Abstract
The DNA-binding protein PhoP controls virulence and Mg2+ homeostasis in the Gram-negative pathogen Salmonella enterica serovar Typhimurium. PhoP regulates expression of a large number of genes that differ both in their ancestry and in the biochemical functions and physiological roles of the encoded products. This suggests that PhoP-regulated genes are differentially expressed. To understand how a bacterial activator might generate varied gene expression behaviour, we investigated the cis-acting promoter features (i.e. the number of PhoP binding sites, as well as their orientation and location with respect to the sites bound by RNA polymerase and the sequences that constitute the PhoP binding sites) in 23 PhoP-activated promoters. Our results show that natural PhoP-activated promoters utilize only a limited number of combinations of cis-acting features – or promoter architectures. We determine that PhoP activates transcription by different mechanisms, and that ancestral and horizontally acquired PhoP-activated genes have distinct promoter architectures.
Introduction
Bacterial activators stimulate gene transcription by binding to specific DNA sequences on target promoters where they make contact with RNA polymerase (RNAP) and/or alter the local DNA structure (Barnard et al., 2004). Activators may bind to a single or to multiple sites at a given promoter, in both possible orientations and at various distances from the site bound by RNAP (Li et al., 1997; Roy et al., 2004). Activator binding to a promoter may require cofactors that increase the sensitivity of the regulation (Pedersen et al., 1991; Browning and Busby, 2004) and/or overcome the silencing effects of nucleoid-associated proteins that predominantly affect genes that were horizontally acquired (Dorman, 2007). Although we understand at atomic resolution levels the interactions that certain transcriptional activators establish with their target sequences (Blanco et al., 2002; Browning and Busby, 2004; Mayo et al., 2006), it is still unclear which arrangement(s) of cis-acting regulatory features, such as the number, orientation, location and sequence of binding sites, can be utilized by a given activator to promote gene transcription. The particular arrangement of these features, which we refer to as promoter architecture, is of special interest for activators that control multiple targets. This is because genes co-regulated by a particular activator protein are often expressed in distinct fashions, and this could be due to the corresponding promoters having different architectures. Here we analyse the promoter architectures of genes directly activated by PhoP, a transcriptional activator that governs virulence and Mg2+ homeostasis in several enteric species (Groisman, 2001).
The PhoP protein regulates expression of ∼ 5% of the genes in the Gram-negative pathogen Salmonella enterica serovar Typhimurium (Harari et al., 2010). It carries out this task both directly, by binding to the corresponding promoter regions (Kato et al., 2003; Lejona et al., 2003; Shi et al., 2004; Bijlsma and Groisman, 2005; Shin and Groisman, 2005; Aguirre et al., 2006; Tu et al., 2006; Perez et al., 2008), and indirectly, by altering the levels and/or activity of regulatory proteins (Kato and Groisman, 2004; 2008; Tu et al., 2006) and RNAs (Moon and Gottesman, 2009; Lee and Groisman, 2010). Analysis of the genes directly activated by the PhoP protein has revealed that the majority exhibit a limited phylogenetic distribution and appear to have been acquired by horizontal gene transfer (Perez et al., 2009). Moreover, the encoded products differ in their biochemical functions and physiological roles (Groisman, 2001) (Table 1). This raises the possibility that they are produced in distinct amounts when Salmonella experiences PhoP-inducing conditions.
Table 1.
Promoter data set corresponding to 23 PhoP-activated promoters
| PhoP box – RNAP distances | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene name | Function of encoded gene product | A | PhoP box sequence | # | O | A/R | Submotif | Score/submotif | −10 | +1 | Length of 5′ leader |
| yrbL | Putative cytoplasmic protein | I | TCGTTTAGGTTTTGTTTAA | 1 | D | A | S1 | 0.90 | 11 | 27 | 32 |
| phoP | Response regulator | I | TGGTTTATTAACTGTTTAT | 1 | D | A | S1 | 0.88 | 12 | 33 | 34 |
| mgtA | Mg2+ transporter | I | TGGTTTATCGTTGGTTTAA | 1 | D | A | S1 | 0.98 | 12 | 33 | 264 |
| slyB | Outer membrane lipoprotein | I | TCGTTTAAGATTGGTTAAT | 1 | D | A | S1 | 0.88 | 12 | 32 | 100 |
| pmrD | Connector of two-component systems | I | CTATTGCCGTTTTGTTTAT | 1 | D | A | S2 | 0.92 | 12 | 31 | 27 |
| orgB | Effector protein of the SPI-1 type III secretion system | I | TTATTGAGGAGGCATTGAA | 1 | D | A | S3 | 0.80 | 12 | 32 | 20 |
| yobG | Putative inner membrane protein | II | ACAGTTACTCCTGGTTTAA | 2 | D | A | S2 | 0.66 | 12 | 31 | 26 |
| GTTTTTAGGAATGATTCAC | R | A | S3 | 0.61 | 62 | ||||||
| virK | Antimicrobial peptide resistance | II | TCGTTGCCTTTACGTTTAA | 2 | D | A | S1 | 0.77 | 12 | 32 | 30 |
| protein | CCATTGATAAACTGTTTAA | D | R | S2 | 0.94 | 74 | |||||
| mig-14 | Putative transcriptional activator | II | ACATTTTTATTTGGTTAAG | 2 | D | A | S2 | 0.60 | 12 | 33 | 27 |
| ATGTTTAGCTTGTATTTAA | R | A | S3 | 0.98 | 57 | ||||||
| ybjX | Cationic peptide resistance protein | II | GTATTGACGATTGGTTAAT | 2 | D | A | S2 | 0.78 | 12 | 31 | 25 |
| TTGTTTAGATACGGTTTAC | R | A | S1 | 0.92 | 77 | ||||||
| pcgL | d-ala-d-ala dipeptidase | II | ATTTTAACCATCTGTTTAA | 2 | D | A | S2 | 0.79 | 12 | 30 | 47 |
