Approximately 1.2 million Americans have moderate-to-severe psoriasis, a chronic, inflammatory disease of the skin and joints that has substantial impact on health-related quality of life and is associated with excess cardiovascular risk and mortality.1 Despite the rapid growth of treatments for psoriasis, insufficient data exist to distinguish between first- and second-line therapies. Thus, as emphasized by the Institute of Medicine, there exists a critical need for comparative effectiveness research (CER) in psoriasis treatment.2 Since a crucial component of CER is to identify the priorities of stakeholders such as physicians, we conducted a study to describe U.S. dermatologists’ preferences for which treatments to compare in future randomized controlled trials (RCTs) in moderate-to-severe psoriasis.
Methods
We surveyed 1000 U.S. dermatologists (500 National Psoriasis Foundation (NPF) members and 500 American Academy of Dermatology (AAD) members who self-reported that they treat psoriasis) as part of a larger study on preferences for psoriasis therapy. Dermatologists were asked to select three treatments they would most like to compare in a RCT for moderate-to-severe psoriasis from a list of 10 Food and Drug Administration (FDA)-approved biologic, oral systemic, and phototherapies (Figure 1). The order of treatment listings was randomized in 6 ways to reduce bias. Detailed data on the survey methods have been published elsewhere.3, 4 The study was approved by the Penn Institutional Review Board and conducted from May to August 2010.
FIGURE 1.
Questionnaire item assessing dermatologists’ preferences for treatments to include in future trials (note: treatment options were randomized in 6 different orders to reduce bias).
The primary outcome was dermatologists’ preferences for treatments to compare in a RCT, as indicated by each treatment’s cumulative frequency of first, second, or third choice selection. Preferences were summarized descriptively and compared with respect to major provider characteristics.
Results
We received questionnaire responses from 387 dermatologists (39.1% response rate). Responding dermatologists were mostly male (72%), NPF members (64%), and private practitioners (70%). Respondents were similar to non-respondents with respect to sex, duration of practice, and geographic region. Additional data on respondents’ baseline characteristics have been previously described.4 Of note, respondents indicated that a median of 90% of their psoriasis patients on systemic medications or phototherapy also concurrently used topicals by prescription.
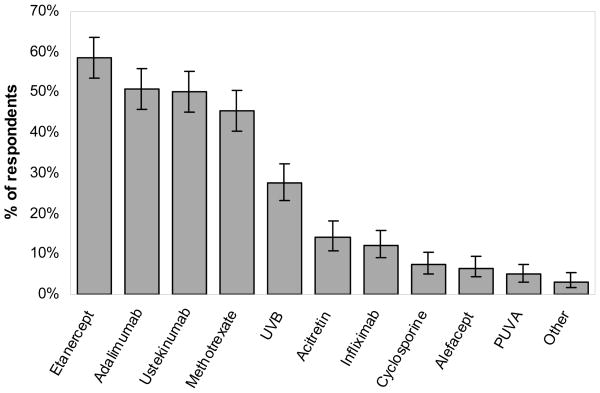
The treatments dermatologists most wanted to compare in a RCT were etanercept (59%; 95% confidence interval (CI) 53.6–63.6%), adalimumab (51%; 95% CI 45.8–56.0%), ustekinumab (50%; 95% CI 45.0–55.2%), and methotrexate (45%; 95% CI 40.4–50.6%) (Figure 2). When preferences were stratified by provider characteristics, including sex, NPF versus AAD membership, region, duration of practice, practice type, patient volume, and infusion center affiliation, the top four overall treatments remained the same although the order of treatments within the top four occasionally differed. The presence of phototherapy units in the practice affected the degree of preference for including ultraviolet (UV) B phototherapy in CER trials [34.1% (95% CI 28.3–40.3%) versus 14.6% (95% CI 8.9–22.1%) of dermatologists without units selected UVB]; however, the top 4 overall treatments were the same regardless of phototherapy availability.
FIGURE 2. Preferences for treatments to compare in a randomized controlled trial.
Error bars indicate 95% confidence interval.
Comment
Our results indicate that U.S. dermatologists who treat psoriasis prefer to compare the newer subcutaneously-administered tumor necrosis factor-inhibitors and interleukin-12/23 inhibitor and the traditional oral systemic methotrexate in future CER trials. Etanercept, adalimumab, and ustekinumab gained FDA approval for plaque psoriasis in 2004, 2008, and 2009, respectively. Methotrexate, however, has been widely used since its approval in 1972.
Notably, we had previously observed that UVB was the most-preferred first-line treatment by dermatologists who treat psoriasis followed by etanercept, adalimumab, and methotrexate;4 however, UVB was only the fifth most-requested treatment for RCT inclusion among these providers. Ustekinumab, on the other hand, was the third most-requested treatment to include in CER trials but was ranked as only the sixth most-preferred first-line treatment for moderate-to-severe psoriasis.4
Our results also indicate that the concurrent use of prescription topicals with systemic or phototherapy is common; however, most previous RCTs have prohibited combination therapy.5 Thus, in order for CER trials to reflect real-world practice, permitting the concomitant use of topical prescription therapy should be considered.
Our findings are highly informative for future trial design as they represent the priorities of hundreds of U.S. dermatologists who actively treat patients with psoriasis. Future studies should examine the priorities of other stakeholders, such as payers and patients, and other elements of CER trial design, such as primary efficacy outcomes, safety endpoints upon which to discriminate, and treatment duration.
