Abstract
Estrogen and progesterone receptor-negative breast cancer disproportionately affects young women and African Americans, has a poor prognosis, and lacks an effective chemoprevention agent. 3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, known as “statins,” are appealing candidate agents for breast cancer chemoprevention because of their demonstrated safety after decades of widespread use. In preclinical studies, statins inhibit multiple cancer-associated pathways in both hormone receptor (HR)–negative and HR-positive cell lines. Epidemiologic studies of statins and breast cancer show inconsistent results, with some suggesting a reduction in HR-negative breast cancer incidence in lipophilic statin users. However, large meta-analyses show no association between statin use and overall risk of breast cancer, although most did not evaluate tumor HR status. Multiple phase 1 and 2 prevention studies of statins for breast cancer risk reduction are ongoing. If results are promising, they may justify a randomized trial of statins for breast cancer chemoprevention, with a focus on HR-negative disease.
Introduction
Over the past decade, randomized trials have shown that medications, specifically the selective estrogen response modulators (SERMs), tamoxifen and raloxifene, may reduce risk of developing breast cancer. However, SERMs prevent the development of estrogen receptor– and progesterone receptor–positive (hormone receptor [HR]–positive) breast cancer only, without reducing risk of HR-negative disease [1-3]. Approximately 20% of breast cancers in the United States are HR-negative, based on data from the Surveillance, Epidemiology, and End Results (SEER) registry [4]. However, recent analyses of the Carolina Breast Cancer Study, California Cancer Registry, and other cohorts have found that nearly 40% of breast cancers in young women and African Americans are HR-negative. These cancers often have higher tumor grade and worse survival [5-7]. Consequently, there is an important and unmet need for prevention of HR-negative breast cancer.
Multiple drug classes are under investigation for their potential ability to prevent HR-negative breast cancer by targeting nonendocrine pathways, including retinoids, selective cyclo-oxygenase-2 inhibitors, and tyrosine kinase inhibitors [8]. However, chemoprevention requires treating cancer-free people over a period of years for a disease they may never develop. Therefore, any candidate agent must be very safe and well tolerated if it is to prove acceptable to healthy patients. These requirements have led investigators to reevaluate medications that have a history of widespread, successful use for noncancer indications, and to focus specifically on statins. Statins have been used in the general US population since 1987 for treatment of hypercholesterolemia [9]. Buchwald [10] initially proposed that cholesterol inhibition could prevent carcinogenesis, after he observed inhibition of tumor growth in cell culture and in vivo experiments. Subsequent preclinical and epidemiologic studies suggested potential efficacy for statins against HR-negative breast cancer and increased enthusiasm for targeted chemoprevention studies. This article reviews evidence from preclinical, epidemiologic, and intervention studies that have prompted consideration of statins for chemoprevention of HR-negative breast cancer. Ongoing trials designed to test their efficacy for this purpose are also summarized.
Preclinical Studies
General statin mechanisms
Statins are small-molecule inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, an enzyme that catalyzes conversion of HMG-CoA to mevalonate and enables subsequent cholesterol synthesis. Despite a shared mechanism, individual statins differ significantly in their structure, pharmacodynamic profile, and lipid-modifying efficacy, influencing water solubility, absorption, metabolism, and excretion [11]. Atorvastatin, simvastatin, lovastatin, fluvastatin, and cerivastatin are categorized as lipophilic statins, whereas pravastatin and rosuvastatin are categorized as hydrophilic. Bioavailability varies significantly among the statins; in general, hydrophilic statins have reduced peripheral tissue uptake [11].
Statins as a drug class inhibit the rate-limiting step in the HMG-CoA reductase pathway, which results in decreased levels of mevalonate and downstream products, including lipid isoprenoid intermediates, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) [12]. These isoprenoid intermediates provide lipid attachments for intracellular G-proteins, such as Ras and Rho, which must undergo post-translational prenylation by FFP or GGPP to enable their translocation from cytoplasm to cell membrane [12]. Statin inhibition of mevalonate synthesis impedes cellular pathways, such as protein synthesis and cell-cycle progression, which are critical for cancer cell growth and survival [12].