| GAGTTTATATTTTGCTTAT | R | R | S3 | 0.58 | 90 | ||||||
| ssrB | Response regulator | II | ACATTAAAAGGCTGTTTAG | 2 | D | A | S2 | 0.80 | 12 | 32 | 150 |
| TGGTGTAGTTTTTGAAGAT | R | R | S2 | 0.55 | −102 | ||||||
| rstA | Response regulator | III | TCGTTTAGAAAAGATTTAT | 1 | D | A | S1 | 0.85 | 23 | 42 | 21 |
| ompX | Outer membrane protein | III | CGGTTGAGGGTTCGTTGAA | 1 | D | A | S1 | 0.86 | 21 | 42 | 237 |
| pagP | Outer membrane lipid A acylase | IV | CTGTTTATAGTTTGTTAAG | 2 | D | A | S1 | 0.82 | 23 | 43 | 16 |
| TTTGTGAAAGCTTATTAAG | D | R | S3 | 0.60 | 126 | ||||||
| pagD | Putative outer membrane protein | IV | TGGTTAACTCTTCGTTGAA | 2 | D | A | S2 | 0.58 | 22 | 42 | 39 |
| GTGTTTAGAGAGAATTTAC | D | R | S3 | 0.89 | 144 | ||||||
| iraP | Regulator of RpoS stability | IV | CCGTTACGATATGGTTTAA | 2 | D | A | S1 | 0.60 | 21 | 39 | 218 |
| TTGTTTTTTGATGGTTTAT | 2 | D | R | S1 | 0.75 | −53 | |||||
| ugtL | Inner membrane protein that modifies the LPS | V | CGGTTGAGCAACTATTTAC | 2 | R | A | S3 | 0.73 | 30 | 51 | 176 |
| AATAATACTTTTAGTTTAA | R | R | S3 | 0.56 | 2 | ||||||
| pagK | Protein delivered to eukaryotic cells via outer membrane vesicles | V | CCATTTATAAAATATTTAA | 2 | R | A | S3 | 0.70 | 37 | 55 | 57 |
| ACGTTTAATATCTATAGTA | D | R | S3 | 0.60 | −2 | ||||||
| pgtE | Outer membrane protease | V | ATTTTTACCTTATATTGAA | 2 | R | A | S3 | 0.60 | 49 | 68 | 45 |
| ATGATTATAGATTGCTTAT | D | R | S3 | 0.55 | 119 | ||||||
| mgtC | Inner membrane virulence protein that aids growth in low Mg2+ | V | CTGTTTAAGTTTGTTTGAT | 2 | R | A | S1 | 0.72 | 48 | 67 | 284 |
| ATGTTTAAACACGCTTTAT | D | R | S1 | 0.72 | −301 | ||||||
| ATGTTTCCTTATATTTTAA | D | R | S3 | 0.60 | −159 | ||||||
| pagC | Outer membrane protein | V | GTGTTTAGAGAGAATTTAC | 2 | R | A | S3 | 0.89 | 47 | 67 | 559 |
| TTATTTACGGTGTGTTTAA | R | R | S2 | 0.88 | −527 | ||||||
| pipD | Pathogenicity island-encoded protein D | V | TTATTGAGGTTGTATTGAT | 2 | R | A | S3 | 0.83 | 58 | 76 | 40 |
| CCGTTACGCTGCTGGGTAT | D | R | S2 | 0.55 | −55 | ||||||
The PhoP protein binds to its DNA targets in vivo only when phosphorylated (Shin and Groisman, 2005; Shin et al., 2006). This process is controlled by the PhoQ protein (Groisman and Mouslim, 2006), which activates the PhoP protein when the bacterium experiences low extracytoplasmic Mg2+ (Garcia Vescovi et al., 1996), antimicrobial peptides (Bader et al., 2005) or acid pH (Prost et al., 2007). Phosphorylated PhoP (PhoP-P) binds to a hexanucleotide direct repeat separated by five nucleotides, designated the PhoP box, in its target promoters (Kato et al., 1999). This suggests that a PhoP-P dimer (Bachhawat and Stock, 2007) binds a PhoP box in a head to tail configuration. Among the relatively small number of PhoP-activated promoters experimentally defined to date, the PhoP box can be found at various distances and in both possible orientations with respect to the −10 hexamer sequence recognized by RNAP (Shi et al., 2004; Shin and Groisman, 2005; Zwir et al., 2005; Perez et al., 2008; Harari et al., 2010), indicative of PhoP utilizing different mechanisms to promote gene transcription (Barnard et al., 2004; Browning and Busby, 2004). Indeed, the C-terminal domain of the α subunit of RNAP is required for transcription of a subset of PhoP-activated promoters (Perez and Groisman, 2009). And the PhoP-activated DNA-binding protein SlyA is necessary to overcome the silencing effects of the histone-like nucleoid-structuring protein (H-NS) at other PhoP-dependent promoters (Kong et al., 2008; Perez et al., 2008; Song et al., 2008; Zhao et al., 2008).
Here we report the spectrum and organization of the cis-acting features present in PhoP-activated promoters (i.e. the promoter architectures), and the different roles that the PhoP protein plays at PhoP-activated promoters. Our analysis reveals that PhoP utilizes five distinct promoter architectures to drive transcription of its activated genes in Salmonella. These architectures are composed of specific (as opposed to arbitrary) combinations of cis-acting regulatory elements. Finally, we establish that Salmonella employs different promoter architectures to control ancestral and horizontally acquired PhoP-activated genes.
Results
Mapping the transcription start sites for PhoP-activated genes
We previously identified a large number of genes directly activated by the PhoP protein in Salmonella using expression microarray analysis of wild-type versus phoP mutant strains and chromatin immunoprecipitation (ChIP) experiments (Harari et al., 2010). However, except for a limited number of cases (Kato et al., 2003; Lejona et al., 2003; Shi et al., 2004; Bijlsma and Groisman, 2005; Zwir et al., 2005; Aguirre et al., 2006; Tu et al., 2006; Perez et al., 2008), the particular sequences recognized by the PhoP protein at the corresponding promoters, as well as the location of these sequences relative to the transcription start sites, have remained unknown. Therefore, to define the scope of PhoP-activated promoters, we first determined the transcription start sites for 15 genes known to be directly activated by the PhoP protein (Harari et al., 2010) by carrying out S1 mapping experiments with RNA harvested from isogenic wild-type and phoP Salmonella strains grown under PhoP-inducing (i.e. 10 µM Mg2+) and -repressing (i.e. 10 mM Mg2+) conditions. Then, we analysed their transcription start sites together with those corresponding to other previously mapped PhoP-dependent promoters.
