Abstract
Lay Abstract
Gastrointestinal dysfunction (GID) in children with autism spectrum disorder (ASD) is not well understood. Differences in factors associated with GID, such as eating habits, have been reported between ASD and non-ASD populations, but relationships between these factors and GID have not been examined. There is also the possibility that what we do know about GID in ASD is influenced by parents’ perceptions of GID in their children. Although parents know their children best, they are not necessarily experts in determining GID. This study examined how well parents and pediatric gastrointestinal clinicians agree on GID in children, and how factors thought to relate to GID in ASD, actually do relate to GID. 121 children were studied, in three groups: co-occurring ASD and GID, ASD without GID, and GID without ASD. Clinical evaluations by pediatric gastroenterologists validated parental reports of GID in ASD, with constipation the leading type of GID in ASD. Presence of GID in ASD was not associated with differences in diet or medications, but was associated with language and social impairments. These findings suggest that healthcare providers of children with ASD should be vigilant for GID, particularly in children who lack the ability to communicate verbally.
Scientific Abstract
The objectives of this study were to characterize gastrointestinal dysfunction (GID) in autism spectrum disorder (ASD), to examine parental reports of GID relative to evaluations by pediatric gastroenterologists, and to explore factors associated with GID in ASD. 121 children were recruited into three groups: co-occurring ASD and GID, ASD without GID, and GID without ASD. A pediatric gastroenterologist evaluated both GID groups. Parents in all three groups completed questionnaires about their child’s behavior and GI symptoms, and a dietary journal. Functional constipation was the most common type of GID in children with ASD (85.0%). Parental report of any GID was highly concordant with a clinical diagnosis of any GID (92.1%). Presence of GID in children with ASD was not associated with distinct dietary habits or medication status. Odds of constipation were associated with younger age, increased social impairment, and lack of expressive language (adjusted odds ratio in nonverbal children: 11.98, 95% CI 2.54 – 56.57). This study validates parental concerns for GID in children with ASD, as parents were sensitive to the existence, although not necessarily the nature, of GID. The strong association between constipation and language impairment highlights the need for vigilance by healthcare providers to detect and treat GID in children with ASD. Medications and diet, commonly thought to contribute to GID in ASD, were not associated with GID status. These findings are consistent with a hypothesis that GID in ASD represents pleiotropic expression of genetic risk factors.
Keywords: Autism, Constipation, Diet, Functional Gastrointestinal Disorders, Nonverbal Communication, Social Behavior
INTRODUCTION
Autism spectrum disorder (ASD) is defined by deficits in social interaction and communication, and a restricted repertoire of activities and interests [Volkmar, 2005]. ASD is heterogeneous and complex, often presenting with behavioral and medical comorbidities, including mood disorders, sleep abnormalities, and gastrointestinal dysfunction (GID) [Geschwind, 2009]. However, a recent consensus report highlighted many unexplored issues in GID and ASD that have important implications for clinical care [Buie et al., 2010].
The absence of rigorous, prospective clinical phenotyping in children with ASD and parent-suspected GID has impeded understanding potential relationships between GID and ASD. Research on GID in ASD has largely been limited to studies relying on parental reports [Valicenti-McDermott et al., 2006; Smith et al., 2009] or retrospective review of records [Black et al., 2002; Xue et al., 2008], lacking comparison groups [Molloy & Manning-Courtney, 2003; Nikolov et al., 2009], or focused on the prevalence of GID in ASD compared to non-ASD populations [Ibrahim et al., 2009; Mouridsen et al., 2010]. It has been suggested that GID in children with ASD does not have a biological basis but rather results from behavior, medications or dietary habits [Ibrahim et al., 2009].
Our laboratory has previously identified genetic differences between individuals with ASD with and without comorbid GID [Campbell et al., 2009], reframing questions of GID in a biologically-reasoned context. Subgroups of individuals with ASD may be at greater risk for GID because of environmental factors that interact with risk-associated genes that are expressed in both the brain and gastrointestinal system. Here we report findings of an ongoing study that further explores this hypothesis. Our objectives were to characterize GID in children with ASD, to compare parental reports of GID symptoms on a validated instrument to results of clinical evaluations by a pediatric gastroenterologist, and to evaluate the relationships of dietary habits and medication status to GID in ASD.
METHODS
Study Procedures
121 children were recruited into three groups: co-occurring ASD and GID (ASD-GID); ASD without any GID (ASD-only); and GID without any ASD (GID-only). At enrollment, parents of children with ASD were queried in a structured interview to assess for ongoing GI complaints and assign GID group status. The ASD-GID and GID-only groups were evaluated by a pediatric gastroenterologist. The ASD-GID and ASD-only groups were assessed with the Autism Diagnostic Observation Schedule (ADOS). Parents in all groups completed questionnaires about their child’s behavior and GI symptoms, and a dietary journal. The research protocol was approved by the Vanderbilt University Institutional Review Board, and written informed consent was obtained from parents of participants.
