Abstract
Objective
The effect of Helicobacter pylori on Barrett’s esophagus is poorly understood. We conducted a meta-analysis to summarize the existing literature examining the effect that H pylori has on Barrett’s esophagus.
Design
We performed a comprehensive search to identify studies pertaining to the association between H pylori and Barrett’s esophagus. We conducted meta-regression analyses to identify sources of variation in the effect of H pylori on Barrett’s esophagus.
Results
Our analysis included a total of 49 studies that examined the effect of H pylori on Barrett’s esophagus and 7 studies that examined the effect of cag A positivity on Barrett’s esophagus. Overall, H pylori, and even more so cag A, tended to be protective for Barrett’s esophagus in most studies; however there was obvious heterogeneity across studies. The effect of H pylori on Barrett’s esophagus varied by geographic location and in the presence of selection and information biases. Only 4 studies were found without obvious selection and information bias, and these showed a protective effect of H pylori on Barrett’s esophagus (Relative Risk = 0.46 [95% CI: 0.35, 0.60]).
Conclusions
Estimates for the effect of H pylori on Barrett’s esophagus were heterogeneous across studies. We identified selection and information bias as potential sources of this heterogeneity. Few studies without obvious selection and information bias have been conducted to examine the effect of H pylori on Barrett’s esophagus, but in these, H pylori infection is associated with a reduced risk of Barrett’s esophagus.
Background
The incidence of esophageal adenocarcinoma has steadily increased over the past three decades in developed countries, while the five-year survival rate continues to be low (only 16% in the US and 10% in Europe).1,2 Barrett’s esophagus, which is estimated to affect less than 2% of the general population,3–5 is considered to be the precancerous lesion for esophageal adenocarcinoma.6–8 Yet little is known about the etiological process leading to Barrett’s esophagus.
Helicobacter pylori has been implicated as a risk factor for precancerous lesions in the stomach which affect the acid producing parietal cells.9–11 However, inconsistent evidence exists regarding the effect of H pylori on gastroesophageal reflux disease, the primary putative risk factor for Barrett’s esophagus and esophageal adenocarcinoma, and the current evidence regarding the effect of H pylori on Barrett’s esophagus remains poorly understood. Some previous studies have reported that H pylori is a risk factor for Barrett’s esophagus,12–14 while other studies have reported that H pylori has no effect on Barrett’s esophagus15–16 and others still report a protective effect.17–19 Previous meta-analyses, using small subsets of studies on this topic, failed to investigate sources of the heterogeneity of effects across studies.20–22 To better understand the conflicting results, we conducted a meta-analysis to evaluate potential sources of heterogeneity for estimates of the effect of H pylori on Barrett’s esophagus, and to summarize the effect that H pylori has on Barrett’s esophagus within homogeneous patient groups.
Methods
We conducted a meta-analysis to summarize the effect that various factors have on Barrett’s Esophagus.4, 12–19, 23–62 However, the study presented here focuses on the analyses examining the effect of H pylori on Barrett’s Esophagus.
A. Data sources
Sources of studies included the literature databases Medline (PubMed and Ovid) and Science Citation Index.63 Studies were searched from the inception of each database through December 31, 2010 using the keywords ‘Barrett’s esophagus,’ ‘Barrett’s metaplasia’ or ‘Barrett’s oesophagus,’ and ‘Helicobacter pylori’ or ‘Campylobacter pylori. Two collaborators used information from the references’ titles and abstracts to identify potentially eligible studies from the literature database searches. We supplemented these searches with backward citation tracking of eligible primary studies, reviews of Barrett’s esophagus, and hand-searches of conference proceedings published in Gut and Gastroenterology.
B. Study selection
All eligible studies satisfied the following inclusion criteria:
Barrett’s esophagus could be used as an outcome in the analysis.
Relevant information was provided to allow the estimation of a relative risk (risk ratio or odds ratio) and a variance measure for the association between H pylori and Barrett’s esophagus.
The individual was used as the unit of analysis for estimating the relative risk.
Information must have been available in English or Spanish (at least as an abstract).
A sample size of more than 4 subjects for each comparison group was utilized.64 Therefore, case reports were not included.
We excluded studies based on the following criteria:
The study was not conducted on humans.
Barrett’s esophagus was not mentioned in the abstract.
The results came from a review article.
When data from multiple reports were identified, we included only the report with the most complete relevant data.
C. Data extraction
All potentially eligible studies were randomly assigned to two of the three primary data extractors for independent preliminary screening. Each extractor first reviewed the report to confirm eligibility, and if ineligible, provided the primary reason for ineligibility. The two assigned primary extractors then independently extracted relevant data from studies judged to be eligible.
We created an extraction database to collect relevant information regarding each eligible study such as citation information; how H pylori and Barrett’s esophagus were measured; the measure of effect and confidence intervals; sample sizes; study location (geographic location, country, etc); design (cross-sectional, basic control or population-based case-control); characteristics of the study population (e.g. prevalence of the modifiable risk factor); comparison group (endoscopic patients, asymptomatic patients; gastroesophageal reflux disease patients; etc); data analyses conducted (adjustment for confounding, etc); likelihood for selection and information bias; and other potential sources of heterogeneity across studies.
Selection bias was considered likely if the comparison group without Barrett’s esophagus did not represent the base population (in terms of the distribution of the exposure) from which the cases of Barrett’s esophagus came. The base population was defined as those who, if they developed Barrett’s esophagus, would be a case with Barrett’s esophagus in the study. For example, if Barrett’s esophagus cases were chosen from patients undergoing an upper endoscopic examination at clinic A in Los Angeles, CA, then the base population would be individuals who, if they had Barrett’s esophagus, would undergo an upper endoscopy examination at clinic A in Los Angeles, CA and would be identified as a case in the study. If the comparison group (without Barrett’s esophagus) was chosen from those undergoing upper endoscopy examinations at clinic A in Los Angeles, CA, then it is likely that selection bias occurred since the distribution of H pylori in endoscopy patients is likely to differ from H pylori in the base population.
Information bias was suggested when the measurement of H pylori or Barrett’s esophagus was likely to be inaccurate. Variables indicating how H pylori and Barrett’s esophagus were measured included an indicator of incident (versus prevalent) measurement, methods used to measure or define H pylori (urea breath test, rapid urease test, culture, histology, serology, fecal test, etc), location of biopsy samples for H pylori measure (gastric biopsies, esophageal biopsies only, or no biopsies), whether the methods for H pylori measurement was consistent for Barrett’s esophagus cases and the non-Barrett’s comparison group, and whether Barrett’s esophagus was diagnosed by first seeing Barrett’s mucosa at endoscopy, and then histologically confirming intestinal metaplasia in biopsy specimens taken at the site where the Barrett’s mucosa was observed.
D. Data cleaning and screening
Studies judged to be eligible by the two data extractors were then assigned to an additional screener for data cleaning and screening. When a discrepancy was observed between the two extractions, the third collaborator reviewed the original report to resolve the discrepancy and correct any errors.
