Abstract
Background
The prevalence of Helicobacter pylori in Western populations has steadily decreased. This has been suggested as one of the factors involved in the recent increase of asthma and allergy. Some studies have reported a negative association between H.pylori and asthma and allergy, but data are inconsistent and there are a few studies in children.
Aim
We investigated whether the prevalence of H.pylori was associated with asthma symptoms, allergic rhinitis, and atopic dermatitis in childhood.
Methods
We determined IgG anti-H.pylori and CagA antibodies in serum of Dutch children, who took part in the PIAMA birth cohort study. Serum was collected from 545 children, aged 7–9 yrs (Dutch ethnicity 91.5%). Symptoms of asthma and atopy were assessed by yearly questionnaires. Chi-square tests and logistic regression were used.
Results
We found 9% H.pylori and 0.9% CagA seropositivity. Twelve (5.9%) children with reported wheezing ever were H.pylori positive, compared to 37 (10.9%) of the non-wheezers (p=0.05). No significant differences in H.pylori prevalence were found between children with or without allergic rhinitis (8.5% vs. 9.5%), atopic dermatitis (8.7% vs. 9.2%), and physician-diagnosed asthma (7.1% vs. 9.4%). Multivariate analysis showed no significant associations between H.pylori seropositivity and wheezing (OR 0.52; 95% CI 0.25–1.06), allergic rhinitis (OR 0.96; 95% CI 0.51–1.81), atopic dermatitis (OR 1.05; 95% CI 0.56–1.98) or physician-diagnosed asthma (OR 0.87; 95% CI 0.37–2.08).
Conclusion
We found a borderline significantly lower H.pylori seropositivity in children with wheezing compared to non-wheezers, but no association between H.pylori serum-antibody status and allergic rhinitis, atopic dermatitis, or asthma.
Keywords: Helicobacter pylori, children, asthma, wheezing, atopy, disappearing microbiota hypothesis
INTRODUCTION
Helicobacter pylori, a Gram-negative bacterium, colonizes the stomach of more than half of the human population. Colonization invariably leads to chronic gastritis, which can give rise to other conditions, in particular peptic ulceration and gastric adenocarcinoma 1. The prevalence of H. pylori has decreased steadily in Western populations over the past decades and has now reached low levels in children (<10% in children aged <10 years) 2–5. Possible contributors to the disappearance of H. pylori are the widespread use of antibiotics, improved hygiene and decreased family size 6.
While this has occurred, the prevalence of atopic disorders such as allergic rhinitis, asthma, and atopic dermatitis has risen dramatically 7. Numerous environmental causes including air pollution, exposure to tobacco smoke, exogenous infections, microbial substances in the environment, ownership of furry pets, and obesity have been proposed to explain this phenomenon 8–9.
In addition to these exogenous factors, a change in our indigenous microflora may have led to the rise in atopic disorders. According to the “disappearing microbiota hypothesis”, ecological changes affecting our ancient indigenous microbiota may have contributed to the increased prevalence of asthma and allergy 10. Changes in the overall pattern of commensals and pathogens in the gastrointestinal tract could be particularly relevant to this mechanism, as the gut associated lymphoid tissue is critical for normal maturation of our immune system, possibly preventing the later development of atopic conditions 11. In line with this hypothesis, a negative association has been observed between H. pylori colonization, the dominant member of the gastric microflora, and the occurrence of asthma or allergy 10, 12. However, data are inconsistent and few studies have been performed in children so far 4, 13.
Therefore, the objective of the present study was to test whether the prevalence of H. pylori is indeed inversely related to the prevalence of asthma symptoms, allergic rhinitis and atopic dermatitis in a cohort of Dutch children.
METHODS
Study population
The study population consisted of a subsample of Dutch children who participated in the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort study; details of this study have been published 14. Expectant mothers were recruited from 52 prenatal health care clinics. Children born between the summer of 1996 and the late fall of 1997 were followed prospectively from birth until the age of 8 years.
The study protocol was approved by the Institutional Review Boards of the participating institutions. The parents of all participants gave written informed consent.