A single start site was detected for most PhoP-activated genes including yrbL, ompX, yobG, pcgL, pagP, pagD, virK, mig-14, pagK, pgtE, mgtC (Fig. S1A–K), pmrD (Kato et al., 2003) and orgB (Aguirre et al., 2006). The corresponding promoters were active in the wild-type strain following growth in low Mg2+ but not in the phoP mutant regardless of the growth condition. A few PhoP-activated genes, however, have additional start sites that are PhoP-independent because they were observed in the phoP mutant strain whether grown in high or low Mg2+ as well as in the wild-type strain grown under non-inducing (i.e. high Mg2+) conditions (Fig. S1L and M). This is the case of the ybjX (Fig. S1L) and iraP (Tu et al., 2006) genes, as well as the slyB and phoP genes, which exhibit two transcription start sites both in Salmonella (Garcia Vescovi et al., 1996) (Fig. S1M) and in Escherichia coli (Minagawa et al., 2003). The slyB and phoP genes differ from the ybjX (Fig. S1L) and iraP (Tu et al., 2006) genes in that the S1 product that is PhoP-independent is present at higher levels after growth in high Mg2+ than in low Mg2+ in both wild-type and phoP mutant strains (Garcia Vescovi et al., 1996) (Fig. S1M). A single PhoP-dependent transcription start site was originally reported for the mgtA gene (Lejona et al., 2003), but it was later found that overexpression of the rob gene promoted mgtA transcription from a different site even in a strain deleted for the phoP and phoQ genes (Barchiesi et al., 2008).
A subset of PhoP-activated mRNAs has unusually long 5′ leader regions
In bacteria, the median length of the 5′ leader region is 30 nt (Lynch et al., 2005; Pinto et al., 2011). Interestingly, it is > 50 bp for nine of the 23 analysed PhoP-dependent transcripts (i.e. mgtA, slyB, ssrB, ompX, iraP, pagK, ugtL, mgtC and pagC) (Table 1; Fig. S2A). This raises the possibility of these genes being subjected to additional regulatory inputs via their 5′ leader regions, which could affect mRNA stability, transcription elongation into the region and/or translation of the transcript. Indeed, the mgtA mRNA includes a 5′ leader that controls elongation into the coding region in response to the cytoplasmic Mg2+ (Cromie et al., 2006) and proline (Park et al., 2011) levels. Likewise, the 5′ leader region of the mgtCBR operon contributes to the Mg2+-dependent regulation of the corresponding coding regions (Spinelli et al., 2008). Finally, the levels of the PagC protein differ significantly between wild-type and hfq mutant Salmonella (Sittka et al., 2008), and this could be due to an sRNA acting on the 559-nt-long leader region of the pagC transcript.
Correlation between PhoP box submotif and PhoP box orientation
We carried out DNase footprinting analysis of the promoter regions corresponding to 15 PhoP-activated genes utilizing phosphorylated PhoP-His6 protein (i.e. PhoP-P). The PhoP-P protein protected sequences of different lengths within each promoter (e.g. 20 nt in the rstA promoter and > 30 nt in the virK and mig-14 promoters), most of which cover both repeats that comprise the PhoP box [i.e. sequences resembling the hexanucleotide direct repeat (T/G)GTTTA separated by five nucleotides (Kato et al., 1999)]. A single region was protected in four promoters (Fig. S3A–D), whereas two sites were protected in the remaining 11 promoters (Fig. S3E–O). In the latter case, PhoP-P exhibited differential affinity for the two sites (Fig. S3E–O). The occurrence of two PhoP boxes had been previously established for the ugtL (Shi et al., 2004) and iraP (Tu et al., 2006) promoters. Here, we generalize our previous findings and bioinformatics analysis (Harari et al., 2010) to 23 promoters including those driving transcription of the pagC, mgtC, pagK, pagP, pgtE, pipD, virK, ybjX, pcgL, yobG and pagD genes where the second PhoP box had not been detected in previous experimental studies (Lejona et al., 2003; Zhao et al., 2008).
The sequences corresponding to the PhoP boxes reflect, on average, the consensus motif (T/G)GTTTA 5 nt (T/G)GTTTA. Based on our DNase footprinting analysis, we redefined distinct subclasses of this consensus – termed PhoP box submotifs – originally established in PhoP-activated promoters (Zwir et al., 2005; Harari et al., 2010) (Fig. 1). For example, the single PhoP box sequence in the phoP, mgtA, yrbL, ompX and slyB promoters resembles the canonical direct repeat (Table 1), which can be further decomposed into three subclasses when considering sequences from other promoters (i.e. (C/T/G)GTTTA, and/or conserved peripheral nucleotides) (Fig. 1A). In the case of the ybjX, pcgL, yobG, virK, mig-14 and ssrB promoters (Table 1), one of the two PhoP boxes is located in the direct orientation (i.e. same orientation as the single PhoP box in the mgtA promoter) and displays a different consensus sequence in the first repeat (i.e. (T/C)ATTTA) (Fig. 1B). Conversely, one of the PhoP boxes in the yobG, mig-14, ugtL, pagK, pgtE, pagC and pipD promoters is located in the opposite relative orientation as the single PhoP box in the mgtA promoter (Table 1) and exhibits a slightly different consensus sequence in the second repeat (i.e. TATTTA) (Fig. 1C). Overall, the PhoP box submotifs correlate well (F statistics, P-value < 0.005, see Experimental procedures) with the orientation of the PhoP box.
Fig. 1.