Beyond this basic mechanism, statins appear to exert pleiotropic effects, including anti-inflammatory, proapoptotic, growth inhibitory, and prodifferentiation properties on malignant cells of diverse origins, including the breast [13]. Despite their plethora of cellular actions, there is no obvious single mechanism by which statins may prevent breast cancer development. There have been multiple studies evaluating the in vitro and in vivo effects of statins, both lipophilic and hydrophilic statin subtypes, including several focused specifically on breast cancer–associated pathways. We review these preclinical studies according to the potential anticancer mechanism investigated.
Proliferation and apoptosis
Statins mediate growth inhibition through multiple pathways, in both HR-negative and HR-positive cell lines: they reduce cell proliferation by promoting G1 cell cycle arrest [14]. Campbell et al. [15•] reported that statins alter regulation of cell survival and proliferation by suppressing the mitogen-activated protein kinase pathway, activating nuclear factor κ B, and increasing synthesis of p21, a cyclin-dependent kinase inhibitor. Growth inhibition was most marked in HR-negative breast cancer cells exposed to lipophilic statins, in vitro and in vivo. By contrast, pravastatin, a hydrophilic statin, did not inhibit growth of any breast cancer cell line, regardless of HR status [15•]. Mueck et al. [16] studied five different statins in HR-positive and HR-negative cell lines. Statins inhibited the growth of HR-negative more potently than HR-positive cell lines, and pravastatin again showed no effect. Independent of the HMG-CoA pathway and HR status, statins can also reduce cell proliferation via proteosome inhibition [17], and induce apoptosis through a nitric oxide and arginine-dependent pathway [18] and via the c-Jun NH2-terminal kinase pathway [19].
Angiogenesis
Statins have been shown to promote and inhibit angiogenesis. Their blockade of the mevalonate pathway appears to inhibit tumor-induced angiogenesis [20,21]; cerivastatin decreases endothelial cell locomotion, an effect that can be reversed by addition of GGPP [20]. Statins also affect other antiangiogenesis pathways. Lovastatin suppresses vascular endothelial growth factor production in transformed breast cancer cell lines, and its effect is potentiated by combination with tumor necrosis factor α [21]. In contrast, one study found that simvastatin may promote angiogenesis by activating protein kinase Akt, and increasing nitric oxide release [22].
Tumor metastasis
Cellular mechanisms such as adhesion, migration, and proteolysis are involved in tumor organization and metastasis. Lovastatin inhibits metastasis in murine mammary tumor models, and blocks tumor cell attachment and cell migration in a highly invasive cell line [23]. Cerivastatin inhibits cell invasiveness by reducing synthesis of phenylated RhoA, a G-protein involved in regulation of the actin cytoskeleton [24]. Statins inhibit migration of breast cancer cells by blocking synthesis of oxysterol, a lipid mediator of migration derived from the mevalonate pathway [25]. An alternate mechanism involves inhibition of matrix metalloproteinases (MMPs), which enable tumor growth, invasion, and metastasis by degrading the extracellular matrix. Cerivastatin blocks RhoA cell-signaling pathways, reducing expression of urokinase and MMPs [13,24].
Recent studies suggest a novel mechanism for statins’ inhibition of MMP expression, dependent on caveolin, a cholesterol-binding protein. These proteins reside in caveolae, cholesterol-rich infoldings of the plasma membrane that are involved in signal transduction and vesicular trafficking and appear to act as tumor suppressors [13]. In breast cancer tissue and in vivo studies, caveolin-1 expression inhibits breast cancer growth and metastasis. When the caveolin-1 gene is disrupted, tumor formation and metastasis result [26,27]. The expression of caveolin-1 and caveolin-2 is associated with the HR-negative, basal-like breast cancer phenotype [28]. Caveolin gene and protein expression appears to differ by statin lipophilicity. In human smooth muscle cells, lipophilic statins stimulate caveolin-1 expression, but hydrophilic statins have no effect [29]. This newly reported caveolin-related mechanism is a high priority for further study because it may in part explain the differences according to statin and tumor subtype observed in observational studies.
Observational Studies of Statins and Breast Cancer in Humans
A potential association between statin treatment and breast cancer risk was initially evaluated in secondary safety analyses of randomized trials of statins for hyperlipidemia [30-36]. Sacks et al. [36] found that women randomly assigned to receive a statin had a higher incidence of breast cancer, with one patient affected in the placebo group and 12 patients affected in the pravastatin group. However, analyses of other randomized trials, including several that used pravastatin, did not confirm an increase in breast cancer risk [30-35]. These secondary analyses of hyperlipidemia trials subsequently prompted dedicated epidemiologic studies of the association between statins and breast cancer incidence.