Questionnaires
Questionnaires for parental completion were sent at the third trimester of pregnancy, at 3 months after birth, at the age of one year and yearly thereafter, up to the age of 8 years 15. In these questionnaires, information on wheezing symptoms, allergic rhinitis, atopic dermatitis, physician-diagnosed asthma, and asthma medication use was collected, using questions based on the International Study of Asthma and Allergies in Childhood (ISAAC) core questionnaires. Furthermore, data on socio-economic status, demographics, and a wide range of possible risk factors for asthma and allergies were collected.
Definitions
Wheezing was assessed with the question: “Has your child had wheezing or whistling in the chest in the last 12 months?”. Allergic rhinitis was assessed with the question: “Has your child had runny nose or sneezing without having a cold in the last 12 months?”. Atopic dermatitis was defined as positive if parents reported the presence of an itchy rash which was intermittent in the prior 12 months on typical eczema sites (the folds of the elbows or behind the knees, around ears or eyes or in front of the ankles). ’Current physician-diagnosed asthma’ was defined as a positive response to both the questions: “Did a physician ever diagnose asthma in your child?” and “Has your child had asthma in the last 12 months?”. Data on prescription of inhalation corticosteroids was collected from pharmacies for children above the age of 2 years.
Unless stated otherwise, all outcome measures refer to the composed outcome ‘ever’, defined as the presence at any point in the first 8 years of life, e.g. wheezing ever was defined as the presence of wheeze at any point in the first 8 years of life. For wheezing, we also examined the effect at each year of age separately (allowing ascertainment of time-trends).
Laboratory tests
Blood samples were collected at a physical examination at the age of 7–9 years. Serum anti-H. pylori IgG antibody levels were determined by enzyme-linked immunosorbent assay (ELISA), using whole cell antigens 16. CagA status was determined by a separate ELISA, based on the presence of serum IgG antibodies against a specific recombinant truncated CagA protein, as described 16–17. Both ELISAs have been validated in adults and children 18, including Dutch adults but not Dutch children. They have been used in previous studies in Dutch children 5. All samples were at least tested in duplicate. The optical density (OD) was determined spectrometrically at 405 nm for each specimen. For each sample, the optical density ratio (ODR) was calculated by dividing the OD of the sample by the mean OD of the positive controls. Specimens were considered positive for H. pylori if the ODR was ≥1.0. The cut-off for CagA positivity was a value of ODR >0.35 based on previous validation.
Statistical analysis
Chi-square tests (for categorical variables) and t-tests (for continuous variables) were used to compare the distribution of socio-economic, demographic, and lifestyle variables in relation to H. pylori status. Univariate and multiple logistic regression was performed to assess a relationship between H. pylori positivity and atopic symptoms. We performed an incomplete case analysis to minimize the risk of selection bias and compared the results with a complete case analysis. Results of both analyses did not differ, so we opted to use the incomplete case analysis to gain statistical power. In the multivariate analysis, odds ratios (ORs) were adjusted for potential confounders, including gender, ethnicity, exposure to indoor smoking, breastfeeding and educational attainment of parents. Children with missing data on any of these covariates were excluded from the multivariate analysis.
A two-sided p-value of <0.05 was considered statistically significant. All analyses were performed using PASW Statistics 17.0 for Windows (SPSS, IBM, New York, United States).
RESULTS
In total, 545 children were studied. Their general characteristics are shown in Table 1. The population consisted of more boys than girls (M/F: 300/245) and children were predominantly of Dutch ethnicity (91.5%).
Table 1.