Subclasses of the PhoP box consensus sequence (submotifs). These subclasses were identified based on footprinting data (Table 1; Fig. S3) as described in Harari et al. (2010) and Zwir et al. (2005), where one sequence can belong to more than one cluster, and a subset of sequences can be hierarchically organized based on their specificity and sensitivity. A. Submotifs corresponding to the canonical direct repeat consensus sequence (S1). Three different subpatterns were identified within this class. B. Submotif S2 corresponding to a conserved variant in the first repeat sequence. C. Submotif S3 corresponding to a conserved variant in the second repeat sequence.
The architectural diversity of PhoP-activated promoters
We analysed 23 PhoP-activated promoters whose transcription start sites (Fig. S1) (Soncini et al., 1995; Garcia Vescovi et al., 1996; Feng et al., 2003; Kato et al., 2003; Lejona et al., 2003; Bijlsma and Groisman, 2005; Aguirre et al., 2006; Tu et al., 2006) and PhoP boxes (Fig. S3) (Kato et al., 2003; Lejona et al., 2003; Shi et al., 2004; Bijlsma and Groisman, 2005; Aguirre et al., 2006; Tu et al., 2006; Perez et al., 2008) had been experimentally determined (Table 1; Fig. 2). This analysis entailed inspecting the promoter sequences for the following features: (i) the number of PhoP boxes, (ii) the location of the PhoP box(es) defined as the distance between the PhoP box and the −10 hexamer sequence predicted to be recognized by RNAP, (iii) the orientation of each PhoP box relative to the predicted −10 hexamer sequence, where direct orientation refers to the half PhoP box sequence 5′ (T/G)GTTTA 3′ pointing towards the −10 hexamer sequence, and reverse orientation where the half PhoP box sequence is pointing away from the predicted −10 hexamer sequence, (iv) the PhoP box rotational phasing along the DNA with respect to the predicted −10 hexamer, and (v) the predicted sequence elements characteristic of promoters recognized by the σ70 form of RNAP, including the −10 hexamer, the −35 hexamer and the spacer length between the two hexamers (Barnard et al., 2004; Shultzaberger et al., 2007) (Fig. 2). Our analysis revealed the presence of five distinct architectures in PhoP-activated promoters (Table 1; Fig. 2).
Fig. 2.

Architecture and DNA sequences of PhoP-activated promoter regions. DNA sequences corresponding to the 23 promoters analysed in this study. To facilitate the comparison of the 23 promoters, we aligned the corresponding sequences with respect to the predicted −10 hexamer using the 5′ most edge of this element as a referential landmark instead of the typically used transcription start site (Gaston et al., 1990; Liu et al., 2004). The transcription start site identified in Fig. S1 is indicated in green. (Transcription start sites for the pipD and rstA promoters are also included, data not shown.) This includes S1 nuclease protection data previously reported for phoP (Soncini et al., 1995; Garcia Vescovi et al., 1996), pmrD (Kato et al., 2003) and orgB (Aguirre et al., 2006) promoters as well as primer extension data reported for mgtA (Lejona et al., 2003), iraP (Tu et al., 2006) and ssrB (Feng et al., 2003; Bijlsma and Groisman, 2005) promoters. The PhoP box corresponding to regions protected by PhoP-P shown in Fig. S3 is indicated in blue. This also includes DNase I footprinting analysis previously reported for phoP (Lejona et al., 2003), ugtL (Shi et al., 2004), pagC (Perez et al., 2008), pmrD (Kato et al., 2003), ssrB (Bijlsma and Groisman, 2005), iraP (Tu et al., 2006) and orgB (Aguirre et al., 2006) promoters [as well as electrophoretic mobility shift assays (EMSA) analysis reported for the mgtA promoter (Lejona et al., 2003), and for the downstream PhoP box in the pagC promoter (this work, data not shown)]. The PhoP box orientation is indicated with blue arrows. The predicted −35 hexamer is underlined in red and the predicted −10 hexamer is indicated in red (Table 2). Activation PhoP boxes sharing common patterns including orientation, phasing and/or location with respect to the RNAP are indicated with grey boxes. The promoters were divided into five classes based on their shared cis-acting features. From top to bottom: Architecture I, Architecture II, Architecture III, Architecture IV and Architecture V.
Architecture I, present in the mgtA, phoP, yrbL, slyB, pmrD and orgB promoters, harbours a single PhoP box in the direct orientation located 12 nt upstream of the predicted −10 hexamer (except for the yrbL promoter where the distance is only 11 nt), i.e. one turn of the DNA helix away from this hexamer. Architecture II, present in the yobG, pcgL, virK, mig-14, ybjX and ssrB promoters, contains two PhoP boxes, one in the direct orientation with location and phasing similar to the single PhoP box in promoters with architecture I, and another PhoP box located upstream of this site (except for the ssrB promoter where the second PhoP box is located downstream of the first PhoP box). Architecture III, present in the rstA and ompX promoters, includes a single PhoP box in the direct orientation located 21–23 nt upstream of the −10 hexamer, i.e. two turns of the DNA helix away from this hexamer. Architecture IV, present in the pagP, pagD and iraP promoters, carries two PhoP boxes, one in the direct orientation with location and phasing similar to the single PhoP box in architecture III promoters, and another PhoP box located either upstream or downstream of this site. Architecture V, present in the ugtL, pagK, pgtE, mgtC, pagC and pipD promoters, also displays two PhoP boxes, one in the reverse orientation, located 30, 37, 49, 48, 47 or 58 nt upstream of the −10 hexamer, separated by half or near half-integral turns of the DNA helix from this hexamer. The other PhoP box either overlaps the −10 hexamer or is located downstream of the transcription start site.
There is a cross-correlation between PhoP box submotif and promoter architecture (F statistics, P-value < 0.01). For instance, promoters with architectures I, III and IV harbour primarily PhoP boxes with sequences resembling submotif S1 (Fig. 1A). By contrast, promoters with architectures II and V mainly harbour PhoP boxes resembling submotifs S2 (Fig. 1B) and S3 (Fig. 1C) respectively.