E. Data analysis
The effect measures of interest were relative risks that compared the risk of Barrett’s esophagus among individuals who tested positive for H pylori to the risk of Barrett’s esophagus among individuals who tested negative for H pylori. We assumed that the Barrett’s esophagus was a rare outcome and therefore used proportions, risk ratios, or odds ratios from eligible studies to estimate this relative risk.
We examined the distribution of the measures of effect using visual and tabular displays and tests of homogeneity to reveal systematic variation in the relative risks of H pylori on Barrett’s esophagus across studies.64 Furthermore, we investigated potential publication bias by using funnel plots.65
We conducted meta-regression analyses to identify factors that influence variation in the estimated relative risks across studies within the pool of available data (previously described under data extraction) and to define subgroups for which the effect of H pylori on Barrett’s esophagus did not show observable residual heterogeneity in the measure of effect across studies. The relative risk estimates were then modeled by the fixed effects of factors that had the greatest potential to explain variation in effects and for which there was sufficient data and variation across studies. Quality scoring, which has well-described methodological shortcomings,66–68 was not included in this meta-analysis. Instead we examined whether specific characteristics of data quality could predict variation in the measure of effect across studies. We also examined the association between Barrett’s esophagus and cag A positive strains of H pylori.
Results
A total of 2,661 abstracts were screened for preliminary eligibility. Of these, 2,487 were judged to be ineligible. Data extraction was performed on the remaining 174 studies, 40 of which were subsequently judged to be ineligible and 134 eligible for the effect of any factor on Barrett’s esophagus; 49 of these pertained to the effect of H pylori on Barrett’s esophagus and were included in this anlaysis. 4, 12–19, 23–62 The most common reasons for ineligibility were lack of reported data to estimate a relevant measure of effect (46%) and sources of data exclusively coming from review articles (41%).
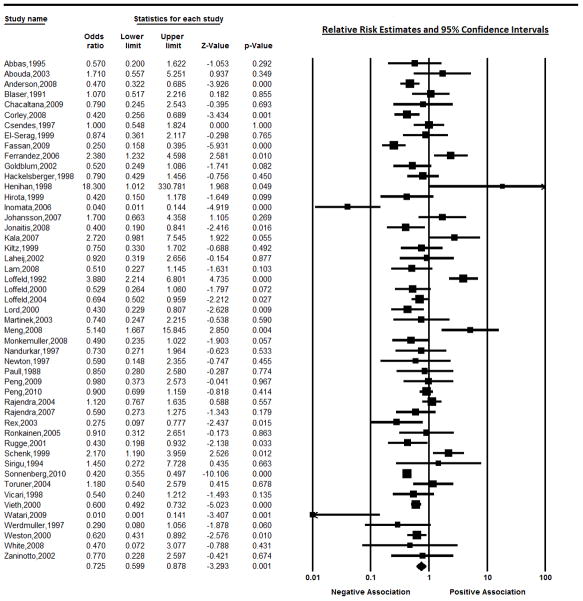
The included studies utilized populations from around the world, including Europe (51%), the United States (20%), Canada, Japan, Malaysia, China, Peru, Chile, Pakistan and Australia. The pooled overall random effects estimate of the relative risk was 0.73 (95% CI 0.60, 0.88). However, heterogeneity was observed both in the visual graph of the measures of effect across studies and the test for heterogeneity (p<0.0001). Figure 1 illustrates the distribution of the estimated effect of H pylori on Barrett’s esophagus across the 49 studies that specifically examined this effect. The funnel plot did not suggest that publication bias was a major concern.
Figure 1.
Estimates of the effect of H pylori on Barrett’s esophagus using estimates from all available studies.
Most of the 49 studies (92%) which examined the effect of H pylori on Barrett’s esophagus used convenience comparison groups or other populations which would not be likely to estimate the true distribution of H pylori in the base population from which the cases of Barrett’s esophagus arose (Table 1), and therefore were considered to have biased estimates of effect due to selection bias. Misclassification of H pylori and Barrett’s esophagus (information bias) was also common; 33% of studies did not specifically state that they diagnosed Barrett’s esophagus by histologically confirming intestinal metaplasia in biopsy specimens taken at the site where the Barrett’s mucosa was observed at endoscopy, and 14% (n=7) diagnosed H pylori histologically but did not use a biopsy sample from either the antrum or the corpus of the stomach to measure H pylori (6 of these 7 studies only measured H pylori in the esophagus or gastro-esophageal junction). The pooled estimate for the studies that used gastric biopsies was 0.66 (95% CI: 0.53, 0.83); test for heterogeneity p<0.0001) while the estimate from studies that measured H pylori only in the esophagus or gastro-esophageal junction was 1.39 (95% CI: 0.52, 3.52; test for heterogeneity p>0.0001).
Table 1.
Summary of studies with estimates for the effect of Helicobacter pylori on Barrett’s esophagus
| 1st Author’s Last Name, Year |
Country | Observed Study Design* |
Gold Standard Measure of BE Reported? |
Measure of H pylori | Comparison Group without BE† |
Selection Bias? |
Factors Adjusted† | N | Relative Risk (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Abbas Z, 1995 | Pakistan | Case-control | No (prevalent) | Rapid urease, histology | Endoscopy patients with reflux esophagitis | Likely | None | 58 | 0.57 (0.20, 1.62) |
| Abouda G, 2003 | UK | Case-control | No (prevalent) | Rapid urease, serology | Endoscopy patients without GERD | Likely | None | 85 | 1.71 (0.56, 5.28) |
| Anderson L, 2008 | Ireland | Population-based Case-control | Yes (newly diagnosed) | Serology | Random sample from General Practice Master Index | Unlikely | None | 468 | 0.47 (0.32, 0.68) |
| Blaser M, 1991 | Canada | Case-control | Not specified | Serology/histology | Healthy hospital or military employees, blood donors, and elderly nursing home residents. | Likely | None | 116 | 1.07 (0.52,2.23) |
| Chacaltan, 2009 | Peru | Case-control | Yes (prevalent) | Histology | Asymptomatic military personnel without erosive esophagitis, and peptic ulcer disease | Likely | None | 922 | 0.79 (0.25, 2.59) |
| Corley D, 2008 | USA | Population-based Case-control | Yes (prevalent) | Serology | Random sample from Kaiser Permanente HMO | Unlikely | Age, gender, location, BMI, ethnicity, smoking, education, multivitamins | 591 | 0.42 (0.26, 0.70) |
| Csendes A, 1997 | Chile | Case-control | No (prevalent) | Histology | Endoscopy patients with dyspepsia, but no GERD | Likely | None | 290 | 1.00 (0.55, 1.83) |
| El-Serag H, 1999 | USA | Case-control | Yes (prevalent) | Histology | Endoscopy patient with erosive esophagitis | Likely | None | 108 | 0.87 (0.36, 2.11) |
| Fassan M, 2009 | Italy | Case-control | Not specified | Histology | Patients with dyspepsia | Likely | None | 420 | 0.25 (0.16, 0.40) |
| Ferrández A, 2006 | Spain | Case-control | Yes (prevalent) | Serology, rapid urease, histology, PCR | Healthy blood donors | Likely | None | 317 | 2.38 (1.23, 4.59) |