Baseline characteristics of study population, according to Helicobacter pylori status
| H. pylori − (n=496) | H. pylori + (n=49) | p-value | |
|---|---|---|---|
| Male gender, n(%) | 274 (55) | 26 (53) | 0.442 |
| Age, mean (SD), y* | 7.9 (0.6) | 7.9 (0.6) | 0.683 |
| Race/ethnicity, n(%) | 0.456 | ||
| Dutch | 459 (93) | 45 (92) | |
| Non-Dutch Caucasian | 13 (3) | 1 (2) | |
| Non-Caucasian | 14 (3) | 3 (6) | |
| Low education of parents, n (%)¥ | 144 (29%) | 21 (43%) | 0.039 |
| Atopy in mother, n (%) | 36 (7) | 1 (2) | 0.166 |
| Atopy in father, n (%) | 156 (31) | 15 (31) | 0.904 |
| Number of older siblings, mean (SD) | 0.86 (1.1) | 0.96 (1.0) | 0.563 |
| Mother smoking during pregnancy, n (%) | 54 (11) | 7 (14) | 0.472 |
| Breastfeeding, n (%) | 428 (86) | 36 (73) | 0.032 |
| Daycare attendance before age 5, n (%)† | 392 (80) | 35 (71) | 0.207 |
| Exposure to indoor smoke, n (%)‡ | 61 (12) | 13 (27) | 0.006 |
Age at time of blood sample collection
Defined as at least one parent with low educational attainment
Defined as at least 4 hours per week in a day-care centre with other children (under 12 year of age) present
Defined as >1 cigarette/week
Children included in the final analyses were less likely to have an atopic mother than children in the original PIAMA population (7% vs. 31%), but they were comparable with respect to ethnicity, percentage having been breastfed, parental socio-economic status, and exposure to indoor smoking with children in the original PIAMA population.
The overall prevalence of H. pylori seropositivity was 9% (95% CI 6.6–11.4%) (n=49) and the prevalence of anti-CagA antibodies was 0.9% (n=5), of which 4 were also H. pylori positive. H. pylori-positive children were significantly more often exposed to indoor smoking (> 1 cigarette/week) (27% vs. 12%, P=0.006), less often received breastfeeding (73% vs. 86%, p=0.03), and their parents had a lower educational attainment (43% vs. 29%, p=0.04) compared to children with negative H. pylori serology.
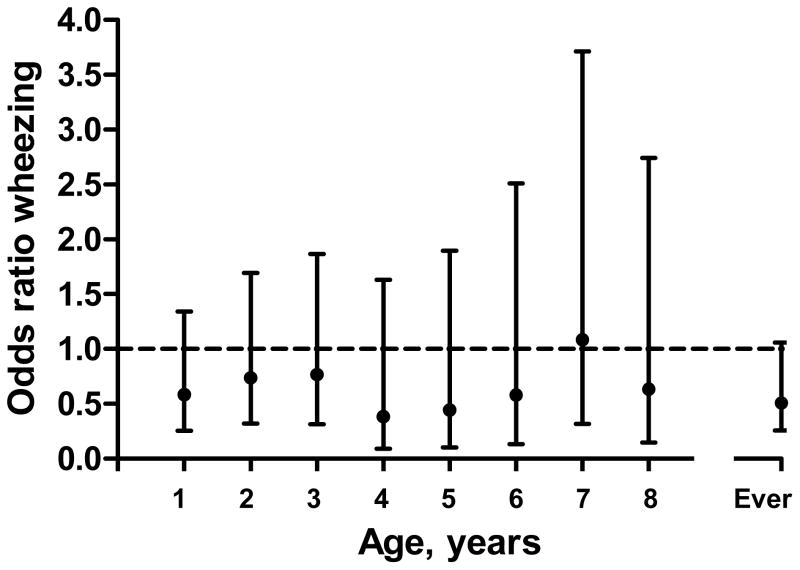
In the univariate analysis, the prevalence of H. pylori in children with ‘wheezing ever’ was 5.9% (n=12) while the H. pylori prevalence in those without wheezing ever was 10.9% (n=37). This difference was borderline significant (p=0.05). When including the one CagA positive H.pylori negative child, the p-value became 0.08. Analysis of the association between H. pylori prevalence and wheezing at separate ages showed a consistent inverse trend from age 1 to 8 years, but this trend did not reach statistical significance (Figure 1). No significant differences in H. pylori seropositivity were found between children with or without allergic rhinitis (8.5% vs. 9.5%), atopic dermatitis (8.7% vs. 9.2%), physician-diagnosed asthma (7.1% vs. 9.4%), and use of inhaled corticosteroids (8.4% vs. 9.1%).