Participants
Children were recruited at Vanderbilt University in Nashville, Tennessee, between 2009 and 2011. Children in both ASD groups were recruited primarily through the hospital’s ASD medical clinic, although some families self-referred after seeing study flyers in other locations, such as family resource events. Children in the GID-only group were recruited through the pediatric gastroenterology outpatient clinic. Exclusion criteria included severe sensory or motor impairment, neurodevelopmental disorders of known etiology (e.g. Fragile X Syndrome), gestational age less than 36 or greater than 42 weeks, and birth weight less than 2500 grams. Inclusion criteria included age between 5 and 18 years, meeting ASD criteria on the ADOS (for the ASD groups), and GI symptoms that had lasted more than a month (for the GID groups).
Measures
Clinical evaluation
Children in both GID groups were evaluated by one of six pediatric gastroenterologists; one of those six (K.C.W.) evaluated 78.9% of participants. The evaluation included a medical history, gastrointestinal symptoms review, physical examination, and examination of growth parameters. Depending on the severity, frequency and duration of signs and symptoms, and presence of associated symptoms such as weight loss or changes in dietary intake, laboratory tests and endoscopic procedures were pursued at the physician’s discretion when non-functional GI disease was suspected. Laboratory tests included a complete blood count with differential (CBC), a comprehensive metabolic panel that included electrolytes and liver function tests (CMP), erythrocyte sedimentation rate (ESR), and celiac screening panel that included total IgA and anti-tissue transglutaminase antibodies (CSP). Endoscopic procedures included esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy (FS), and colonoscopy; all endoscopic procedures included tissue biopsies with clinical histopathological examination. Participants found to have non-functional disease by endoscopy were not excluded from this study. Participants with mild clinical presentations and histories who were also negative on screening laboratories did not receive further gastroenterological evaluation due to the low yield in children with this symptomatology [Di Lorenzo et al., 2005; Dhroove et al., 2010]. Because of pronounced behavioral manifestations, blinding the gastroenterologist to ASD status was not possible.
ADOS
Children in both ASD groups had ASD clinical diagnoses at enrollment, that were confirmed by assessment with the ADOS, a standardized instrument for diagnosing ASD [Lord et al., 1999]. ADOSs were performed within two years of the study by a research-certified assessor who was blinded to GID status, and the revised scoring algorithm was used [Gotham et al., 2007]. Item scores were not available for one participant, and one participant was assessed with a Module 4, which does not have a revised scoring algorithm; for these two participants, the original scoring algorithm was used.
Expressive language
Item A1 on Module 1 of the ADOS rates non-echoed language. A score of either 3 or 8 was considered nonverbal for analyses.
Social Responsiveness Scale (SRS)
The SRS is a 65-item, parent-report instrument that provides a validated continuous measure of social impairment [Constantino & Gruber, 2005]. SRS data was not available for one participant.
Questionnaire on Pediatric Gastrointestinal Symptoms - Rome III Version (QPGS)
The QPGS is a 71-item parent-report instrument that assesses GI symptoms, classifies functional GI disorders (FGIDs) according to Rome-III criteria [Walker et al., 2000; Drossman, 2006], and is widely used in research on pediatric GID [Caplan et al., 2005b, 2005a; Baber et al., 2008]. While organic GI disorders result from a clear anatomic, metabolic or pathologic process that is readily identified clinically, FGIDs instead have no overt pathology which can be identified. For analysis, missing responses on the QPGS were interpreted as a lack of evidence for the assessed symptom. If eight or more items were missing, the QPGS was omitted from analysis; instrument data from two participants were excluded by this criterion. Three participants who were initially enrolled in the ASD-only group subsequently met criteria for one or more FGID classifications; these participants were moved to the ASD-GID group and evaluated by a pediatric gastroenterologist. Four participants who were enrolled in the ASD-only group subsequently met criteria for fecal incontinence on the QPGS, but no other FGID; we interpreted this to be a toilet-training issue, rather than indicative of GID, and thus these individuals remained in the ASD-only group. The instrument is available online at romecriteria.org.
Dietary journal
Parents were asked to record all food eaten by the participating child for seven days, into 11 categories but without serving size information. For analysis, we required complete data from at least five days. Two journals were excluded based on this criterion, and 20 parents did not complete the journal.
Medication classification
Medications were queried in the Micromedex database (Thomson Reuters, New York, NY) and classified as having potential GI side effects if adverse effects of abdominal pain, constipation, indigestion, nausea, vomiting, or diarrhea were reported in greater than 10% of patients. Supplemental Table 1 lists medications and corresponding classifications.