| Goldblum J, 2002 | USA | Case-control | Yes (newly diagnosed) | Rapid urease, histology, culture | Endoscopy VA patients without GERD | Likely | None | 130 | 0.52 (0.25, 1.09) |
| Hackelsberger A, 1998 | Germany | Case-control | Yes (prevalent) | Rapid urease, histology | Endoscopy patients | Likely | None | 363 | 0.79 (0.43, 1.46) |
| Henihan R, 1998 | Ireland | Case-control | Yes (prevalent) | Histology (esophagus) | Reflux esophagitis | Likely | None | 122 | 18.3 (1.01,329.99) |
| Hirota W, 1999 | USA | Cross-sectional | Yes (prevalent) | Histology (esophagus) | Endoscopy patients | Likely | None | 842 | 0.42 (0.15, 1.18) |
| Inomata Y, 2008 | Japan | Case-control | Yes (prevalent) | Serology, rapid urease, histology | Endoscopy patients with esophagitis | Likely | None | 227 | 0.40 (0.19, 0.84) |
| Johansson J, 2007 | Sweden | Case-control | Yes (prevalent) | Histology (gastrooesphageal junction) | Endoscopy patients (H pylori was not measured for the population controls) | Likely | Age, gender, BMI, heartburn, smoking and alcohol | 519 | 1.7 (0.7, 4.6) |
| Jonaitis L, 2008 | Lithuania | Case-control | Yes (prevalent) | Rapid urease | Endoscopy patients with esophagitis | Likely | None | 227 | 0.40 (0.19, 0.84) |
| Kala Z, 2007 | Czech Republic | Case-control | No (prevalent) | Rapid urease | Endoscopy patients with esophagitis | Likely | None | 86 | 2.72 (0.98, 7.54) |
| Kiltz U, 1999 | Germany | Case-control | Yes (prevalent) | Rapid urease, serology | Endoscopy patients | Likely | None | 355 | 0.75 (0.33, 1.70) |
| Laheij R, 2002 | Netherlands | Cross-sectional | No (prevalent) | Rapid urease, histology, culture | Endoscopy patients without reflux esophagitis | Likely | Gender, corpus gastritis | 551 | 0.92 (0.30, 2.50) |
| Lam K, 2008 | USA | Case-control (nested within a Cross-sectional study) | Yes (prevalent) | Serology | Asymptomatic endoscopy patients | Likely | None | 269 | 0.51 (0.32, 0.68) |
| Loffeld R, 1992 | Netherlands | Case-control | Yes (prevalent) | Histology (esophagus) | Healthy blood donors | Likely | None | 469 | 3.88 (2.21, 6.79) |
| Loffeld R, 2000 | Netherlands | Case-control | No (newly diagnosed) | Serology | Endoscopy patients | Likely | None | 490 | 0.53 (0.26, 1.06) |
| Loffeld R, 2004 | Netherlands | Case-control | No (prevalent) | Histology | Endoscopy patients | Likely | None | 4154 | 0.69 (0.50, 0.96) |
| Lord R, 2000 | Australia | Case-control | Yes (prevalent) | Histology | Endoscopy patients | Likely | None | 305 | 0.43 (0.23, 0.81) |
| Martinek J, 2003 | Czech Republic | Case-control | Not Specified | Histology, rapid urease | Endoscopy patients | Likely | None | 290 | 0.74 (0.25. 2.24) |
| Meng X, 2008 | Not stated | Case-control | Not Specified | PCR | Endoscopy patients with dyspepsia | Likely | None | 132 | 5.14 (1.67, 15.87) |
| Monkemuller K, 2008 | Germany | Case-control | Yes (prevalent) | Histology | Non-erosive reflux disease patients with heartburn | Likely | None | 194 | 0.94 (0.23, 1.00) |
| Nandurkar S, 1997 | Australia | Cross-sectional | Yes (prevalent) | Histologyof biopsy tissue from the gastrooesphageal junction | Endoscopy patients | Likely | None | 158 | 0.73 (0.27, 1.98) |
| Newton M, 1997 | England | Case-control | Yes (prevalent) | Histology, rapid urease | Asymptomatic endoscopy patients | Likely | None | 41 | 0.59 (0.15, 2.39) |
| Paull G, 1988 | USA | Case-control | Yes (prevalent) | Histology | Endoscopy patients | Likely | None | 51 | 0.85 (0.28, 2.58) |
| Peng S, 2009 | China | Case-control | Yes (prevalent) | Rapid urease | Endoscopy patients with esophagitis | Likely | None | 137 | 0.98 (0.37, 2.55) |
| Peng, 2010 | China | Case-control | Yes (prevalent) | Rapid urease | Endoscopy patients with uninvestigated reflux symptoms | Likely | None | 469 | 0.90 (0.70, 1.16) |
| Rajendra S, 2004 | Malaysia | Case-control | Yes (prevalent) | Rapid urease, histology | Symptomatic endoscopy patients | Likely | None | 1864 | 1.12 (0.77, 1.64) |
| Rajendra S, 2007 | Malaysia | Case-control | Yes (prevalent) | Serology | Endoscopy patients without GERD | Likely | None | 108 | 0.59 (0.27, 1.26) |
| Rex D, 2003 | USA | Case-control | Yes (newly diagnosed) | Rapid urease | Asymptomatic Colonoscopy patients | Unlikely | None | 812 | 0.27 (0.10, 0.77) |
| Ronkainen J, 2005 | Sweden | Cross-sectional | Yes (prevalent) | Serology, histology, culture | Random sample of the adult population | Unlikely | None | 1000 | 0.91 (0.31, 2.63) |
| Rugge M, 2001 | Italy | Case-control | Yes (prevalent) | Histology | Endoscopy patients with non-ulcer dyspepsia | Likely | None | 106 | 0.43 (0.20, 0.94) |
| Schenk B, 1999 | Netherlands | Case-control | No (prevalent) | Histology | Endoscopy patients with GERD | Likely | None | 137 | 2.17 (1.19, 3.96) |
| Sirigu F, 1994 | Italy | Case-control | Yes (prevalent) | Histology | Age and gender matched endoscopy patients | Likely | None | 82 | 1.45 (0.27, 7.67) |
| Sonnenberg A, 2010 | USA | Cross-sectional | Yes (prevalent) | Histology | Endoscopy patients | Likely | Age, sex, state of residence and insurance type | 78985 | 0.42 (0.35, 0.49) |
| Toruner M, 2004 | Turkey | Case-control | Yes (prevalent) | Histology | Endoscopy patients | Likely | None | 335 | 1.18 (0.54, 2.58) |
| Vicari J, 1998 | USA | Case-control | No (prevalent) | Serology, histology | Endoscopy patients without GERD | Likely | None | 105 | 0.54 (0.24, 1.21) |
| Vieth M, 2000 | Germany | Case-control | No (prevalent) | Histology | Endoscopy patients with non-ulcer dyspepsia | Likely | None | 1766 | 0.60 (0.49, 0.73) |
| Watari J, 2009 | Japan | Case-control | Yes (prevalent) | Histology, culture | Patients with gastric metaplasia | Likely | None | 140 | 0.01 (0.001, .20) |
| Werdmuller B, 1997 | Netherlands | Case-control | Not specified | Histology, culture, serology, rapid urease | Endoscopy patients with GERD | Likely | None | 412 | 0.29 (0.08, 1.06) |
| Weston A, 2000 | USA | Case-control | Yes (prevalent) | Histology | Endoscopy patients with GERD | Likely | None | 506 | 0.62 (0.43, 0.89) |
| White N, 2008 | Canada | Case-control | Yes (prevalent) | Histology (esophagus) | Endoscopy patients | Likely | None | 68 | 0.47 (0.07, 3.00) |
| Zaninotto G, 2002 | Italy | Case-control | Yes (prevalent) | Histology | Endoscopy patients with GERD | Likely | None | 66 | 0.77 (0.23, 2.58) |
If not specified, studies are not population-based. Study design observed by data extraction may differ from the study design reported in the manuscript.