Figure 1.
Odds ratios (ORs) and confidence intervals for the inverse association between Helicobacter pylori and wheezing. ORs given for the overall effect and for each year of age, separately.
In the multivariate analyses the inverse association between H. pylori prevalence and wheezing ever remained borderline significant (OR 0.52; 95% CI 0.25–1.06; p=0.07). No significant associations were found with allergic rhinitis (OR 0.96; 95% CI 0.51–1.81), atopic dermatitis (OR 1.05; 0.56–1.98), physician-diagnosed asthma (OR 0.87; 95% CI 0.37–2.08), or inhaled corticosteroid use (OR 1.00; 95% CI 0.42–2.40) (Table 2). The seropositivity rate for CagA was too low for statistical analysis.
Table 2.
Association between Helicobacter pylori status and asthma, asthmatic symptoms, allergic rhinitis, and atopic dermatitis
| H.pylori − (n=496) | H.pylori + (n=49) | Crude OR (95% CI) | P-value | Adjusted OR (95% CI)* | P-value | |
|---|---|---|---|---|---|---|
| Wheezing | 0.51 (0.26–1.01) | 0.05 | 0.52 (0.25–1.06) | 0.07 | ||
| No | 304 | 37 | ||||
| Yes | 192 | 12 | ||||
| Allergic rhinitis | 0.88 (0.49–1.58) | 0.67 | 0.96 (0.51–1.81) | 0.89 | ||
| No | 227 | 24 | ||||
| Yes | 269 | 25 | ||||
| Atopic dermatitis | 0.94 (0.52–1.70) | 0.84 | 1.05 (0.56–1.98) | 0.88 | ||
| No | 276 | 28 | ||||
| Yes | 220 | 21 | ||||
| Doctors diagnosed asthma | 0.74 (0.32–1.70) | 0.48 | 0.87 (0.37–2.08) | 0.76 | ||
| No | 405 | 42 | ||||
| Yes | 91 | 7 | ||||
| Inhaled corticosteroid use | 0.92 (0.40–2.13) | 0.85 | 1.00 (0.42–2.40) | 1.00 | ||
| No | 420 | 42 | ||||
| Yes | 76 | 7 | ||||
Abbreviations: CI: confidence interval, OR: odds ratio,
OR and 95% CI correspond to the ratio of the odds of outcome in H. pylori positivity vs. the odds of outcome in H. pylori negativity.
OR adjusted for gender, ethnicity, exposure to indoor smoking, breastfeeding and educational attainment of parents, excluding participants with unknown data for one of the covariates (n=40).
DISCUSSION
This study shows an inverse trend in children between colonization with H. pylori and wheezing
The present observations to some extent support the “disappearing microbiota hypothesis” 10. According to this hypothesis, the disappearance of our ancestral indigenous microbiota is proposed to contribute to the rise in prevalence of asthma and other atopic conditions. H. pylori, the ancient dominant member of the gastric niche, is one of these disappearing microorganisms in the Western world. Although probably not exclusively responsible, H. pylori can be considered as a model to study this hypothesis. One reason is that colonization with H. pylori is mostly persistent and stable over time. H. pylori status can easily and reliably be assessed with non-invasive methods, such as serology and stool antigen detection.
One of the suggested underlying molecular mechanisms of this possible preventive effect of H. pylori is that the neutrophil-activating protein of H. pylori (HP-NAP) not only plays a key role in driving T-helper type 1 (Th1) inflammation, but is also able to inhibit T-helper type 2 (Th2)-mediated bronchial inflammation of allergic bronchial asthma 19. More details come from a recent mouse model study, in which models of allergic airway disease were experimentally infected with H. pylori. Mice infected neonatally were found to exhibit significantly less tissue inflammation and goblet cell metaplasia than uninfected controls. Their bronchoalveolar inflammation, eosinophilia, and IL-5 and IL-13 secretion were clearly reduced and the pulmonary infiltration of Th2 and Th17 cells were diminished in infected animals. Asthma protection was further associated with impaired maturation of lung-infiltrating dendritic cells and the induction of regulatory T cells 20.