Observational studies of statins and breast cancer in humans, including case-control, cohort studies, and meta-analyses, are summarized in Table 1. Study heterogeneity renders comparison difficult. In particular, studies differ in their definitions of statin users and controls (some studies compare ever versus never users and other studies compare current versus former); measures of statin use (with recall bias a major potential confounder among case-control studies dependent upon subject reporting of exposures); and classification of statin subtypes (with some studies defining atorvastatin as hydrophilic, but others as lipophilic). Multiple meta-analyses have attempted to clarify the conflicting results of individual reports, yet are limited by the variables collected in the contributing studies (for example, many studies did not collect information on HR status, which reduces the power of meta-analyses to address associations by tumor subtype). Despite these limitations, observational studies remain informative for investigating an association between statin use and breast cancer risk because no randomized clinical trial has yet evaluated this question. Below, we discuss results of observational studies according to overall findings, consideration of HR status, and consideration of statin lipophilicity.
Table 1.
Epidemiologic studies of statins and breast cancer incidence
| Study | Design | Patients, n | Breast cancer odds or hazard ratio (95% CI) |
Hormone receptor subset analysis odds or hazard ratio (95% CI) |
Statin subset analysis odds or hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Beck et al. [39] | Case control | 67,472 | 1.09 (0.93–1.28) | None | None |
| Blais et al. [55] | Case control | 6721 | 0.67 (0.33–1.38) | None | None |
| Graaf et al. [37] | Case control | 20,105 | 1.07 (0.65–1.74) | None | None |
| Kaye and Jick [40] | Case control | 18,088 | 0.9 (0.6–1.3) | None | None |
| Kochhar et al. [41] | Case control | 40,421 | 0.49 (0.38–0.62) | None | None |
| Boudreau et al. [9] | Case control | 1982 | 0.9 (0.7–1.2) | None | Lipophilic 0.8–1.2; hydrophilic 1.0 (0.5–1.7) |
| Pocobelli et al. [38] | Case control | 8620 | 1.0 (0.8–1.2) | None | Lipophilic 1.0 (0.8–1.2); (fl uvastatin 0.5 [0.3–0.8]); hydrophilic 1.2 (0.9–1.8) |
| Kumar et al. [50••] | Case only | 2141 | None | Negative 0.63 (0.43–0.92) | None |
| Cauley et al. [45] | Cohort | 7528 | 0.30 (0.10–0.94) | None | None |
| Eliassen et al. [43] | Cohort | 75,828 | 0.91 (0.76–1.08) | Positive 0.98 (0.79–1.22); negative 0.96 (0.60–1.51) |
None |
| Boudreau et al. [44] | Cohort | 92,788 | 1.07 (0.88–1.29) | Positive 1.06 (0.85–1.32); negative 1.28 (0.78–2.08) |
Lipophilic 1.0 (0.8–1.26); hydrophilic 1.01 (0.48–2.13) |
| Cauley et al. [42••] | Cohort | 156,351 | 0.91 (0.80–1.05) | Positive 0.97 (0.83–1.13); negative 0.83 (0.55–1.25) |
Lipophilic 0.82 (0.70–0.97) |
| Meta-analyses of statins and breast cancer incidence | |||
|---|---|---|---|
| Study | Studies included | Breast cancer odds or risk ratio (95% CI) | Statin subset analysis odds or hazard ratio (95% CI) |
| Bonovas et al. [46] | 7 randomized trials; 9 observational studies |
1.03 (0.93–1.14) | None |
| Dale et al. [49] | 5 randomized trials | 1.33 (0.79–2.26) | Lipophilic 1.01 (0.82–1.25); hydrophilic 1.00 (0.90–1.11) |
| Browning and Martin [48] |
7 randomized trials; 9 observational studies |
Randomized trials: 1.01 (0.79–1.30); observational studies: 0.96 (0.90–1.04) | Lipophilic 0.89 (0.62–1.27); hydrophilic 1.15 (0.81–1.64) |
| Kuoppala et al. [47•] | 17 total studies | 1.04 (0.74–19) | Lipophilic 0.74–1.4; hydrophilic 3.3 (1.7–6.3) |
Overall breast cancer incidence