BMI= body mass index; GERD = Gastroesophageal reflux disease
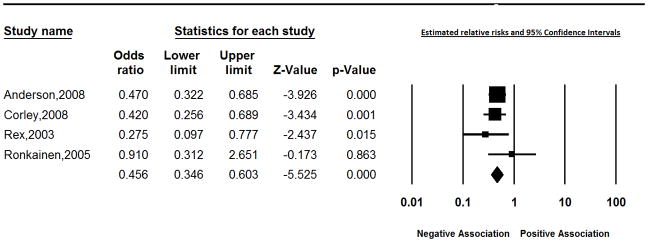
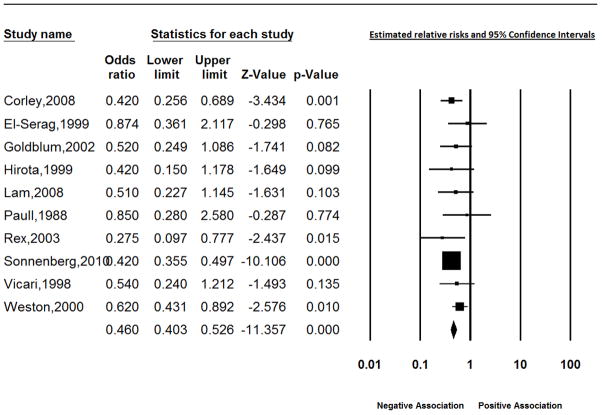
In weighted meta-regression analyses using all 49 studies, selection bias, H pylori infection measured only using a biopsy sample from the stomach, and study location were identified as sources of heterogeneity. As illustrated in Figure 2, the effect within the group of 4 studies with appropriate measurement of H pylori and without a likely source of selection bias was consistently protective for BE; the random effects summary estimate for the relative risk was 0.46 (95% CI: 0.35, 0.60).4,17,25,51 Likewise, the effect of H pylori on BE was consistently protective in studies conducted in the United States; 0.46 (95% CI: 0.40, 0.53), (heterogeneity test p=0.50) (Figure 3). Strongly protective measures of effect (RR = 0.01 and 0.04) were reported for the two studies from Japan. Residual heterogeneity was observed for all other strata of these variables; effect estimates ranged from 0.25 to 5.14 for studies conducted outside of the United States or Japan with a likely source of selection bias which used gastric biopsy samples from the antrum or corpus to measure H pylori.
Figure 2.
Estimates of the effect of H pylori on Barrett’s esophagus in studies with appropriate measurement of H pylori and without a likely source of selection bias..
Figure 3.
Estimates of the effect of Helicobacter pylori on Barrett’s esophagus using estimates from studies in the United States.
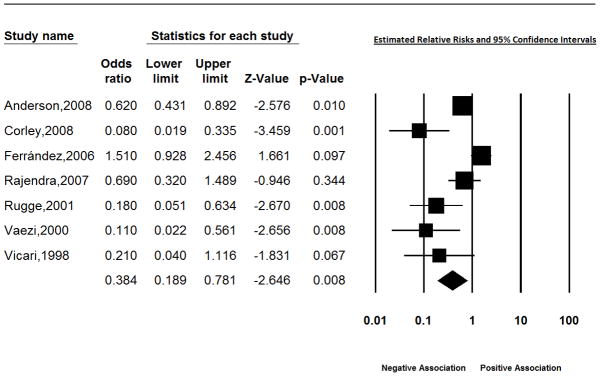
We also examined the effect of cag A positivity on Barrett’s esophagus. Data from the subgroup of 7 studies which examined the effect of cag A positivity on BE, suggested a protective effect of cag A on BE in all but 1 study (Table 2, Figure 4).17,25,29,49,52,56,69 The pooled random estimate for the relative risk in these 7 studies was 0.38 (95% CI: 0.19, 0.78), but the relative risk for the study by Ferrández et al. (estimated to be 1.5 [95% CI: 0.93, 2.46]) differed greatly from the other studies.29
Table 2.
Summary of studies with estimates for the effect of CagA + Helicobacter pylori on Barrett’s esophagus
| 1st Author’s Last Name, Year | Country | Observed Study Design | Gold Standard Measure of BE Reported? | Comparison Group without BE† | Selection Bias? | Factors Adjusted† | N | Measure of Effect (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Anderson L, 2008 | Ireland | Population-based Case-control | Yes | General Practice Master Index from 4 general practices in the region matched by age and gender | Likely, since age and gender were not adjusted for in the analysis | None | 468 | 0.62 (0.43, 0.89) |
| Corley D, 2008 | USA | Population-based Case-control | Yes | Kaiser Permanente members in Northern California | Unlikely | Age, gender, location, BMI, ethnicity, smoking, education, multivitamins | 516 | 0.08 (0.02, 0.35) |
| Ferrández A, 2006 | Spain | Case-control | Yes | Healthy blood donors | Likely | None | 317 | 1.51 (0.93, 2.46) |
| Rajendra S, 2007 | Malaysia | Case-control | Yes | Endoscopy patients without GERD and without acid suppression therapy | Likely | None | 108 | 0.69 (0.32, 1.49) |
| Rugge M, 2001 | Italy | Case-control | Yes | Endoscopy patients | Likely | None | 54 | 0.18 (0.05, 0.62) |
| Vicari J, 1998 | USA | Case-control | No | Endoscopy patients with Non-ulcer dyspepsia | Likely | None | 41 | 0.21 (0.04, 1.13) |
| Vaezi M, 2000 | USA | Case-control | No | Endoscopy patient without symptomatic GERD | Likely | None | 143 | 0.11 (0.02, 0.52) |
If not specified, studies are not population-based.