Acknowledgments
Funding/Support: This study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases RC1-AR058204 (J.M.G.), the Doris Duke Clinical Research Fellowship (K.A.), and National Institutes of Health Training Grant T32-AR07465 (J.W., D.B.S.).
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Ms. Wan and Dr. Gelfand had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Abuabara, Van Voorhees, Bebo, Krueger, Callis Duffin, Gelfand. Acquisition of data: Abuabara, Shin, Gelfand. Analysis and interpretation of data: Wan, Troxel, Shin, Gelfand. Drafting of the manuscript: Wan. Critical revision of the manuscript for important intellectual content: Wan, Abuabara, Troxel, Shin, Van Voorhees, Bebo, Krueger, Callis Duffin, Gelfand. Statistical analysis: Wan, Troxel, Shin, Gelfand. Obtained funding: Gelfand. Administrative, technical, or material support: Wan, Abuabara, Shin. Study supervision: Gelfand.
Financial Disclosures:
- Relevant to this manuscript: Dr. Van Voorhees served on the advisory board of and was an investigator and speaker for Amgen and Genentech, receiving honoraria and grants; was a consultant for Incyte, Leo, VGX, and Xtrac, receiving honoraria; served on the advisory board and was a speaker for Abbott, Centocor, and Connetics, receiving honoraria; served on the advisory board and was an investigator for Bristol Myers Squibb and Warner Chilcott, receiving honoraria and grants; was an investigator for Astellas, IDEC, and Roche, receiving grants; served as a consultant for Amgen; and received honoraria from Synta. Dr. Bebo has had relationships with Abbott, Amgen, Astellas, Centocor, Galderma, Genentech, PhotoMedix, Stiefel/GSK, Warner Chilcott, and Wyeth, receiving other benefits. Dr. Krueger served on the steering committees for Centocor/Phoenix 2 and Golimumab/psoriatic arthritis, receiving no compensation; served on the steering committee for PSOLAR, receiving other financial benefit; served on the data monitoring board for Novartis and as chair of the data monitoring safety board for Pfizer, receiving other financial benefit; was a consultant and/or advisory board member for Abbott, Almirall, Amgen, Anacor, Astellas, Boehringer Ingleheim, Bristol Myers Squibb, Centocor, CombinatoRx, Genzyme, Isis, Lilly, L’Oreal, Lupin Limited, MedaCorp, Medicis, Novartis, Nova Nordisk, Pfizer, Schering Plough, Somagenics, theDerm.org, Synvista, Warner Chilcot, UCB, Vascular Biogenics Limited, and ZARS, receiving honoraria or other financial benefit; was a stockholder in ZARS, receiving stock options; was a speaker for Abbott, Amgen, Astellas, Centocor, and the National Psoriasis Foundation, receiving honoraria; and was an investigator for Abbott, Amgen, and Centocor, receiving grants. Dr. Callis Duffin was an investigator, consultant, and speaker for Abbott, Amgen, and Centocor, receiving honoraria and salary; served on the advisory board of Amgen; and received residency/fellowship program funding from Abbott and Amgen. Dr. Gelfand served as consultant and investigator with Abbott, Amgen, Centocor, Genentech, Novartis, and Pfizer, receiving grants and honoraria; was a consultant with Celgene, Covance, Galderma, Shire Pharmaceuticals, and Wyeth, receiving honoraria; and was an investigator with Shionogi, receiving grants. Drs. Abuabara and Troxel, Mr. Shin, and Ms. Wan have no financial disclosures to report.
- All other relationships: None
Contributor Information
Joy Wan, Email: joywan@mail.med.upenn.edu.
Katrina Abuabara, Email: katrina.abuabara@uphs.upenn.edu.
Andrea B. Troxel, Email: atroxel@mail.med.upenn.edu.
Daniel B. Shin, Email: dbshin@mail.med.upenn.edu.
Abby S. Van Voorhees, Email: abby.vanvoorhees@uphs.upenn.edu.
Bruce F. Bebo, Jr., Email: bbebo@psoriasis.org.
Gerald G. Krueger, Email: gerald.krueger@hsc.utah.edu.
Kristina Callis Duffin, Email: kristina.callis@hsc.utah.edu.
Joel M. Gelfand, Email: joel.gelfand@uphs.upenn.edu.
References
- 1.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 2.Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med. 2009;151(3):203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. [DOI] [PubMed] [Google Scholar]
- 3.Wan J, Abuabara K, Shin DB, Troxel AB, Bebo BF, Jr, Gelfand JM. Dermatologist response rates to a mailed questionnaire: a randomized trial of monetary incentives. J Am Acad Dermatol. doi: 10.1016/j.jaad.2011.03.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan J, Abuabara K, Troxel AB, et al. Dermatologist preferences for first-line therapy of moderate-to-severe psoriasis in healthy adult patients. J Am Acad Dermatol. doi: 10.1016/j.jaad.2011.03.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naldi L, Svensson A, Zenoni D, et al. Comparators, study duration, outcome measures and sponsorship in therapeutic trials of psoriasis: update of the EDEN Psoriasis Survey 2001–2006. Br J Dermatol. 2010;162(2):384–389. doi: 10.1111/j.1365-2133.2009.09515.x. [DOI] [PubMed] [Google Scholar]