Data Analysis
Study data were managed using REDCap, a secure, research-oriented, web-based application [Harris et al., 2009]. Statistical analyses were computed using SPSS version 18.0.3 (IBM, Somers, NY). Participant characteristics were described by mean and standard deviation for continuous measures (age and BMI percentile) and by percent for nonparametric measures (sex, ethnicity, race, ADOS classification, and nonverbal status). ADOS classification and nonverbal status were compared using χ2 tests; BMI percentiles were compared by a one-way analysis of variance (ANOVA). Prevalences of GI diagnoses were reported as percentages. Kappa coefficients (κ) were calculated to assess parent-physician agreement for a diagnosis of constipation, and parent-physician percent agreement for presence of any GID was compared with a χ2 test. SRS T-scores were plotted as boxplots and compared with an ANOVA; T-scores of nonverbal versus verbal subgroups were compared with a two-tailed t-test. Dietary habits were plotted as group means of percent of food category eaten with 95% confidence intervals (CI), and compared with an ANOVA. The proportion of subgroups taking medications with potential GI side effects was compared with a χ2 test. To examine factors associated with the outcome of constipation, an exploratory logistic regression model was developed to test the additive effect of factors with potential clinical relevance (SRS T-score, nonverbal status, BMI percentile, and medication status). After univariate logistic regression models were tested, factors with significant unadjusted odds ratios (ORs) were retained and then entered into a final model, with sex and age as covariates. Adjusted ORs for constipation are reported. For all statistical tests, a P value of less than 0.05 was considered significant. When applicable, Tukey’s HSD post hoc test was computed.
RESULTS
Participant Characteristics
121 children were enrolled into three groups (ASD-GID = 40; ASD-only = 45; GID-only 36; Table 1). Participants were predominantly male (range: 63.9 to 86.7%) and of non-Hispanic white racial and ethnic origin (range: 77.5 to 88.9%), and ranged in age from 5.1 to 17.9 years. There was no difference in ADOS classification between the ASD groups (proportion who met criteria for autism: ASD-GID 95.0%; ASD-only 91.1%; P = 0.48).
Table 1.
Characteristics of Study Participants
| ASD-GID {n = 40) | ASD-only (n=45) | G ID-only (n = 36) | |
|---|---|---|---|
| Age in years, mean (SD) | 10.8(3.7) | 12.4(3.4) | 11.0(3.4) |
| Male sex, % (n) | 72.5 (29) | 86.7 (39) | 63.9 (23) |
| Ethnicity and race, % (n) | |||
| Hispanic | 12.5 (5) | 0(0) | 2.8(1) |
| Non-Hispanic white | 77.5(31) | 86.7 (39) | B8.9 (32) |
| Non-Hispanic black | 7.5 (3) | 8.9 {4} | 8.3 (3) |
| Non-Hispanic other | 2.5(1) | 4.4 (2) | 0(0} |
| ADOS Classification, % (n) | |||
| Autism | 95.0 (38) | 91.1 (41) | n/a |
| Autism Spectrum | 5.0 (2) | 8,9 {4) | n/a |
| Nonverbal, % (n)A | 30.0(12) | 6.7 (3) | 0(0} |
| BMI-for-age percentile, mean (SD)B | 76.2 (30.2) | 68.9(31.0) | 57.2 (36.3) |
ASD-GID versus other groups, P< 0.01
ASD-GID versus GID-only, P = 0.03
Gastrointestinal Diagnoses
Functional constipation (FC) was the most common diagnosis of GID in ASD (85.0%; Figure 1) when evaluated by a pediatric gastroenterologist. Although FC was also common in the GID-only group (44.4%), a comparison of prevalence of FC in ASD versus non-ASD was not appropriate, as this study was not designed to be epidemiologically representative. Instead, the diagnoses in the GID-only group show that this group had primarily FGIDs and was thus similar to the ASD-GID group in nature of GID, to enable subsequent comparisons across groups for other factors. Reflux was the next most common diagnosis in the ASD-GID group (20.0%), and three children were found to have non-functional, organic disease underlying their GI symptoms (one case each of eosinophilic esophagitis, H. pylori, and celiac disease). Findings on endoscopic examination were consistent with organic disease for each of the three ASD-GID non-functional participants. Seven children in the GID-only group were found to have non-functional GI disease (four cases of eosinophilic esophagitis, and one case each of H. pylori, celiac disease, and Crohn’s disease). All seven cases had findings by EGD or colonoscopy that were consistent with these diagnoses. Percentages of study groups examined endoscopically were, for ASD-GID and GID-only, respectively: EGD, 35.0% and 69.4%; FS, 7.5% and 2.8%; colonoscopy, 10.0% and 36.1%. Other than the three ASD-GID and seven GID-only non-functional cases listed above, endoscopic examination did not yield remarkable findings in the other participants.