BMI = body mass index; GERD = Gastroesophageal reflux disease
Figure 4.
Estimated effect of Cag A status on Barrett’s esophagus.
Discussion
In the current analysis we examined the effect of H pylori on Barrett’s esophagus across 49 studies. Our analyses suggest that H pylori tended to be protective for Barrett’s esophagus. However, heterogeneity was easily observable across studies, and the effect varied in the presence of selection bias, with the use of esophageal tissue instead of gastric tissue to diagnose H pylori, and in different geographic locations.
The effect of information bias through inaccurate measurement of H pylori using tissue from the esophagus instead of the stomach was observed to be a source of heterogeneity. Studies that defined H pylori exclusively from esophageal biopsies were more likely to find a positive association between H pylori and Barrett’s esophagus, whereas the rest of the studies that measured H pylori using gastric biopsies tended to show a protective association between H pylori and Barrett’s esophagus. Since the occurrence of H pylori in the esophagus would not likely reflect the occurrence of H pylori in the stomach, a person’s true H pylori status is likely to be misclassified when tissue from the esophagus is used.
A strong protective effect of gastric H pylori on Barrett’s esophagus was observed without observable heterogeneity within the subgroup of 4 studies without a likely source of selection or information bias 0.46 (95% CI: 0.35, 0.60), where the definitions of Barrett’s esophagus as well as H pylori adhere to internationally accepted standards.70 However, 45 of the 49 studies had obvious sources of selection bias (some with information bias as well), so only 4 studies were used to estimate this measure of effect.4,17,25,51 Additional studies are needed with appropriately chosen comparison groups. For example, for case-control studies, the controls should be chosen to represent the exposure distribution of the base population from which the cases arose. Endoscopy patients, healthy blood donors, and patients with conditions positively or negatively associated with H pylori would not be appropriate comparison groups. Controls from identified catchment populations from which the cases arose would be the most appropriate choice.
The effect of cag A positive H. pylori on Barrett’s esophagus also tended to be protective. Only one of the seven studies examining the association between cag A and Barrett’s esophagus did not show a protective effect. In the study by Ferrández et al which used healthy blood donors as controls, the relative risk was estimated to be 1.5 (95% CI: 0.93, 2.46).29 Blood donors typically are healthier in many ways and younger than most populations and therefore may have led to selection bias since they may have a lower prevalence of H pylori than the base population from which the cases arose. On the other hand, the protective effect observed in 5 of the 6 other studies was also likely affected by selection bias.
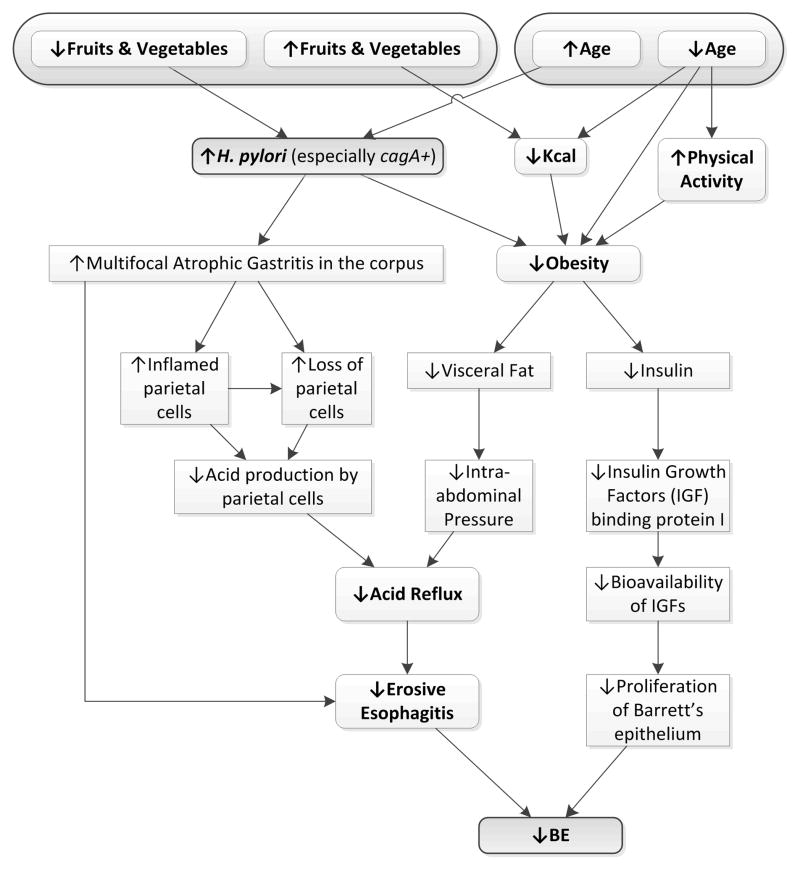
Potential candidates for sources of heterogeneity for the effect of H pylori on Barrett’s esophagus include corpus inflammation and atrophy. Some evidence suggests these factors may modify the effect of H pylori on related disease outcomes such as symptomatic GERD and erosive esophagitis.71 However, existing studies examining the effect of H pylori on Barrett’s esophagus did not evaluate effect modification by type or distribution of gastritis on the risk of Barrett’s esophagus. Figure 5 illustrates possible mechanisms whereby H pylori may decrease the risk for Barrett’s esophagus. H pylori infection’s hypothesized protective effect on Barrett’s esophagus may occur due to its association with multifocal atrophic gastritis and the resulting damaging effect it has on the acid producing parietal cells.9–11 With a loss of parietal cells, the esophagus is less likely to be exposed to the harmful effects of gastric acid and acid reflux, erosive esophagitis and then Barrett’s esophagus is less likely to occur. Also in this hypothesized protective pathway for Barrett’s esophagus, both a low intake of fruits and vegetables and older age is associated with H pylori infection,72–746 while younger age is associated with lower calorie consumption and increased physical activity, both leading to a lower risk for obesity.75,76 With a lower risk for obesity, lower visceral fat and lower insulin levels both may lead to a reduction in Barrett’s esophagus (Figure 5).77–80 Using this figure, after eliminating intermediates and controlling for age and dietary factors such as fruit and vegetable consumption, there may be no remaining potential confounders for the effect of H pylori on Barrett’s esophagus. Therefore, age and dietary factors such as fruit and vegetable intake should be controlled for, yet only 3 (7%) of the studies adjusted for age,17,19.34 and only 1 adjusted for a relevant dietary factor (Corley, et al).17
Figure 5.
The putative pathways involving Helicobacter pylori and a decreased risk for Barrett’s Esophagus.