The majority of observational studies, and all meta-analyses, found no significant association between statin use and overall risk of breast cancer (Table 1). These include case-control studies in the Netherlands’ Pharmo Database [37], the Three States Cancer Registries [38], the Cancer Surveillance System [9], the Saskatchewan Health Group [39], and the United Kingdom General Practice Research Database [40]. By contrast, a case-control study in the Veterans Affairs Health System, presented in abstract form, reported a significant reduction in breast cancer risk among statin users (OR, 0.49) [41]. Cohort studies in the Women’s Health Initiative [42••], the Nurses’ Health Study [43], and the Group Health System in Washington State [44] found no association between overall breast cancer incidence and statin use, whereas the Study of Osteoporotic Fractures Group found a significant reduction in breast cancer risk (multivariate-adjusted OR, 0.30), but the age-adjusted OR was not significant [45]. Meta-analyses have encompassed both randomized clinical trials and observational studies, including most of the individual studies in Table 1. Most meta-analyses evaluated overall cancer incidence in statin users and included subgroup analyses of breast cancer, with the exception of Bonovas et al. [46], which evaluated breast cancer risk as a primary outcome. To date, no meta-analysis has reported a significant association between statin use and overall breast cancer incidence [46,47•,48,49]. This absence of an overall effect has prompted investigators to evaluate potential associations by tumor HR subtype and statin lipophilicity.
Hormone receptor status
The majority of observational studies that investigated breast cancer incidence by HR status found no association (Table 1). These included cohort studies in the Women’s Health Initiative [42••], the Nurses’ Health Study [43], and the Group Health System in Washington State [44]. In contrast, a case-only study in the Kaiser Permanente Northern California cohort reported a statistically significant reduction in incidence of HR-negative breast cancer in women who used statins for more than 1 year (OR, 0.63). Notably, users of lipophilic statins only were included because only lipophilic statins were approved by the formulary of the Kaiser Permanente Health Maintenance Organization [50••]. It has been suggested that the relatively small number of HR-negative breast cancer cases in many studies may have limited the statistical power to observe any association with statin use. To date, no meta-analysis has evaluated breast cancer incidence by tumor HR status.
Statin lipophilicity
Although most studies that evaluated statins by drug subtype found no difference in their association with breast cancer incidence (Table 1), a case-control study in the Three States Cancer Registries reported that the lipophilic fluvastatin was associated with a statistically significant reduction in the risk of breast cancer (OR, 0.5 in current users) [38]. Similarly, the Women’s Health Initiative cohort study reported a statistically significant reduction in breast cancer risk among lipophilic statins users (HR, 0.82) [42••]. However, several meta-analyses, some of which included the Women’s Health Initiative cohort study [47•,48,49], have evaluated breast cancer risk by statin subtype and reported no significant association with breast cancer incidence [47•,48,49].
Clinical Trials of Statins for Breast Cancer Chemoprevention
Most epidemiologic studies have not found a significant protective association between statins and breast cancer; however, observational studies are subject to bias by unmeasured confounders and cannot definitively test the question of whether statins reduce breast cancer incidence. Preclinical studies that suggest mechanisms for statins in breast cancer prevention, and the suggestion from observational studies that statins might selectively prevent HR-negative disease, have guided ongoing clinical trials.