PhoP uses different mechanisms to activate transcription from promoters harbouring a single PhoP box
Activator proteins promote gene transcription in at least two ways depending on the location of their binding sites relative to those of the RNAP (Barnard et al., 2004; Browning and Busby, 2004). In Class I activation, the activator binds to a sequence located upstream of the −35 hexamer and recruits RNAP by interacting with the C-terminal domain of its α subunit (α-CTD). In Class II activation, the activator binds to a sequence that overlaps with the −35 hexamer, and the interaction is established with the σ subunit of RNAP, although additional RNAP subunits may also be contacted.
The single PhoP box in the mgtA, phoP, yrbL, slyB, pmrD and orgB promoters is located in the direct orientation c. 12 nt upstream of the predicted −10 hexamer (architecture I; Fig. 2). These promoters are predicted to behave as Class II promoters because their respective PhoP boxes overlap the predicted −35 hexamer (Table 2; Fig. 2). This was demonstrated for the mgtA promoter because the α-CTD of RNAP is not required for PhoP-dependent activation of mgtA in vitro (Perez and Groisman, 2009). The ompX and rstA promoters (architecture III; Fig. 2) also harbour a single PhoP box in the direct orientation. However, the PhoP box is located 21 nt and 23 nt upstream from the predicted −10 hexamer in the ompX and rstA promoters respectively. This is one turn of the DNA helix upstream from the location of the PhoP box in the architecture I promoters (Fig. 2). We determined that the α-CTD subunit of RNAP is required to promote PhoP-dependent activation of transcription from the rstA gene (Fig. 3). The production of spurious transcripts by the alpha Δ-235 (αΔ-CTD) RNAP indicates that this mutant RNAP is competent for transcription. The bands below the rstA transcripts, likely originating from cryptic sites, are commonly observed in in vitro transcription assays, particularly when naked linear DNA is used as template. The spurious transcripts originate within 20 nt of the rstA transcription start site. The data indicate that PhoP and RNAP most likely occlude these cryptic sites to favour rstA transcription. PhoP and mutant RNAP, on the other hand, are unable to promote rstA transcription and, thus, do not occlude the cryptic sites. The spurious transcripts observed in the two lanes where the PhoP protein was omitted demonstrate that both wild-type and mutant RNAP are able to promote gene transcription (i.e. the inability of the mutant RNAP to promote PhoP-dependent rstA transcription cannot be due simply to the enzyme being dead). In addition, we have demonstrated that same preparation of mutant RNAP could promote transcription from the mgtA promoter (Perez and Groisman, 2009). Cumulatively, these data indicate that the location of the single PhoP box affects the mechanism by which PhoP promotes gene transcription.
Table 2.
Summary of the RNAP features in 23 PhoP-activated promoters
| Distance | Sequence | Mismatches | Sequence | Mismatches | Spacer length | RNAP | |
|---|---|---|---|---|---|---|---|
| Promoter | −10 to +1 | −10 | −10 | −35 | −35 | −35 to −10 | Score |
| mgtA | 7 | TATGAT | 1 | TCGTTG | 4 | 17 | 0.74 |
| phoP | 7 | CATAAT | 1 | TTAACT | 2 | 17 | 0.82 |
| yrbL | 2 | CATACT | 2 | TAGGTT | 4 | 18 | 0.62 |
| slyB | 6 | TATGAT | 1 | AAGATT/TTAAGA | 4/2 | 18/20 | 0.68/0.68 |
| pmrD | 5 | TAACGT | 3 | CCGTTT/TTGCCG | 5/2 | 18/21 | 0.53/0.53 |
| orgB | 7 | TAGAAA | 2 | GAGGCA | 3 | 16 | 0.68 |
| yobG | 5 | TAATTT | 3 | TACTCC/GTTACT | 4/3 | 19/21 | 0.56/0.54 |
| virK | 6 | TACCGT | 3 | TTTACG | 2 | 16 | 0.70 |
| mig-14 | 7 | TACAAA | 2 | TTTATT | 3 | 19 | 0.65 |
| ybjX | 5 | TTCAAT | 2 | ACGATT/TTGACG | 4/1 | 18/21 | 0.60/0.60 |
| pcgL | 4 | TAATCT | 3 | ACCATC/TAACCA | 5/4 | 18/20 | 0.56/54 |
| ssrB | 6 | CAGACT | 3 | AAGGCT | 4 | 17 | 0.61 |
| rstA | 5 | TATGTT | 2 | TGGAAG | 3 | 17 | 0.75 |
| ompX | 7 | TAAAAT | 1 | TTGAAA | 1 | 19 | 0.79 |
| pagP | 6 | TATTAT | 1 | ATTTTA/AAGATT | 4/4 | 16/19 | 0.69/0.68 |
| pagD | 6 | CATAAT | 1 | AATAAA/TGAATA/TTGAAT | 4/3/2 | 17/19/20 | 0.72/0.70/0.66 |
| iraP | 4 | TCCGTG | 5 | TTAAAT/TTTAAA | 3/2 | 18/19 | 0.53/0.54 |
| ugtL | 7 | TAAAAG | 2 | TTGTCT/TAGAAA | 3/3 | 15/21 | 0.66/0.64 |
| pagK | 4 | TAATAT | 2 | CTGATG/ACCTGA | 3/5 | 15/17 | 0.65/0.61 |
| pgtE | 5 | CAAGAT | 3 | TGTACT | 3 | 17 | 0.68 |
| mgtC | 5 | TATAAT | 0 | TTTACG | 2 | 16 | 0.83 |
| pagC | 6 | TATAAC | 1 | TGGTGT | 4 | 17 | 0.71 |
| pipD | 5 | TCCAAT | 2 | CTGGCT | 3 | 17 | 0.72 |
The RNAP score of a query sequence is based on its similarity with the RNAP consensus pattern (−10 sequence, −35 sequence and the distance between them) identified from examples in the RegulonDB database (Harari et al., 2009).
Fig. 3.