Figure 1. Gastrointestinal Dysfunction in Children With and Without ASD, by Physician’s Evaluation and Parent’s Report.
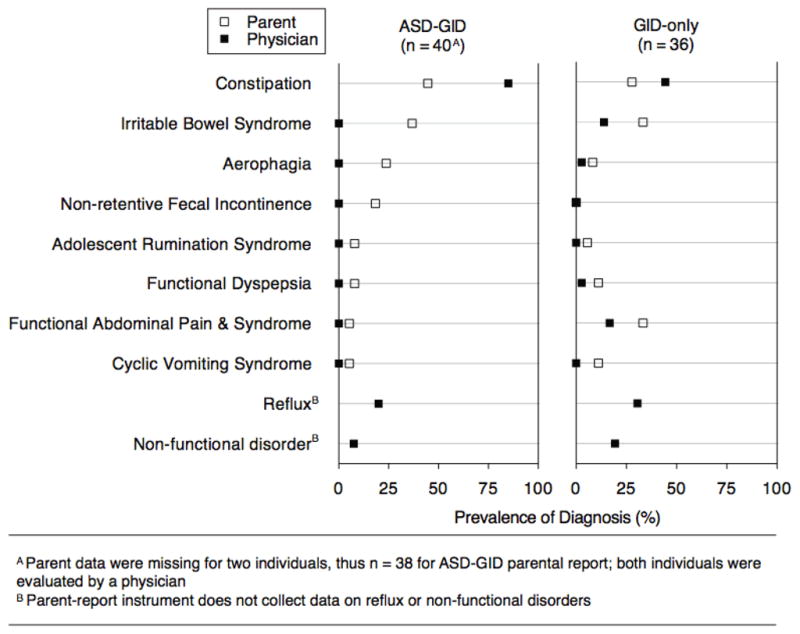
Diagnoses of gastrointestinal dysfunction, by both a pediatric gastroenterologist’s clinical evaluation and a parent’s report on a symptom-based classification instrument, for children with and without ASD. Constipation was the most prevalent diagnosis, and concordance between parents and clinicians was fair to moderate for a specific diagnosis of constipation, but high when considering presence versus absence of any gastrointestinal dysfunction.
Percentages of study groups with laboratory studies completed were, for ASD-GID and GID-only, respectively: CBC, 75.0% and 75.0%; CMP, 77.5% and 72.2%; ESR, 45.0% and 50.0%; CSP, 60.0% and 61.1%. CBC and CMP results were clinically unremarkable in all participants. Six children (three in each group) had mildly elevated ESRs; four of the six were endoscopically examined, resulting in two children with reflux esophagitis, one with Crohn’s disease, and one with no remarkable findings. Three children had positive CSPs; EGD findings in these children supported a diagnosis of celiac disease in two children, and the third child did not have any significant pathological features consistent with celiac disease.
FC by the parent-reported QPGS was also the most common diagnosis in the ASD-GID group (44.7%). Parent-physician agreement for a diagnosis of FC was fair for the ASD-GID group (κ = 0.26) and moderate for the GID-only group (κ = 0.42) [Landis & Koch, 1977]. However, percent agreement between a physician’s diagnosis for any GID and a parent’s report yielding any diagnosis on the QPGS was 92.1% in the ASD-GID group, compared to 88.9% in the GID-only group (P = 0.64).
Language & Social Impairments
To explore the functional significance of comorbid GID, we investigated group differences in language and social function. The percentage of nonverbal children was larger in the ASD-GID group, compared to the ASD-only group (Table 1; ASD-GID 30.0%; ASD-only 6.7%; P < 0.01).
Children in the GID-only group did not show an elevated SRS T-score above that in typically-developing children (normed with mean 50 and SD 10 [Constantino & Gruber, 2005]; GID-only mean 51.3; Figure 2). As expected [Constantino et al., 2003], children in the ASD-only group showed an elevated T-score compared to the GID-only group (ASD-only mean 77.7; GID-only mean 51.3; P < 0.001). Interestingly, children in the ASD-GID group showed an elevated T-score compared to children in both ASD-only and GID-only groups, indicating the most severe social impairment in the co-occurring group (ASD-GID mean 89.2; ASD-only 77.7; P = 0.001). The elevated SRS T-score in the ASD-GID group was not associated with impaired language, as scores of nonverbal and verbal children in this group were not different (nonverbal mean 86.2; verbal mean 90.4; P = 0.35).
Figure 2. Social Impairment Measured by SRS T-Scores.