Two previous meta-analyses have reported a protective effect of H pylori on Barrett’s esophagus across only 5 and 9 studies.20,21 These summary measures of effect were reported in the presence of heterogeneous effects without searching for sources of the variation of effects across studies. As noted by Wang et al in a more recent meta-analysis, the reported protective summary measure of effect may have been affected by the types of subjects used as the comparison group.22 Wang et al limited their meta-analysis of the effect of H pylori on Barrett’s esophagus to studies which used “normal healthy subjects as controls,” which included blood donors in 3 studies and endoscopy controls in 9 studies.22 As we discussed previously, the use of blood donors as controls is likely to lead to a biased (higher) estimate of effect due to selection bias. Likewise, the prevalence of H pylori infection among endoscopy patients would not likely represent the prevalence of H pylori in the base population from which the cases arose. The current meta-analysis is the first to examine the effect of selection and other biases on the observed effect of H pylori on Barrett’s esophagus across studies.
Conclusion
Our meta-analysis of 49 studies suggests that H pylori infection is associated with a reduced risk of Barrett’s esophagus. However, the effect is heterogeneous across studies. We identified methodological issues such as selection and information bias as potential sources of heterogeneity. In total, we found four studies without obvious selection and information bias and these showed a protective effect of H pylori on Barrett’s esophagus.
Acknowledgments
Statement of Interests
This study was funded in part by NIH R01 116845 (PI – El-Serag) and NIDDK K24-04-107 (PI El-Serag), and in part by the Houston VA HSR&D Center of Excellence (HFP90-020) and the NIH/National Institute of Diabetes and Digestive and Kidney Disease, Center Grant P30 DK56338. We would to thank Eva Evans and Rohit Ojha for their contributions to this project.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC, Petersen N, Key CR. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50:368–72. doi: 10.1136/gut.50.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesphagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2007;57:1354–59. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 4.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterol. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 5.Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar-lined (Barrettt’s) Esophagus comparison of population-based clinical and autopsy findings. Gastroenterol. 1990;99:918–22. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 6.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373:850–61. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 7.Spechler SJ. Clinical practice. Barrett’s Esophagus. N Engl J Med. 2002;346:836–42. doi: 10.1056/NEJMcp012118. [DOI] [PubMed] [Google Scholar]
- 8.Cook MB, Wild CP, Everett SM, et al. Risk of mortality and cancer incidence in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2095–96. doi: 10.1158/1055-9965.EPI-07-0432. [DOI] [PubMed] [Google Scholar]
- 9.Correa P. The epidemiology of gastric cancer. World J Surg. 1991;15:228–34. doi: 10.1007/BF01659057. [DOI] [PubMed] [Google Scholar]
- 10.Correa P. The human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–60. [ http://cancerres.aacrjournals.org/cgi/reprint/48/13/3554] [PubMed] [Google Scholar]
- 11.Fischbach LA, Correa P, Feldman M, Fontham E, Priest EL, Goodman KJ, Jain R. Increased Reflux Symptoms after Calcium Carbonate Supplementation and Successful Helicobacter pylori Treatment: Results from a Randomized Clinical Trial in a Population with a High Prevalence of Multifocal Atrophic Gastritis. Dig Dis Sci. 2003;48:1487–94. doi: 10.1023/a:1024751420515. [DOI] [PubMed] [Google Scholar]
- 12.Henihan RD, Stuart RC, Nolan N, et al. Barrett’s esophagus and the presence of Helicobacter pylori. Am J Gastroenterol. 1998;93:542–6. doi: 10.1111/j.1572-0241.1998.162_b.x. [DOI] [PubMed] [Google Scholar]
- 13.Loffeld RJ, Ten Tije BJ, Arends JW. Prevalence and significance of Helicobacter pylori in patients with Barrett’s esophagus. Am J Gastroenterol. 1992;87:1598–1600. [PubMed] [Google Scholar]
- 14.Meng X, Scheer MA, Tsang TK. GERD, Barrett’s esophagus and Helicobacter pylori infection. Gastroenterol. 2008;134:A443. [Google Scholar]
- 15.Csendes A, Smok G, Cerda G, Burdiles P, Mazza D, Csendes P. Prevalence of Helicobacter pylori infection in 190 control subjects and in 236 patients with gastroesophageal reflux, erosive esophagitis or Barrett’s esophagus. Dis Esophagus. 1997;10:38–42. doi: 10.1093/dote/10.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Peng S, Cui Y, Xiao YL, et al. Prevalence of erosive esophagitis and Barrett’s esophagus in the adult Chinese population. Dis of the Esophagus. 2009;41:1011–17. doi: 10.1055/s-0029-1215291. [DOI] [PubMed] [Google Scholar]
- 17.Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, et al. Helicobacter pylori infection and the risk of Barrett’s oesophagus: a community-based study. Gut. 2008;57:727–33. doi: 10.1136/gut.2007.132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fassan M, Rugge M, Parente P, Tieppo C, Rugge M, Battaglia G. The role of Helicobacter pylori in the spectrum of Barrett’s carcinogenesis. Cancer Prev Res. 2009;2:94. doi: 10.1158/1940-6207.CAPR-08-0194. [DOI] [PubMed] [Google Scholar]
- 19.Sonnenberg A, Lash R, Genta R. A national study of Helicobacter pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139:1894–1901. doi: 10.1053/j.gastro.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Rokkas T, Pistiolas D, Sechopoulous P, et al. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1413–7. doi: 10.1016/j.cgh.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Gisbert JP, Pajares JM. Prevalence of Helicobacter pylori infection in gastroesophageal reflux disease and Barrett’s esophagus. Med Clin (Barc) 2002;119:217–23. doi: 10.1016/s0025-7753(02)73368-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:494–500. doi: 10.1038/ajg.2008.37. [DOI] [PubMed] [Google Scholar]
- 23.Abbas Z, Hussainy AS, Ibrahim F, Jafri S, Shaikh H, Khan AH. Barrett’s oesophagus and Helicobacter pylori. J Gastroenterol Hepatology. 1995;10:331–3. doi: 10.1111/j.1440-1746.1995.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 24.Abouda GF, Cotton JC, Dillon JF. Prevalence of Helicobacter pylori virulence factors in patients with reflux oesophagitis and Barrett’s oesophagus. Gut. 2003;52:A411. [Google Scholar]