The α-CTD subunit of RNA polymerase is required to promote transcription from the rstA promoter. Single-round in vitro transcription assays with linear templates corresponding to the rstA promoter using wild-type RNAP or mutant alpha Δ235 (αΔ-CTD) RNAP, and increasing amounts of phosphorylated Salmonella PhoP-His-6 protein (0, 65, 130 and 325 nM). Arrows show PhoP-dependent transcripts. Additional bands correspond to spurious transcripts that are not PhoP-activated.
PhoP-activated promoters with two PhoP boxes
In contrast to the eight promoters discussed above, the remaining 15 promoters (i.e. corresponding to the yobG, pcgL, virK, mig-14, ybjX, ssrB, pagP, pagD, iraP, ugtL, pagK, pgtE, mgtC, pagC and pipD genes) harbour two PhoP boxes, which can be found in the direct or reverse orientations (Table 1; Fig. 2). One of the PhoP boxes is invariably located at or farther upstream of the site normally occupied by the predicted −35 hexamer (Fig. 2). In nine of these promoters (i.e. yobG, pcgL, virK, mig-14, ybjX, ssrB, pagP, pagD and iraP), corresponding to architectures II and IV, one of the PhoP boxes is in the same orientation, location and phasing as that present in promoters with architectures I and III respectively. Promoters with architectures II and IV differ from promoters with architectures I and III in harbouring a second PhoP box (Fig. 2). In architecture V promoters (i.e. ugtL, pagK, pgtE, mgtC, pagC and pipD), one of the PhoP boxes is located farther upstream from the location of the single PhoP box in the promoters with architectures I and III (Table 1; Fig. 2). This PhoP box is invariably in the reverse orientation and separated by half-integral turns from the predicted −10 hexamer (Table 1; Fig. 2), which distinguishes these promoters from architecture II and IV promoters.
The location of the second PhoP box varies among promoters with architectures II, IV and V (Fig. 2). For all architecture V promoters except the pgtE promoter, the location of the second PhoP box suggests it functions as a repression site despite being part of PhoP-activated promoters. For example, the second PhoP box in the ugtL promoter overlaps with the predicted −10 hexamer (Fig. 2), suggesting that PhoP-P binding to this sequence likely blocks transcription initiation. In the case of the mgtC promoter, the second PhoP box is downstream of the mapped transcription start site (Fig. 2), implying it operates as a roadblock to hinder transcription elongation (Browning and Busby, 2004).
The cross-correlation between the sequence of the PhoP box and its predicted role reveals that PhoP boxes predicted to participate in activation display primarily the S1 and S2 submotifs whereas those anticipated to act in repression display mainly the S3 submotif (Table 1; Fig. 1; F statistics, P-value < 0.008).
The location and orientation of a PhoP box are critical for PhoP-promoted transcription
To define the combinations of PhoP box location and orientation that enable PhoP-dependent gene transcription, we measured the fluorescence produced by strains harbouring engineered plasmids carrying fusions between wild-type or mutant PhoP-activated promoters and a promoterless gfp gene. Promoters labelled with the suffix ‘4’ (e.g. phoP4) are shortened derivatives of the natural promoters that recapitulate PhoP-dependent gene transcription. They include the minimum DNA sequence corresponding to the PhoP box(es) identified in DNase footprinting assays (Fig. S3) and to the −10 and −35 hexamers predicted from the S1 mapping experiments (Fig. S1). For example, cells harbouring a plasmid with the promoter phoP4, containing only 52 nt upstream and 5 nt downstream of the PhoP-dependent transcription start site, displayed a behaviour that was virtually identical to that of cells harbouring a plasmid with a longer PhoP promoter fragment that included 112 additional bp upstream (Fig. 4A). Therefore, all the sequence information necessary for Mg2+-regulated PhoP-dependent transcription appears to be present in the cloned 58 bp DNA fragment.
Fig. 4.

Transcription of PhoP-activated promoters is affected by the location, orientation and phasing of the PhoP box. GFP expression produced by Salmonella harbouring plasmids with a promoterless gfp gene driven by derivatives of the phoP promoter. Each construct is a shortened derivative of the corresponding PhoP-activated promoters that includes the most upstream PhoP box and the RNAP binding site. The resulting DNA fragment was cloned in front of promoterless gfp gene in a plasmid pMS201 and introduced into a Salmonella strain expressing an HA-tagged PhoP (A and B) from its normal chromosomal location (EG13918) or wild-type PhoP (C and D). Cells were grown in N-minimal media containing 10 mM MgCl2 and then switched to media containing 50 µM MgCl2. Promoter activity (y-axis) was measured by normalizing GFP expression to cell density and pre-proceeded as described in Experimental procedures. Shown are the mean and S.D. values of at least three independent experiments. A. The phoP (blue line) promoter compared with its shortened derivative phoP4 (green line). The phoP4 (green line) and rstA4 (cyan line) promoters compared with their derivatives phoP4/rev (red line) and rstA4/rev (orange line), respectively, where the PhoP box orientation was reversed from their original locations. B. The phoP4/revK (blue line) and the phoP4/revC (green line) promoters, where the orientation of the PhoP box was reversed and moved to positions of the PhoP box in the natural pagK (blue line) and mgtC (green line) promoters (see Fig. 2). C. The PhoP box moved upstream/downstream from its normal location and orientation in the phoP4 promoter at 7, 12, 17, 21, 22, 23, 24, 25, 26, 27, 28, 33, 36 and 48 bp from the predicted −10 hexamer. D. The oppositely oriented PhoP box in the phoP4 promoter was moved upstream/downstream at 12, 23, 27, 28, 29, 30, 31, 32, 36, 37, 47 and 48 bp from the predicted −10 hexamer.
We believe that PhoP is initiating transcription from the phoP4 promoter from the same location as when the promoter is in the chromosome (as opposed to utilizing alternative −10 sequences). This is because mutation of the predicted −10 hexamer significantly compromised expression (Fig. S4).
