Neurol. 2009 [Google Scholar]
- 160.Rizzi M, Knoth R, Hampe CS, Lorenz P, Gougeon ML, Lemercier B, Venhoff N, Ferrera F, Salzer U, Thiesen HJ, Peter HH, Walker UA, Eibel H. Long-lived plasma cells and memory B cells produce pathogenic anti-GAD65 autoantibodies in Stiff Person Syndrome. PLoS One. 2010;5(5):e10838. doi: 10.1371/journal.pone.0010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jarius S, Stich O, Speck J, Rasiah C, Wildemann B, Meinck HM, Rauer S. Qualitative and quantitative evidence of anti-glutamic acid decarboxylase-specific intrathecal antibody synthesis in patients with stiff person syndrome. J Neuroimmunol. 2010;229(1–):219–224. doi: 10.1016/j.jneuroim.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 162.Hanninen A, Soilu-Hanninen M, Hampe CS, Deptula A, Geubtner K, Ilonen J, Knip M, Reijonen H. Characterization of CD4+ T cells specific for glutamic acid decarboxylase (GAD65) and proinsulin in a patient with stiff-person syndrome but without type 1 diabetes. Diabetes Metab Res Rev. 2010;26(4):271–279. doi: 10.1002/dmrr.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burton AR, Baquet Z, Eisenbarth GS, Tisch R, Smeyne R, Workman CJ, Vignali DA. Central nervous system destruction mediated by glutamic acid decarboxylase-specific CD4+ T cells. J Immunol. 2010;184(9):4863–4870. doi: 10.4049/jimmunol.0903728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Skorstad G, Hestvik AL, Vartdal F, Holmoy T. Cerebrospinal fluid T cell responses against glutamic acid decarboxylase 65 in patients with stiff person syndrome. J Autoimmun. 2009;32(1):24–32. doi: 10.1016/j.jaut.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 165.Holmoy T, Skorstad G, Roste LS, Scheie D, Alvik K. Stiff person syndrome associated with lower motor neuron disease and infiltration of cytotoxic T cells in the spinal cord. Clin Neurol Neurosurg. 2009;111(8):708–712. doi: 10.1016/j.clineuro.2009.06.005. [DOI] [PubMed] [Google Scholar]