- 25.Anderson LA, Murphy SJ, Johnston BT, Watson RG, Ferguson HR, Bamford KB. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: results from the FINBAR case-control study. Gut. 2008;57:734–9. doi: 10.1136/gut.2007.132662. [DOI] [PubMed] [Google Scholar]
- 26.Blaser MJ, Perez-Perez GL, Lindenbaum J, et al. Association of infection due to Helicobacter pylori with specific upper gastrointestinal pathology. Reviews Inf Dis. 1991;13(Suppl 8):S704–8. doi: 10.1093/clinids/13.supplement_8.s704. [DOI] [PubMed] [Google Scholar]
- 27.Chacaltana A, Urday C, Ramon W, Rodríguez C, Espinoza J, Velarde H. [Prevalence, clinical-endoscopic characteristics and predictive factors of Barrett’s Esophagus in endoscopic screening for gastric cancer] Rev Gastroenterol Peru. 2009;29:24–32. [PubMed] [Google Scholar]
- 28.El-Serag HB, Sonnenberg A, Jamal MM, Kunkel D, Crooks L, Feddersen RM. Characteristics of intestinal metaplasia in the gastric cardia. Am J Gastroenterol. 1999;94:622–7. doi: 10.1111/j.1572-0241.1999.00924.x. [DOI] [PubMed] [Google Scholar]
- 29.Ferrández A, Benito R, Arenas J, García-González MA, Sopeña F, Alcedo J. CagA-positive Helicobacter pylori infection is not associated with decreased risk of Barrett’s esophagus in a population with high H. pylori infection rate. BMC Gastroenterol. 2006;6:7. doi: 10.1186/1471-230X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldblum JR, Richter JE, Vaezi M, Falk GW, Rice TW, Peek RM. Helicobacter pylori infection, not gastroesophageal reflux, is the major cause of inflammation and intestinal metaplasia of gastric cardiac mucosa. Am J Gastroenterol. 2002;97:302–11. doi: 10.1111/j.1572-0241.2002.05462.x. [DOI] [PubMed] [Google Scholar]
- 31.Hackelsberger A, Günther T, Schultze V, Manes G, Dominguez-Muñoz JE, Roessner A. Intestinal metaplasia at the gastro-oesophageal junction: Helicobacter pylori gastritis or gastro-oesophageal reflux disease? Gut. 1998;43:17–21. doi: 10.1136/gut.43.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirota WK, Loughney TM, Lazas DJ, Maydonovitch CL, Rholl V, Wong RK. Specialized intestinal metaplsia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterol. 1999;116:277–285. doi: 10.1016/s0016-5085(99)70123-x. [DOI] [PubMed] [Google Scholar]
- 33.Inomata Y, Koike T, Ohara S, et al. Preservation of gastric acid secretion may be important for the development of gastroesophageal junction adenocarcinoma in Japanese people, irrespective of the H pylori infection status. Am J Gastroenterol. 2006;101:926–933. doi: 10.1111/j.1572-0241.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 34.Johansson J, Håkansson HO, Mellblom L, Kempas A, Johansson KE, Granath F. Risk factors for Barrett’s oesophagus: a population-based approach. Scand J Gastroenterol. 2007;42:148–56. doi: 10.1080/00365520600881037. [DOI] [PubMed] [Google Scholar]
- 35.Jonaitis L, Kriukas D, Sakalauskas L, Janciauskas D, Kupcinskas L. Prevalence of H-pylori among patients with erosive esophagitis and Barrett’s esophagus among routinely endoscoped patients in high H-pylori prevalence area. Helicobacter. 2008;13:440–1. [Google Scholar]
- 36.Kala Z, Dolina J, Marek F, Izakovicova Holla L. Polymorphisms of glutathione S-transferase M1, T1 and P1 in patients with reflux esophagitis and Barrett’s esophagus. J Hum Genet. 2007;52:527–34. doi: 10.1007/s10038-007-0148-z. [DOI] [PubMed] [Google Scholar]
- 37.Kiltz U, Baier J, Schmidt WE, Adamek RJ. Barrett’s metaplasia and Helicobacter pylori infection (letter) Am J Gastroenterol. 1999;94:1985–6. doi: 10.1111/j.1572-0241.1999.01985.x. [DOI] [PubMed] [Google Scholar]
- 38.Laheij RJ, Van Rossum LG, De Boer WA, Jansen JB. Corpus gastritis in patients with endoscopic diagnosis of reflux oesophagitis and Barrett’s oesophagus. Aliment Pharmacol Ther. 2002;16:887–91. doi: 10.1046/j.1365-2036.2002.01245.x. [DOI] [PubMed] [Google Scholar]
- 39.Lam KD, Phan JT, Garcia RT, Trinh H, Nguyen H, Nguyen K. Low proportion of Barrett’s esophagus in Asian Americans. Am J Gastroenterol. 2008;103:1625–30. doi: 10.1111/j.1572-0241.2008.01891.x. [DOI] [PubMed] [Google Scholar]
- 40.Loffeld RJ, Werdmuller BF, Kuster JG, Pérez-Pérez GI, Blaser MJ, Kuipers EJ. Colonization with cagA-positive Helicobacter pylori strains inversely associated with reflux esophagitis and Barrett’s esophagus. Digestion. 2000;62:95–9. doi: 10.1159/000007801. [DOI] [PubMed] [Google Scholar]
- 41.Loffeld RJ, van der Putten AB. Helicobacter pylori and gastro-oesophageal reflux disease: a cross-sectional epidemiological study. Neth J Med. 2004;62:188–91. [PubMed] [Google Scholar]
- 42.Lord RV, Frommer DJ, Inder S, Tran D, Ward RL. Prevalence of Helicobacter pylori infection in 160 patients with Barrett’s oesophagus or Barrett’s adenocarcinoma. Aust NZ J Surg. 2000;70:26–33. doi: 10.1046/j.1440-1622.2000.01737.x. [DOI] [PubMed] [Google Scholar]
- 43.Martinek J, Hucl T, Spicak J. The prevalence of Helicobacter pylori infection in some disease of the esophagus, stomach and duodenum – a retrospective analysis. Cesk Slov Gastroenterol Hepatol. 2003;57:228–32. [Google Scholar]
- 44.Monkemuller K, Neumann H, Nocon M, et al. Serum gastrin and pepsinogens do not correlate with the different grades of severity of gastro-oesophageal reflux disease: a matched case-control study. Aliment Pharm Ther. 2008;28:491–6. doi: 10.1111/j.1365-2036.2008.03769.x. [DOI] [PubMed] [Google Scholar]
- 45.Nandurkar S, Talley NJ, Martin CJ, Ng TH, Adams S. Short segment Barrett’s oesophagus: prevalence, diagnosis and associations. Gut. 1997;40:710–5. doi: 10.1136/gut.40.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newton M, Bryan R, Burnham WR, Kamm MA. Evaluation of Helicobacter pylori in reflux oesophagitis and Barrett’s oesophagus. Gut. 1997;40:9–13. doi: 10.1136/gut.40.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paull G, Yardley JH. Gastric and esophageal Campylobacter pylori in patients with Barrett’s esophagi. Gastroenteroloy. 1988;95:216–8. doi: 10.1016/0016-5085(88)90316-2. [DOI] [PubMed] [Google Scholar]
- 48.Peng S, Xiong LS, Xiao YL, et al. Prompt upper endoscopy is an appropriate initial management in uninvestigated patients with typical reflux symptoms. Am J Gastroenterol. 2010;105:1947–52. doi: 10.1038/ajg.2010.121. [DOI] [PubMed] [Google Scholar]
- 49.Rajendra S, Ackroyd R, Robertson IK, Ho JJ, Karim N, Kutty KM. Helicobacter pylori, ethnicity, and the gastroesophageal reflux disease spectrum: a study from the East. Helicobacter. 2007;12:177–83. doi: 10.1111/j.1523-5378.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 50.Rajendra S, Kutty K, Karim N. Ethnic differences in the prevalence of endoscopic esophagitis and Barrett’s esophagus: the long and short of it all. Dig Dis Sci. 2004;49:237–42. doi: 10.1023/b:ddas.0000017444.30792.94. [DOI] [PubMed] [Google Scholar]