Classical promoter enhancers retain the ability to activate gene transcription when placed in the opposite orientation (Kadener et al., 2002). Thus, we tested whether a PhoP box that is normally in one orientation can function when present at the same position but in the opposite orientation. We made derivatives of the natural phoP and rstA promoters where the 17 nucleotides corresponding to each of their single PhoP boxes in the direct orientation were placed in the opposite orientation (phoP4-rev and rstA4-rev). The wild-type strain harbouring the resulting plasmids produced no fluorescence (Fig. 4A), in contrast to the fluorescence displayed by the strains harbouring the isogenic plasmids with the original phoP and rstA promoters (Fig. 4A). These data demonstrate that the PhoP box functions in an orientation-dependent manner, at least when located 12 nt or 23 nt upstream of the −10 hexamer sequence of RNAP. Importantly, the PhoP box present in the phoP promoter is competent to mediate transcription when present in the reverse orientation at distances from the predicted −10 region found in natural PhoP-activated promoters. For example, the single PhoP box from the phoP promoter could functionally replace the PhoP box in the pagK promoter located 37 nucleotides from the predicted −10 region (phoP-revK1, Fig. 4B), and the PhoP box in the mgtC promoter located 48 nucleotides from the predicted −10 region (phop-revC, Fig. 4B).
We then explored the range of distances over which a directly oriented PhoP box can promote gene activation. The PhoP box mediated transcription when located 12, 23 or 33 nt upstream of the predicted −10 hexamer (Fig. 4C). Similar fluorescence values were produced by strains carrying constructs with the phoP PhoP box 12 or 23 nt upstream of the predicted −10 hexamer (Fig. 4C), which correspond to the normal locations of the PhoP box in the phoP and rstA promoters respectively (Fig. 2). The PhoP box could still drive gfp transcription when located 33 nt upstream of the −10 hexamer even though there are no examples of natural PhoP-activated promoters with a directly oriented PhoP box at this location (Fig. 4C). Yet, the strain with the latter construct produced only half as much fluorescence as the strains with the former two plasmids (Fig. 4C).
The three promoters described above share the orientation and sequence of the PhoP box. However, they differ in the location of the PhoP box, which, curiously, is one or two integral turns of a DNA helix away from the position of the PhoP box in the other promoters. This suggests that the PhoP-P protein promoting transcription from these three promoters is positioned on the same face of the DNA. If being on the same face of the DNA is critical for PhoP-activated gene transcription, then altering the distance by a number of nucleotides different from 10–11 (corresponding to one turn of a DNA helix) should abolish gene transcription. As predicted, strains harbouring plasmids where gfp transcription was driven by a promoter with a directly oriented PhoP box located 7, 17 or 28 nt upstream of the predicted −10 hexamer sequence produced little fluorescence (Fig. 4C). Similarly low levels of fluorescence were displayed by strains where the PhoP box was situated 25, 26 or 27 nt upstream from the predicted −10 hexamer sequence (Fig. 4C). And when the PhoP box was positioned 21, 22 or 24 nt upstream from the predicted −10 hexamer, expression was ∼ 25% of that observed when the PhoP box was at position 23 (Fig. 4C).
Next, we examined the locations at which the PhoP box could promote gene transcription when present in the reverse orientation. We created a set of promoters analogous to those described above, except that the PhoP box was present in the reverse orientation at various distances from the −10 hexamer, including some corresponding to the location of natural PhoP-dependent promoters (Fig. 2). The PhoP box promoted transcription when placed 27, 37 or 48 nt upstream of the predicted −10 hexamer (Fig. 4D). Interestingly, expression increased as the distance of the PhoP box to the −10 hexamer increased (Fig. 4D). Consistent with the notion that being on a particular face of the DNA is necessary for PhoP-promoted gene transcription, there was little expression when the PhoP box was present 23, 28, 29, 30, 31 or 32 nt upstream of the −10 hexamer sequence (Fig. 4D). In contrast to promoters harbouring the PhoP box in the direct orientation, the PhoP box in the three promoters with the PhoP box in the opposite orientation is separated by half-integral turns of the DNA helix from the predicted −10 hexamer. These results support the idea that it is the combination of location and orientation of the PhoP box that determines on which face of the DNA helix PhoP-P is found and the ability of PhoP-P bound to such PhoP box to establish productive interactions with RNAP.
A second PhoP box modulates transcription in a promoter with two PhoP boxes
Most promoters with architectures II and IV harbour two PhoP boxes located at the position corresponding to the predicted −35 hexamer and/or upstream of it, suggesting the PhoP binding sites operate as activation sites (Fig. 2). To test this hypothesis, we measured the fluorescence produced by wild-type Salmonella harbouring a medium copy number plasmid with a promoterless gfp gene driven by the natural architecture II ybjX promoter or by derivatives with nucleotide substitutions in each of the two PhoP boxes (Fig. 5A). Nucleotide substitutions in the PhoP box proximal to the predicted −10 hexamer abolished expression (ybjX4-mut-dn; Fig. 5B). By contrast, mutation of the upstream PhoP box, which is in the reverse orientation and located 30 nt upstream of the first PhoP box (Fig. 5A), dramatically reduced fluorescence (i.e. > 8-fold) but did not eliminate it (ybjX4-mut-up-rev; Fig. 5B). Because having a second PhoP box upstream of a PhoP box located 12 nt upstream of the −10 hexamer increases gene transcription in the ybjX promoter, we wondered whether adding a second PhoP box to a promoter that normally has a single PhoP box increases its expression levels. As hypothesized, adding a second PhoP box 26 nt upstream of the single PhoP box in the phoP promoter, which is the location of the architecture II virK promoter (Fig. 2), enhanced its transcription levels originating from the engineered promoter (phoP4-2; Fig. 5C).
Fig. 5.