Several large cross-sectional and case-control studies demonstrate an inverse relationship between asthma and H.pylori especially for CagA+ strains and early onset asthma and allergic rhinitis 4, 12–13, 21–22. Others have reported no associations 23–25 or, in line with our findings, weak inverse associations 22.
To our knowledge, this is the first study with longitudinal data on asthma and atopy in relation to H. pylori status. There was no risk of recall bias, since parents were not aware of H. pylori status of their child at the time of completion of the questionnaires. Furthermore, we achieved a very high participation grade on repeated questionnaires. Only 1.5% to 6.2% of the yearly questionnaires were (partly) missing.
In an observational study, it is not possible to firmly demonstrate causality, as correlations may be explained by other mechanisms 8. A crucial requirement for causality is that the H. pylori infection precedes the start of symptoms. Since there are reliable data that H. pylori is acquired almost exclusively in childhood, and usually persists for life if not eliminated by antibiotics, it might be speculated that H. pylori precedes the diagnosis of asthma 26–27. However, asthma commonly has its onset early in life as well. In the present study, questionnaires were collected from very young age, however H. pylori was only tested at 8 years old. Therefore, temporality of wheezing symptoms and H. pylori infection could not be examined in this study.
In the absence of specific data on antibiotic use, one can speculate that the use of antibiotics is a potential confounder, since children with asthma may receive antibiotic treatment for respiratory infection. However elimination of H. pylori by antimicrobial monotherapy is infrequent, which likely limited the effect of this potential confounder 28. Furthermore, successful removal of H. pylori cannot be detected for many months after eradication as IgG titres fall with about 50% per 6–12 months 29. Arguments against a substantial confounding effect of antibiotics come from studies in which a specific inverse association was found between H. pylori and early onset asthma, but not late onset asthma 12. If antibiotic therapy would act as a notable confounder, the effect would be expected to increase with age similar to the increased cumulative exposure to antibiotics.
Asthma is difficult to diagnose in infants and young children for whom testing of reversible airway obstruction is technically difficult. Therefore, wheezing is often used as a proxy for asthma in children in epidemiologic studies. Especially in the first years of life this could lead to considerable misclassification, due to the high prevalence of transient wheezing symptoms during common respiratory infections 30. The specificity of wheezing as a proxy for asthma increases with age. We found an inverse trend between wheezing and H. pylori, which was consistent over age. In fact, the association at the age of 4–6 years was somewhat stronger than at 1–3 years. The inverse association between H. pylori and asthma (OR 0.87) was not significant, possibly due to the low numbers of children with a physician-diagnosis of asthma.
The current study has some potential limitations. First, we largely relied on selfreported data on asthmatic symptoms and atopic conditions. This may have led to some (non-differential) misclassification and thereby to bias toward the null. This would indicate that the true association may be larger than observed. Second, our data do not allow conclusions on the exact age of H. pylori acquisition, because children were tested only once at the age of 8 years. Third, it is possible that the observed inverse association between wheezing and H. pylori arose by chance, as multiple associations were examined. This is less likely, considering that we found a very consistent trend over time and over the different parameters defining atopy. A plausible explanation is that our study was not adequately powered to detect a moderate inverse association. Finally, although both ELISAs have been validated in adults and children, including Dutch adults, and have been used in previous studies in Dutch children 5, they have not been separately validated in Dutch children specifically.
Identification of similar prevalences in larger groups will allow more solid conclusions to be drawn. If we gain better understandings of not only the relations between H. pylori and atopy, but also the mechanisms underlying the possibly protective effects of H. pylori for atopic disorders, we could investigate whether there are disease contexts in which gastric carriage of H. pylori in early life could yield benefits 31.
Acknowledgments
Supported by T-R01 DK090989 from the National Institutes of Health and the Diane Belfer Program for Human Microbial Ecology.