- 51.Rex DK, Cummings OW, Shaw M, Cumings MD, Wong RK, Vasudeva RS. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterol. 2003;125:1670–7. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 52.Rugge M, Russo V, Busatto G, et al. The phenotype of gastric mucosa coexisting with Barrett’s oesophagus. J Clin Pathol. 2001;54:456–60. doi: 10.1136/jcp.54.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, Eskes SA, Meuwissen SG. Helicobacter pylori and the efficacy of omeprazole therapy for gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:884–7. doi: 10.1111/j.1572-0241.1999.982_e.x. [DOI] [PubMed] [Google Scholar]
- 54.Sirigu F, Capeccioni S, Dessì A, Masia AM. Helicobacter pylori in Barrett’s esophagus and in normal or inflamed esophageal mucosa: a retrospective study. Riv Eur Sci Med Farmacol. 1994;16:131–4. [PubMed] [Google Scholar]
- 55.Toruner M, Soyikan I, Ensari A, Kuzu I, Yurdaydin C, Ozden A. Barrett’s esophagus: prevalence and its relationship with dyspeptic symptoms. J Gastroenterol Hepatol. 2004;19:535–40. doi: 10.1111/j.1440-1746.2003.03342.x. [DOI] [PubMed] [Google Scholar]
- 56.Vicari JJ, Peek RM, Falk GW, et al. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115:50–7. doi: 10.1016/s0016-5085(98)70364-6. [DOI] [PubMed] [Google Scholar]
- 57.Vieth M, Masoud B, Meining A, Stolte M. Helicobacter pylori infection: protection against Barrett’s mucosa and neoplasia? Digestion. 2000;62:225–231. doi: 10.1159/000007820. [DOI] [PubMed] [Google Scholar]
- 58.Watari J, Morichi K, Tanabe H, et al. Differences in genetic instability and cellular phenotype among Barrett’s, cardiac and gastric intestinal metaplasia in a Japanese population with Helicobacter pylori. Histopathology. 2009;55:261–9. doi: 10.1111/j.1365-2559.2009.03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Werdmuller BF, Loffeld RJ. Helicobacter pylori infection has no role in pathogenesis of reflux esophagitis. Dig Dis Sci. 1997;42:103–5. doi: 10.1023/a:1018841222856. [DOI] [PubMed] [Google Scholar]
- 60.Weston AP, Badr AS, Topalovski M, Cherian R, Dixon A, Hassanein RS. Prospective evaluation of the prevalence of gastric Helicobacter pylori infection in patients with GERD, Barrett’s esophagus, Barrett’s dysplasia, and Barrett’s adenocarcinoma. Am J Gastroenterol. 2000;95:387–94. doi: 10.1111/j.1572-0241.2000.01758.x. [DOI] [PubMed] [Google Scholar]
- 61.White NM, Gabril M, Ejeckam G, et al. Barrett’s esophagus and cardiac intestinal metaplasia: two conditions within the same spectrum. Can J Gastroenterol. 2008;22:369–75. doi: 10.1155/2008/243254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaninotto G, Portale G, Parenti A, Lanza C, Costantini M, Molena D. Role of acid and bile reflux in development of specialized intestinal metaplasia in distal oesophagus. Dig Liver Dis. 2002;34:251–7. doi: 10.1016/s1590-8658(02)80144-x. [DOI] [PubMed] [Google Scholar]
- 63.Kuper H, Nicholson A, Hemingway H. Searching for observational studies: what does citation tracking add to PubMed? A case study in depression and coronary heart disease. BMC Med Res Methodol. 2006;6:4. doi: 10.1186/1471-2288-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenland S. Meta-analysis. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. 2. Philadelphia, PA: Lippincott-Raven Publishers; 1998. pp. 643–73. [Google Scholar]
- 65.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 66.Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol. 1994;140:290–6. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 67.Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2:463–71. doi: 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- 68.Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–60. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 69.Vaezi MF, Falk GW, Peek RM, Vicari JJ, Goldblum JR, Perez-Perez GI. CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am J Gastroenterol. 2000;95:2206–11. doi: 10.1111/j.1572-0241.2000.02305.x. [DOI] [PubMed] [Google Scholar]
- 70.Sampliner RE The Practice Parameters Committee of The American College of Gastroenterology. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s Esophagus. Am J Gastroenterol. 1998;93:1028–32. doi: 10.1111/j.1572-0241.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 71.El-Serag HB, Sonnenberg A, Jamal MM, Inadomi JM, Crooks LA, Feddersen EM. Corpus gastritis is protective against reflux oesophagitis. Gut. 1999;45:181–5. doi: 10.1136/gut.45.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodman KJ, Correa P, Tengana Aux HJ, DeLany JP, Collazos T. Nutritional factors and Helicobacter pylori infection in Colombian children. J Pediatr Gastroenterol Nutr. 1997;25:507–15. doi: 10.1097/00005176-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Moujaber T, MacIntryre CR, Backhouse J, Gidding H, Quinn H, Gilbert GL. The seroepidemiology of Helicobacter pylori infection in Australia. Int J Infect Dis. 2008;12:500–4. doi: 10.1016/j.ijid.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Lehours P, Yilmaz O. Epidemiology of Helicobacter pylori infection. Helicobacter. 2007;12(Suppl 1):1–3. doi: 10.1111/j.1523-5378.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 75.Flood VM, Burlutsky G, Webb KL, Wang JJ, Smith WT, Mitchell P. Food and nutrient consumption trends in older Australians: a 10-year cohort study. Eur J Clin Nutr. 2010;64:603–13. doi: 10.1038/ejcn.2010.34. [DOI] [PubMed] [Google Scholar]
- 76.Chang UJ, Hong YH, Suh HJ, Jund EY. Lowering the energy density of parboiled rice by adding water rich vegetables can decrease total energy intake in a parboiled rice-based diet without reducing satiety on healthy women. Appetite. 2010;55:338–42. doi: 10.1016/j.appet.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Jacobson BC, Chan AT, Giovannucci El, et al. Body mass index and Barrett’s oesophagus in women. Gut. 2009;58:1460–6. doi: 10.1136/gut.2008.174508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abrams JA. Obesity and Barrett’s oesophagus: more than just reflux. Gut. 2009;58:1437–8. doi: 10.1136/gut.2009.183285. [DOI] [PubMed] [Google Scholar]
- 79.Sugerman HJ. Increased intra-abdominal pressure and GERD/Barrett’s esophagus. Gastroenterology. 2007;133:2075. doi: 10.1053/j.gastro.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 80.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. [DOI] [PubMed] [Google Scholar]