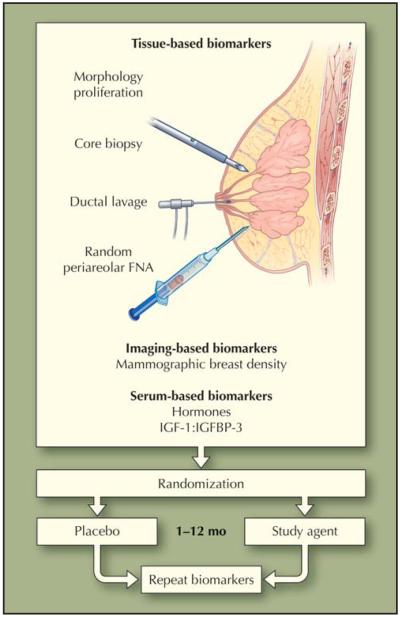
Clinical trials designed to prove that an agent prevents breast cancer require thousands of participants and multiple years of follow-up. The National Surgical Adjuvant Breast and Powel Project’s large, randomized phase 3 trials evaluating tamoxifen (P1) and raloxifene (P2) enrolled 13,388 and 19,747 women, respectively [2,3]. In 2007, the US National Cancer Institute declined to fund the STELLAR (Study to Evaluate Letrozole and Raloxifene) trial, a similarly designed study that aimed to compare raloxifene to the aromatase inhibitor letrozole for breast cancer prevention in postmenopausal women. The substantial cost of large, phase 3 chemoprevention trials has proved a deterrent, prompting investigators to initiate early-stage, biomarker-based trials that aim to evaluate an agent’s potential in a limited number of patients over a short time before more substantial resources are committed. Clinical settings for early-stage trials of statins in breast cancer chemoprevention include the presurgical window in patients who have undergone a biopsy diagnosing breast cancer and are awaiting definitive surgery; primary prevention in patients at increased risk of developing breast cancer; and secondary prevention in breast cancer survivors (Table 2). These trials evaluate breast cancer–associated biomarkers either directly in breast tissue, or indirectly in serum or by breast imaging. Fabian et al. [51] developed a method for repeated sampling of bilateral breast tissue, the random periareolar fine-needle aspiration (rpFNA) technique, and demonstrated that women with atypical cytology on rpFNA have a two- to fivefold increased risk of subsequently developing breast cancer, compared with women without cytologic atypia. Consequently, Fabian et al. [51] and other investigators have defined models of early-stage chemoprevention trials that measure changes in risk-associated biomarkers, such as cellular proliferation, atypical cytology on rpFNA, or mammographic density, before and after short-term use of a candidate agent (Fig. 1) [52]. All current trials use a lipophilic statin. Although no open trial designates reduction in HR-negative breast cancer as a primary end point, several enroll participants at high risk specifically of HR-negative disease, or evaluate outcomes associated with tumor HR status.
Table 2.
Ongoing clinical trials of statins for breast cancer chemoprevention*
| Population | Setting | Design | Primary end point | Secondary end points | Statin; daily dose; duration |
Participating centers |
|---|---|---|---|---|---|---|
| Primary breast cancer: stage 0–1 | Presurgical | Randomized | Ki-67 | C-reactive protein; cleaved caspase 3; CD68; CD25 |
Fluvastatin; 20–80 mg; 3–6 wk |
University of California, San Francisco; University of Chicago Memorial Sloan- Kettering; Dana-Farber Cancer Institute [53] |
| Primary breast cancer: postmeno- ȃpausal; stage I |
Presurgical | Single-arm | Ki-67 | Apoptosis | Atorvastatin; 80 mg; 2 wk |
Lund University, Sweden |
| Primary breast cancer: stage I–III | Presurgical | Single-arm | Proliferation; apoptosis |
Gene expression* | Simvastatin; 20 mg; 2–3 wk |
National University, Singapore |
| High-risk: 5 y > 1.67% (Gail model); atypical hyperplasia; carcinoma in situ |
Primary | Randomized | Ki-67 | Breast cytology; apoptosis; proliferation |
Atorvastatin; 3 mo | M. D. Anderson Cancer Center |
| High risk: BRCA1 or BRCA2 mutation; strong family history; prior breast cancer (hormone- receptor negative) |
Primary or secondary | Single-arm | Breast cytology* | Ki-67; breast density; cancer incidence; DNA damage |
Lovastatin; 80 mg; 6 mo |
Stanford University Cancer Center [54] |
| Primary breast cancer: stage 0–111 | Secondary | Single-arm | C-reactive protein; lipid profile; breast density |
DNA methylation; PI3 kinase |
Simvastatin; 6-7 mo | Sidney Kimmel Cancer Center; Dana-Farber Cancer Institute |
Trials listed at http://clinicaltrials.gov; accessed May 25, 2009. All trials are currently recruiting except for the trial at National University Hospital, Singapore.
Secondary end points include evaluation of gene expression and other simvastatin effects on CD44-positive/CD24-negative basal-like breast cancer cells.
Breast cells are collected by two-quadrant random perioareolar fine-needle aspiration [51].
Figure 1.