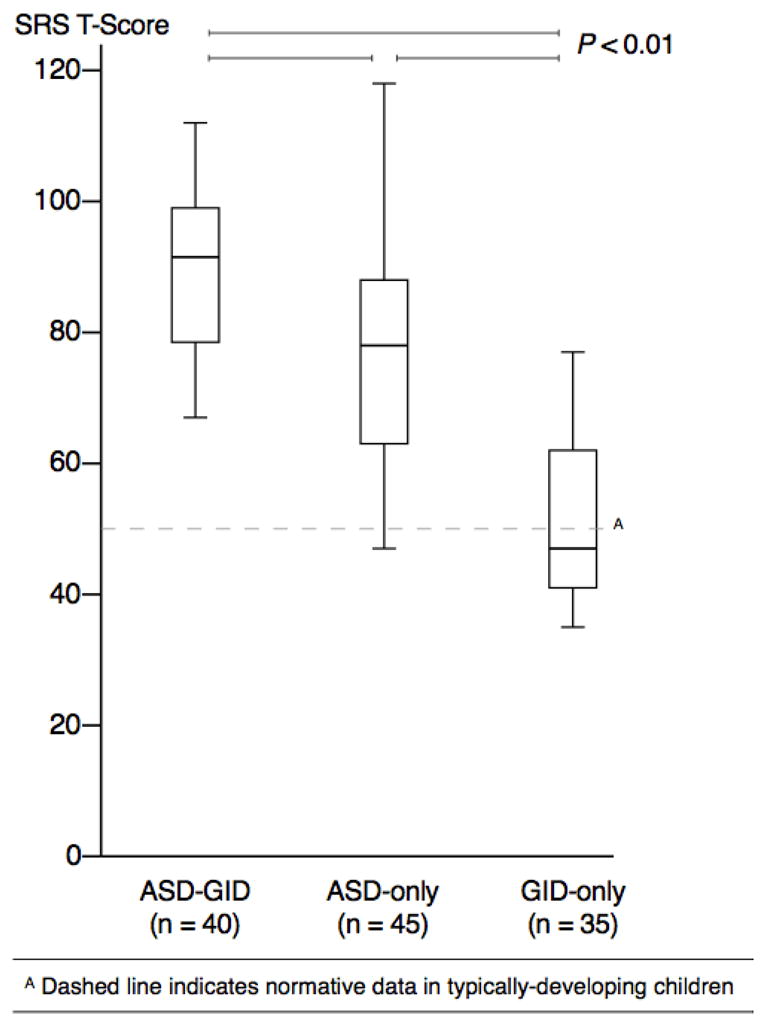
Social impairment, measured on the Social Responsiveness Scale, was significantly greater in both ASD groups, compared to the GID-only group; the most social impairment was seen in the ASD-GID group.
Indistinguishable Diets
To explore whether GID in ASD was associated with a restricted or limited diet, parents were asked to record 11 categories of food that their child ate during the course of seven days (Figure 3). Comparison of the relative distributions of food categories showed no significant differences across groups.
Figure 3. Percent of Food Categories Eaten During Seven Days.
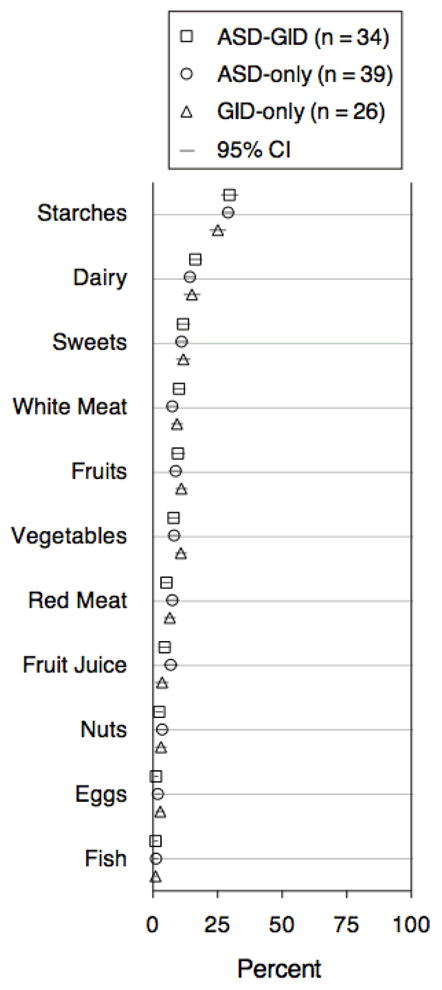
Parents recorded foods eaten by their children for seven days, in 11 different food categories. Relative distributions of food categories eaten did not differ significantly across any of the three groups.