References
- 1.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roosendaal R, Kuipers EJ, Buitenwerf J, et al. Helicobacter pylori and the birth cohort effect: evidence of a continuous decrease of infection rates in childhood. Am J Gastroenterol. 1997;92:1480–2. [PubMed] [Google Scholar]
- 3.Banatvala N, Mayo K, Megraud F, et al. The cohort effect and Helicobacter pylori. J Infect Dis. 1993;168:219–21. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–60. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Hoed CM, Vila AJ, Holster IL, et al. Helicobacter pylori and the birth cohort effect: evidence for stabilized colonization rates in childhood. Helicobacter. 2011;16:405–9. doi: 10.1111/j.1523-5378.2011.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287:3096–102. doi: 10.1001/jama.287.23.3096. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Sheikh A, Strachan DP, et al. Time trends in allergic disorders in the UK. Thorax. 2007;62:91–6. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561–7. doi: 10.1136/gut.2007.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strachan DP, Carey IM. Home environment and severe asthma in adolescence: a population based case-control study. BMJ. 1995;311:1053–6. doi: 10.1136/bmj.311.7012.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–94. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matricardi PM, Rosmini F, Riondino S, et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ. 2000;320:412–7. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–7. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 13.Reibman J, Marmor M, Filner J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;3:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunekreef B, Smit J, de Jongste J, et al. The prevention and incidence of asthma and mite allergy (PIAMA) birth cohort study: design and first results. Pediatr Allergy Immunol. 2002;13 (Suppl 15):55–60. doi: 10.1034/j.1399-3038.13.s.15.1.x. [DOI] [PubMed] [Google Scholar]
- 15.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 16.Everhart JE, Kruszon-Moran D, Perez-Perez G. Reliability of Helicobacter pylori and CagA serological assays. Clin Diagn Lab Immunol. 2002;9:412–6. doi: 10.1128/CDLI.9.2.412-416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]
- 18.Drumm B, Perez-Perez GI, Blaser MJ, et al. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990;322:359–63. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- 19.D’Elios MM, Codolo G, Amedei A, et al. Helicobacter pylori, asthma and allergy. FEMS Immunol Med Microbiol. 2009;56:1–8. doi: 10.1111/j.1574-695X.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 20.Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011 doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCune A, Lane A, Murray L, et al. Reduced risk of atopic disorders in adults with Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2003;15:637–40. doi: 10.1097/00042737-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Shiotani A, Miyanishi T, Kamada T, et al. Helicobacter pylori infection and allergic diseases: epidemiological study in Japanese university students. J Gastroenterol Hepatol. 2008;23:e29–33. doi: 10.1111/j.1440-1746.2007.05107.x. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis D, Luczynska C, Chinn S, et al. The association of hepatitis A and Helicobacter pylori with sensitization to common allergens, asthma and hay fever in a population of young British adults. Allergy. 2004;59:1063–7. doi: 10.1111/j.1398-9995.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 24.Bodner C, Anderson WJ, Reid TS, et al. Childhood exposure to infection and risk of adult onset wheeze and atopy. Thorax. 2000;55:383–7. doi: 10.1136/thorax.55.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang KW, Lam WK, Chan KN, et al. Helicobacter pylori sero-prevalence in asthma. Respir Med. 2000;94:756–9. doi: 10.1053/rmed.2000.0817. [DOI] [PubMed] [Google Scholar]
- 26.Malaty HM, El-Kasabany A, Graham DY, et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931–5. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- 27.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 28.Broussard CS, Goodman KJ, Phillips CV, et al. Antibiotics taken for other illnesses and spontaneous clearance of Helicobacter pylori infection in children. Pharmacoepidemiol Drug Saf. 2009;18:722–9. doi: 10.1002/pds.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosunen TU, Seppala K, Sarna S, et al. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori. Lancet. 1992;339:893–5. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 30.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 31.Blaser MJ. Helicobacter pylori and gastric diseases. BMJ. 1998;316:1507–10. doi: 10.1136/bmj.316.7143.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]