Design of early-stage biomarker-based chemoprevention trials in women at high risk for breast cancer. FNA—fine-needle aspiration; IGF—insulin-like growth factor; IGFBP—insulin-like growth factor binding proteins. (From Fabian et al. [52]; with permission.)
Presurgical window trials
The presurgical model involves women with ductal carcinoma in situ or early-stage breast cancer. Participants receive the study agent from the time of cancer diagnosis by needle biopsy until surgery removes the tumor 3 to 6 weeks later. Three ongoing statin studies use this design (Table 2), and two evaluate change in Ki-67, a proliferation marker associated with tumor grade, as the primary end point. Concerns raised about this study design include slow accrual due to patients’ potential reluctance to wait 3 to 6 weeks for their definitive cancer surgery, the short drug-exposure period, and possible heterogeneity of Ki-67 across different parts of the tumor. To date, preliminary results of one such study have been reported by Garwood et al. [53]. Of 40 participants enrolled, 29 had pre- and post-treatment samples adequate for evaluation. Participants with grade 3, mostly HR-negative tumors had a statistically significant decline in Ki-67, but no significant difference was noted in a comparison between HR-positive and HR-negative tumors [53]. A trial led by the National University Hospital in Singapore aims to evaluate the effect of simvastatin on genomic changes associated with the basal, HR-negative subtype of breast cancer, but no results have yet been presented [1].
Primary and secondary prevention trials
Phase 2 biomarker-based chemoprevention trials enroll participants at high risk of developing a first or second breast cancer, and treat them with a study drug over a period of months. As in the presurgical window setting, phase 2 prevention trials use a biomarker-based primary end point, and may have a single intervention arm or a randomized, placebo-controlled design (Fig. 1). These novel designs represent appealing strategies for first evaluations of a drug’s potential as a clinical chemopreventive agent; however, caveats include uncertainty as to the reproducibility, inter- and intrasubject variability, and clinical significance of surrogate end points, such as atypical cytology on rpFNA. Concerns remain about participant accrual because cancer-free women are asked to undergo repeated needle biopsies of the breast and they may be less willing to tolerate intervention than are patients with cancer. There are three ongoing phase 2 biomarker-based trials of statins for breast cancer chemoprevention. Entry criteria vary, but generally include women at high risk due to a known cancer-susceptibility gene mutation, strong family history of breast cancer, or a previous HR-negative breast cancer. Study durations range over a period of months and primary end points include change in Ki-67 or the prevalence of atypical cytology on rpFNA (Table 2). In 2007, Kurian et al. [54] presented initial feasibility results of an ongoing single-arm study of lovastatin in high-risk women. On continued accrual, most participants have had no change or an improvement in the primary end point of cytology, and have tolerated the rpFNA procedure and study drug well.
Conclusions
Several drug classes are under evaluation for chemoprevention of HR-negative breast cancer, given the clear need to prevent this poor-prognosis disease. Statins are attractive for breast cancer chemoprevention given their tolerability and safety, but their efficacy remains to be proven.
Statins inhibit multiple cancer-associated pathways in preclinical studies. Lipophilic statins appear particularly active, especially in HR-negative breast cancer cells. In contrast, only one of several epidemiologic studies reported a statistically significant association between lipophilic statin use and breast cancer risk reduction [42••], and only one found a significant decrease in HR-negative breast cancer among statin users [50••]. Meta-analyses have found no association between statins and breast cancer, but none so far has investigated breast cancer incidence by HR status [46,47•,48,49]. Preliminary results of two ongoing early-stage chemoprevention studies have been reported to date and suggest favorable effects on breast cancer risk–associated biomarkers [53,54]. If final results from the ongoing statin intervention studies prove promising, they may justify a randomized clinical trial evaluating breast cancer prevention as the primary outcome, with stratification by tumor HR status.
Whether or not the pending results from current statin studies eventually prompt a phase 3 randomized trial, the experience to date of statins as chemoprevention candidates, from their selection based on suggestive epidemiologic and preclinical studies, to the design of novel biomarker-based trials for their initial evaluation, has much to teach investigators committed to developing drugs that are effective and practical for cancer prevention.
Footnotes
Disclosure
Dr. Kurian has received research funding from BiPar Sciences to conduct a clinical trial unrelated to this article.