Factors Associated with Constipation
Logistic regression was used to determine what factors were associated with functional constipation, the most prevalent GI diagnosis in our sample. Elevated BMI has been associated with FGIDs in typically-developing children [Pashankar & Loening-Baucke, 2005; Teitelbaum et al., 2009], and obesity prevalence in ASD has been reported to be 30% [Curtin et al., 2010]. Mean BMI-for-age percentile [Kuczmarski et al., 2002] was not statistically different between ASD-GID and ASD-only children (Table 1), and in univariate logistic regression, BMI was not significantly associated with a diagnosis of constipation in ASD (OR 1.01, 95% CI 0.99 – 1.02). There were no differences among ASD subgroups in the proportion taking medications with potential constipating side effects (67.6% of ASD-GID with constipation; 83.3% of ASD-GID with GID other than constipation; 64.4% of ASD-only; P = 0.65). In univariate logistic regression, potentially constipating medications were not associated with constipation in ASD (OR 1.04, 95% CI 0.42 – 2.64). Thus, in our final multivariate logistic regression model, we adjusted for age and sex (due to the large developmental age range and preponderance of males in our sample), and included SRS T-score and nonverbal status, as those were different between ASD-GID and ASD-only groups. Table 2 reports adjusted ORs, showing that younger (OR 0.81, 95% CI 0.69 – 0.94; P = 0.01), more socially impaired (OR 1.05, 95% CI 1.01 – 1.09; P = 0.02), and nonverbal (OR 11.98, 95% CI 2.54 – 56.57; P = 0.002) children with ASD had increased odds for functional constipation.
Table 2.
Factors Associated With a Diagnosis of Constipation in Children With ASD (n = 85)
| Adjusted Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Sex | 0.79 | 0.20–3.11 | NS |
| Age | 0.81 | 0.69–0.94 | 0.01 |
| SRS T-score | 1.05 | 1.01–1.09 | 0.02 |
| Nonverbal | 11.98 | 2.54 – 56.57 | 0.002 |
Multivariate logistic regression, adjusting for sex and age, showed younger, more socially impaired (by SRS T-score), and nonverbal children with ASD had increased odds for functional constipation.
DISCUSSION
For the first time, data obtained from clinical specialists and parents were integrated in the same study to address gaps in understanding GID in ASD [Buie et al., 2010]. A number of unique findings address what we suggest are misconceptions of GID in ASD. The majority of children in the ASD-GID group had functional, rather than organic, GID; children with ASD had the same types of GID as children without ASD. For constipation, the most common diagnosis in the ASD-GID group, we saw fair parent-physician agreement (κ = 0.26), which is similar to reported agreement among pediatric gastroenterologists (κ = 0.36) [Saps et al., 2010]. Percent agreement between any type of parent-reported GID and any physician diagnosis, however, was high (92.1% in ASD-GID), and no different than that in the GID-only group. This suggests that parents of children with ASD are similar to parents of children without ASD in reporting GID, and parents from both groups are limited in their ability to discriminate subtypes of GID. Moreover, parental report of GI symptoms in a child requires inference that may by limited by communication deficits in ASD. The parental report data in Figure 1 suggest a variety of FGIDs are present in ASD, whereas by a physician’s evaluation, most children were given a working diagnosis of functional constipation. This suggests that in children with ASD the manifestations of functional constipation can be variable and the expertise of a gastroenterologist is needed to determine the nature of the GID.
Interestingly, some children were initially enrolled into the ASD-only group, as parents did not report ongoing GID. However, upon completing the QPGS, 19 children met criteria for one or more FGIDs. To properly re-assign them to the ASD-GID group, we requested that the children be evaluated by a pediatric gastroenterologist. Several families could no longer be contacted and other families chose not to be evaluated; thus, no data from these 19 children are included in this report. This finding, combined with high parent-physician concordance of GID presence, suggests that parents of children with ASD do not over-report GID, and in fact may under-report GID.
We suggest that comorbid GID has implications beyond medical status for children with ASD. Our data show a large portion of children with co-occurring ASD and GID lack expressive language; as a novel finding, this association warrants further study. This study did not assess for behavioral problems, such as aggression or self-injurious acts. However, it will be important to clarify the relationship between externalizing behaviors, language level, and GI disorders, in a rigorous manner. Children in the ASD-GID group also showed increased social impairment on the SRS, compared to the ASD-only group. Because GID-only children did not show increased SRS scores, this suggests an interaction between ASD and GID statuses. Additional insight may derive from monitoring SRS scores or other behaviors during GID treatment, to determine whether improved medical status can enhance child responsiveness to established ASD behavioral treatments or decrease problematic behaviors [Bauman, 2010]. In addition, observation of parent-child communication regarding the toileting needs of nonverbal children with ASD will clarify the extent to which lack of expressive language may itself contribute to constipation by limiting appropriate toileting behavior.