No further potential conflicts of interest were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Li Y, Brown PH. Translational approaches for the prevention of estrogen receptor-negative breast cancer. Eur J Cancer Prev. 2007;16:203–215. doi: 10.1097/CEJ.0b013e328011ed98. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 4.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21:28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 5.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–607. [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 7.Telli ML, Kurian AW, Chang ET, et al. Differences in breast cancer subtype distribution exist among ethnic subgroups of Asian women in California [abstract 2088]; Presented at the 31st annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 10-14, 2008. [Google Scholar]
- 8.Castrellon AB, Gluck S. Chemoprevention of breast cancer. Expert Rev Anticancer Ther. 2008;8:443–452. doi: 10.1586/14737140.8.3.443. [DOI] [PubMed] [Google Scholar]
- 9.Boudreau DM, Gardner JS, Malone KE, et al. The association between 3-hydroxy-3-methylglutaryl coenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer. 2004;100:2308–2316. doi: 10.1002/cncr.20271. [DOI] [PubMed] [Google Scholar]
- 10.Buchwald H. Cholesterol inhibition, cancer, and chemotherapy. Lancet. 1992;339:1154–1156. doi: 10.1016/0140-6736(92)90744-n. [DOI] [PubMed] [Google Scholar]
- 11.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 13.Mannello F, Tonti GA. Statins and breast cancer: may matrix metalloproteinase be the missing link. Cancer Invest. 2009;27:466–470. doi: 10.1080/07357900802491444. [DOI] [PubMed] [Google Scholar]
- 14.Rao S, Lowe M, Herliczek TW, Keyomarsi K. Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene. 1998;17:2393–2402. doi: 10.1038/sj.onc.1202322. [DOI] [PubMed] [Google Scholar]
- 15.•.Campbell MJ, Esserman LJ, Zhou Y, et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–8714. doi: 10.1158/0008-5472.CAN-05-4061. This preclinical study showed more potent growth inhibition with statins in HR-negative cell lines compared with HR-positive cell lines.
- 16.Mueck AO, Seeger H, Wallwiener D. Effect of statins combined with estradiol on the proliferation of human receptor-positive and receptor-negative breast cancer cells. Menopause. 2003;10:332–336. doi: 10.1097/01.GME.0000055485.06076.00. [DOI] [PubMed] [Google Scholar]
- 17.Rao S, Porter DC, Chen X, et al. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci U S A. 1999;96:7797–7802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotamraju S, Williams CL, Kalyanaraman B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67:7386–7394. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- 19.Koyuturk M, Ersoz M, Altiok N. Simvastatin induces apoptosis in human breast cancer cells: p53 and estrogen receptor independent pathway requiring signalling through JNK. Cancer Lett. 2007;250:220–228. doi: 10.1016/j.canlet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Vincent L, Chen W, Hong L, et al. Inhibition of endothelial cell migration by cerivastatin, an HMG-CoA reductase inhibitor: contribution to its anti-angiogenic effect. FEBS Lett. 2001;495:159–166. doi: 10.1016/s0014-5793(01)02337-7. [DOI] [PubMed] [Google Scholar]
- 21.Feleszko W, Balkowiec EZ, Sieberth E, et al. Lovastatin and tumor necrosis factor-alpha exhibit potentiated antitumor effects against Ha-ras-transformed murine tumor via inhibition of tumor-induced angiogenesis. Int J Cancer. 1999;81:560–567. doi: 10.1002/(sici)1097-0215(19990517)81:4<560::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alonso DF, Farina HG, Skilton G, et al. Reduction of mouse mammary tumor formation and metastasis by lovastatin, an inhibitor of the mevalonate pathway of cholesterol synthesis. Breast Cancer Res Treat. 1998;50:83–93. doi: 10.1023/a:1006058409974. [DOI] [PubMed] [Google Scholar]
- 24.Denoyelle C, Vasse M, Korner M, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22:1139–1148. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 25.Silva J, Beckedorf A, Bieberich E. Osteoblast-derived oxysterol is a migration-inducing factor for human breast cancer cells. J Biol Chem. 2003;278:25376–25385. doi: 10.1074/jbc.M301233200. [DOI] [PubMed] [Google Scholar]
- 26.Sloan EK, Stanley KL, Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23:7893–7897. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- 27.Williams TM, Medina F, Badano I, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 28.Elsheikh SE, Green AR, Rakha EA, et al. Caveolin 1 and Caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br J Cancer. 2008;99:327–334. doi: 10.1038/sj.bjc.6604463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plenz GA, Hofnagel O, Robenek H. Differential modulation of caveolin-1 expression in cells of the vasculature by statins. Circulation. 2004;109:e7–e8. doi: 10.1161/01.CIR.0000111128.83347.7A. [DOI] [PubMed] [Google Scholar]