We were surprised to find no differences in dietary habits between the three groups. Previous studies have reported increased food selectivity in younger children with ASD [Bandini et al., 2010; Emond et al., 2010]. The data reported here focus on older children, and do not show evidence of food selectivity beyond that seen in a non-ASD population. We suggest that it is unlikely that dietary habits play a large causal role in GID status in our study population.
Potential medication side effects and BMI did not contribute to a diagnosis of constipation. Although it has been suggested that constipation in ASD is caused by such factors [Ibrahim et al., 2009], our data do not support this conclusion. For any given child, medications may contribute to GID. However, group-level analysis did not detect an association between potential medication side effects and GID in ASD. Associations between impaired language and social function remained after adjustment for age and sex. Although the OR for the SRS is modest (1.05), this indicates a 5% increase in odds of constipation for each point increase in the SRS T-score. The OR for nonverbal status is large (11.98), and has important implications for those involved in the clinical care of children with ASD who lack traditional communication abilities. Consistent with the recent consensus report [Buie et al., 2010], the data presented here affirm the need for healthcare providers to evaluate their patients for latent constipation, and to consider empiric treatment of constipation in nonverbal children.
An important limitation of this study was that the physician’s evaluation was often limited to a single encounter. Laboratory tests and endoscopic procedures were ordered as clinically-indicated on a per-patient basis, to determine if organic disease was underlying a child’s GID. Thus, it is likely that the diagnoses presented here are functional. As FGIDs are symptom-based diagnoses of exclusion, working through the differential diagnosis may require multiple visits to determine the specific underlying FGID (for example, determining if intermittent abdominal pain is due to functional constipation or functional abdominal pain). The diagnoses presented here are the most likely diagnoses given the child’s clinical presentation at study enrollment. Additionally, laboratory tests and endoscopic examinations were not completed for all study participants. However, it has been reported that for children with abdominal pain, tests and procedures have low diagnostic yields beyond what a detailed history and physical examination can determine [Di Lorenzo et al., 2005; Dhroove et al., 2010]. To address these limitations, future studies will benefit from follow-up evaluations of participants to determine whether original diagnoses are confirmed after subsequent clinical evaluations.
This report characterized GID in children with ASD, evaluated by pediatric gastroenterologists and compared to parental report of GI symptoms, for the first time in comparison to ASD-only and GID-only groups. Parental report of presence of any GID in ASD was highly concordant with a physician’s diagnosis of any GID, validating parental concerns for GID in children with ASD. Parents were sensitive to the existence, although not necessarily the nature, of GID. Functional constipation was the most prevalent GID in ASD. Odds for constipation were significantly associated with younger age, increased social impairment, and lack of expressive language. Factors previously suggested as causes of GID in ASD, diet and medications, were not associated with GID. These data support our evolving hypothesis [Campbell et al., 2009] that, like altered brain-based function characteristic of ASD, GID in children with ASD represents expression of genetic risk factors that interact with environmental components to influence GI function. In the context of the current findings, future studies are warranted to determine whether there are fundamental differences in gastrointestinal biology between individuals with ASD with and without comorbid GID.
Supplementary Material
Acknowledgments
Funding:
Grant sponsor National Institutes of Health/National Institute of Child Health and Human Development; Grant numbers R21HD065289, R01HD23264
Grant sponsor National Institutes of Health/National Institute of General Medical Sciences; Grant number T32GM07347
Grant sponsor National Institutes of Health/National Center for Research Resources; Grant numbers TL1RR024978, UL1RR024975
Grant sponsor Marino Autism Research Institute
We are grateful to the families who participated in this study. This work was supported by NICHD grant R21HD065289 (P.L.), and in part by NIGMS grant T32GM07347 for the Vanderbilt Medical-Scientist Training Program (P.G.), NCRR grant TL1RR024978 (P.G.), NICHD grant R01HD23264 (L.W.), the Vanderbilt Clinical and Translational Science Award UL1RR024975 from NCRR, the Marino Autism Research Institute, the Pediatric Clinical Research Center at Vanderbilt University, and through the Vanderbilt Autism Treatment Network Site, a program funded by Autism Speaks. We are grateful to Loren Tilson for expert managerial oversight and Christianne Lane, PhD for assistance with statistical analysis. Data used in the preparation of this article reside in the NIH-supported National Database for Autism Research, in collection #1450. This manuscript reflects the views of the authors and does not reflect the opinions or views of the NIH.