- 30.Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 31.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 32.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 33.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/ TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 34.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 35.Strandberg TE, Pyorala K, Cook TJ, et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364:771–777. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 36.Sacks FM, Pfeffer MA, Moye LA, et al. Cholesterol and Recurrent Events Trial investigators The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 37.Graaf MR, Beiderbeck AB, Egberts AC, et al. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 38.Pocobelli G, Newcomb PA, Trentham-Dietz A, et al. Statin use and risk of breast cancer. Cancer. 2008;112:27–33. doi: 10.1002/cncr.23129. [DOI] [PubMed] [Google Scholar]
- 39.Beck P, Wysowski DK, Downey W, Butler-Jones D. Statin use and the risk of breast cancer. J Clin Epidemiol. 2003;56:280–285. doi: 10.1016/s0895-4356(02)00614-5. [DOI] [PubMed] [Google Scholar]
- 40.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635–637. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochhar R, Khurana V, Bejjanki H, et al. Statins reduce breast cancer risk: a case control study in U.S. female veterans [abstract 514]; Presented at the American Society of Clinical Oncology Annual Meeting; Orlando, FL. May 13-17, 2005. [Google Scholar]
- 42.••.Cauley JA, McTiernan A, Rodabough RJ, et al. Statin use and breast cancer: prospective results from the Women’s Health Initiative. J Natl Cancer Inst. 2006;98:700–707. doi: 10.1093/jnci/djj188. This cohort study in the Women’s Health Initiative showed statistical significant reduction in breast cancer risk among lipophilic statin users.
- 43.Eliassen AH, Colditz GA, Rosner B, et al. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch Intern Med. 2005;165:2264–2271. doi: 10.1001/archinte.165.19.2264. [DOI] [PubMed] [Google Scholar]
- 44.Boudreau DM, Yu O, Miglioretti DL, et al. Statin use and breast cancer risk in a large population-based setting. Cancer Epidemiol Biomarkers Prev. 2007;16:416–421. doi: 10.1158/1055-9965.EPI-06-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cauley JA, Zmuda JM, Lui LY, et al. Lipid-lowering drug use and breast cancer in older women: a prospective study. J Womens Health (Larchmt) 2003;12:749–756. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 46.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23:8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- 47.•.Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer. 2008;44:2122–2132. doi: 10.1016/j.ejca.2008.06.025. A large, comprehensive meta-analysis of observational and randomized trials, evaluating the association between statins and cancer.
- 48.Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120:833–843. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 49.Dale KM, Coleman CI, Henyan NN, et al. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 50.••.Kumar AS, Benz CC, Shim V, et al. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarkers Prev. 2008;17:1028–1033. doi: 10.1158/1055-9965.EPI-07-0726. A case-only study in the Kaiser Permanente Northern California cohort that showed a statistically significant reduction in HR-negative breast cancer incidence among statins users.
- 51.Fabian CJ, Kimler BF, Zalles CM, et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J Natl Cancer Inst. 2000;92:1217–1227. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 52.Fabian CJ, Kimler BF, Mayo MS, Khan SA. Breast-tissue sampling for risk assessment and prevention. Endocr Relat Cancer. 2005;12:185–213. doi: 10.1677/erc.1.01000. [DOI] [PubMed] [Google Scholar]
- 53.Garwood ER, Kumar AS, Baehner F, et al. Fluvastatin has biologic effects on stage 0 and 1 breast cancer [abstract 4122]; Presented at the 31st annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 10-14, 2008. [Google Scholar]
- 54.Kurian AW, Sharma VB, Schwartz EJ, et al. A phase II breast cancer chemoprevention study of lovastatin in high-risk women: initial feasibility data [abstract 1502]; Presented at the American Society of Clinical Oncology Annual Meeting; Chicago, IL. June 1-5, 2007. [Google Scholar]
- 55.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]