References
- Baber KF, Anderson J, Puzanovova M, Walker LS. Rome II versus Rome III classification of functional gastrointestinal disorders in pediatric chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:299–302. doi: 10.1097/MPG.0b013e31816c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157:259–264. doi: 10.1016/j.jpeds.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. 2010;7:320–327. doi: 10.1016/j.nurt.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C, Kaye JA, Jick H. Relation of childhood gastrointestinal disorders to autism: nested case-control study using data from the UK General Practice Research Database. BMJ. 2002;325:419–421. doi: 10.1136/bmj.325.7361.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ, 3rd, Furuta GT, Levy J, Vandewater J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Buie TM, Winter H, Bauman M, Sutcliffe JS, Perrin JM, et al. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123:1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]
- Caplan A, Walker L, Rasquin A. Development and preliminary validation of the questionnaire on pediatric gastrointestinal symptoms to assess functional gastrointestinal disorders in children and adolescents. J Pediatr Gastroenterol Nutr. 2005a;41:296–304. doi: 10.1097/01.mpg.0000172748.64103.33. [DOI] [PubMed] [Google Scholar]
- Caplan A, Walker L, Rasquin A. Validation of the pediatric Rome II criteria for functional gastrointestinal disorders using the questionnaire on pediatric gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2005b;41:305–316. doi: 10.1097/01.mpg.0000172749.71726.13. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale: Manual. Western Psychological Services; 2005. [Google Scholar]
- Curtin C, Anderson SE, Must A, Bandini L. The prevalence of obesity in children with autism: a secondary data analysis using nationally representative data from the National Survey of Children’s Health. BMC Pediatr. 2010;10:11. doi: 10.1186/1471-2431-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr. 2010;51:579–583. doi: 10.1097/MPG.0b013e3181de0639. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo C, Colletti RB, Lehmann HP, Boyle JT, Gerson WT, Hyams JS, et al. Chronic abdominal pain in children: a clinical report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:245–248. doi: 10.1097/01.mpg.0000155367.44628.21. [DOI] [PubMed] [Google Scholar]
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics. 2010;126:e337–342. doi: 10.1542/peds.2009-2391. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009;124:680–686. doi: 10.1542/peds.2008-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002:111–190. [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule: Manual. Western Psychological Services; 1999. [Google Scholar]
- Molloy CA, Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003;7:165–171. doi: 10.1177/1362361303007002004. [DOI] [PubMed] [Google Scholar]
- Mouridsen SE, Rich B, Isager T. A longitudinal study of gastrointestinal diseases in individuals diagnosed with infantile autism as children. Child Care Health Dev. 2010;36:437–443. doi: 10.1111/j.1365-2214.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- Nikolov RN, Bearss KE, Lettinga J, Erickson C, Rodowski M, Aman MG, et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord. 2009;39:405–413. doi: 10.1007/s10803-008-0637-8. [DOI] [PubMed] [Google Scholar]
- Pashankar DS, Loening-Baucke V. Increased prevalence of obesity in children with functional constipation evaluated in an academic medical center. Pediatrics. 2005;116:e377–380. doi: 10.1542/peds.2005-0490. [DOI] [PubMed] [Google Scholar]
- Saps M, Chogle A, Sztainberg MO, Dhroove G, Di Lorenzo C. Inter-Rater Reliability of the ROME III Criteria in Children. Gastroenterology. 2010;138:S-109–S-110. [Google Scholar]
- Smith RA, Farnworth H, Wright B, Allgar V. Are there more bowel symptoms in children with autism compared to normal children and children with other developmental and neurological disorders?: A case control study. Autism. 2009;13:343–355. doi: 10.1177/1362361309106418. [DOI] [PubMed] [Google Scholar]
- Teitelbaum JE, Sinha P, Micale M, Yeung S, Jaeger J. Obesity is related to multiple functional abdominal diseases. J Pediatr. 2009;154:444–446. doi: 10.1016/j.jpeds.2008.09.053. [DOI] [PubMed] [Google Scholar]
- Valicenti-McDermott M, McVicar K, Rapin I, Wershil BK, Cohen H, Shinnar S. Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. J Dev Behav Pediatr. 2006;27:S128–136. doi: 10.1097/00004703-200604002-00011. [DOI] [PubMed] [Google Scholar]
- Volkmar FR. Handbook of autism and pervasive developmental disorders. 3. Hoboken: John Wiley & Sons; 2005. [Google Scholar]
- Walker LS, Caplan-Dover A, Rasquin-Weber A. Manual for the Questionnaire on Pediatric Gastrointestinal Symptoms. 2000. [Google Scholar]
- Xue M, Brimacombe M, Chaaban J, Zimmerman-Bier B, Wagner GC. Autism spectrum disorders: concurrent clinical disorders. J Child Neurol. 2008;23:6–13. doi: 10.1177/0883073807307102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