Different roles for the two PhoP boxes in PhoP-activated promoters with two PhoP boxes. A. DNA sequences of a set of natural and mutant PhoP-activated promoters. All constructs harbour the predicted −10 and −35 hexamers identified in the S1 mapping experiments (Fig. S1). The predicted −10 sequences and the transcription start sites are indicated in red and green respectively (Table 2). PhoP boxes and their orientation are indicated in bold and blue respectively (Table 1). Nucleotide substitutions introduced in the PhoP boxes are indicated in bold italics. Cells in all GFP expression experiments were grown in N-minimal media containing 10 mM MgCl2 and switched to media with 50 µM MgCl2. The promoter activity (y-axis) was determined by normalizing GFP expression to cell density and pre-processed as described in Experimental procedures. Shown are the mean and S.D. values of at least three independent experiments. B. GFP expression displayed by a Salmonella strain expressing HA-tagged PhoP from its normal chromosomal location (EG13918) harbouring plasmids with a promoterless gfp gene driven by the wild-type ybjX promoter (blue line) or derivatives ybjX-mut-dn (red line) and ybjX-mut-up-rev (green line) where the two PhoP boxes were individually mutated. C. GFP expression displayed by a Salmonella strain expressing HA-tagged PhoP from its normal chromosomal location (EG13918) harbouring plasmids with a promoterless gfp gene driven by the phoP4 promoter (blue line) or its derivative phoP4-2 (green line), with a second PhoP box 26 nt upstream of the first PhoP box. D. GFP expression displayed by a Salmonella strain expressing HA-tagged PhoP from its normal chromosomal location (EG13918) harbouring plasmids with a promoterless gfp gene driven by the wild-type virK promoter (blue line) or derivatives virK-mut-dn (red line), virK-mut-up-for (green line) and virK-mut-up-rev (cyan line) where the two PhoP boxes were individually mutated. E. Run-off in vitro transcription assays with linear templates corresponding to the virK promoter region (virK-wt), and an equivalent DNA fragment with point mutations in the first (virK-mut-dn) and second (virK-mut-up-for) PhoP boxes as indicated in (A), RNAP and increasing amounts of PhoP-His protein (0, 25, 50 and 100 pmol). The transcripts are indicated with arrows. Shown are the mean and S.D. values of at least two independent experiments. F. Quantification of the in vitro transcription assays in (E), where the induction fold was calculated with respect to samples lacking PhoP-His protein. G and H. Run-off in vitro transcription assays with linear templates corresponding to the virK promoter region (virK-wt) and an equivalent DNA fragment with point mutations in the second PhoP box (virK-mut-up-for) as indicated in (A), RNAP, PhoP-His protein only (0, 10, 20, 30 and 40 pmol) and in combination with H-NS (0 and 10 pmol). The transcripts are indicated with arrows. Shown are the mean and S.D. values of at least two independent experiments. I and J. Quantification of the in vitro transcription assays in (G) and (H), where the induction fold was calculated with respect to samples lacking PhoP-His protein. Blue and red lines indicate transcripts with PhoP protein only and in combination with H-NS respectively.
Architecture V promoters also harbour two PhoP boxes, one of which is located at the position corresponding to the −10 hexamer or downstream of the transcription start site, suggesting they operate as repression sites (Fig. 2). This is the case in the ugtL promoter, which harbours one PhoP box overlapping the −10 sequence (Shi et al., 2004) and is anticipated to function as a repression site despite being part of a PhoP-activated promoter.
A PhoP box necessary to overcome silencing by H-NS
Why are two PhoP boxes needed for activation in architecture II and IV promoters instead of a single high-affinity site for PhoP-P? A possible explanation is that one of the PhoP boxes has a role(s) other than interacting with RNAP. For example, transcription of many horizontally acquired genes, including some that are PhoP-activated, is silenced by nucleoid-associated proteins (Dorman, 2007; Perez et al., 2008). This raises the possibility of the second PhoP box being involved in overcoming gene silencing.
We chose as a model for this group of promoters the architecture II virK promoter, which harbours two PhoP boxes (Fig. 2) and is silenced by the H-NS protein (Navarre et al., 2006). Then, we measured the fluorescence produced by wild-type Salmonella harbouring a medium copy number plasmid with a promoterless gfp gene driven by the natural virK promoter or by derivatives with nucleotide substitutions in one or the other PhoP box (Fig. 5A). Nucleotide substitutions in the PhoP box proximal to the predicted −10 hexamer abolished expression (virK4-mut-dn; Fig. 5D). By contrast, mutation of the second PhoP box in the virK promoter, which is in the direct orientation and located 26 nt upstream of the first PhoP box (Fig. 5A), dramatically reduced fluorescence but did not eliminate it (virK4-mut-up-for; Fig. 5D). These behaviours mimic those exhibited by derivatives of the ybjX promoter with mutations in each of its two PhoP boxes (Fig. 5B).
We further evaluated the virK promoter by carrying out in vitro transcription reactions with wild-type or mutant DNA templates, purified PhoP protein and RNAP. In agreement with the in vivo results, there was no transcription when the virK-mut-dn mutant DNA was used (Fig. 5E and F). Surprisingly, the virK4-mut-up-for mutant template was transcribed as well as the wild-type template (Fig. 5E and F), which is in contrast to the defective transcription displayed in vivo (Fig. 5D). However, when H-NS was added to the reactions, the virK4-mut-up-for mutant template was not transcribed as efficiently as the wild type (Fig. 5G–J). These data suggest that transcriptional activation of the virK promoter requires binding of PhoP to one PhoP box to help overcome H-NS-mediated silencing and to another PhoP box to recruit RNAP.
It has been proposed that PhoP overcomes H-NS silencing at the virK promoter by binding to a PhoP box that is different from the two PhoP boxes described above (Zhao et al., 2008). To test this notion, we evaluated the fluorescence produced by a strain with an isogenic plasmid harbouring nucleotide substitutions in the proposed PhoP box (i.e. virK4-mut-up-rev; Fig. 5A). The resulting strain produced similar fluorescence as the strain with the wild-type virK promoter (Fig. 5E). This in vivo result suggests that the proposed PhoP box is neither necessary to activate the virK promoter nor necessary to overcome silencing by H-NS.
Promoter architectures differ between ancestral and horizontally acquired genes
We analysed the genes corresponding to the 23 PhoP-activated promoters investigated in this work to determine their ancestral versus horizontally acquired nature based on the Conservation Scores (CS) (Janky and van Helden, 2007) of the corresponding ORFs (Fig. 6A) and their respective GC contents (Ochman et al., 2000). The CS is a measure of the degree of sequence identity for each pair of homologues by calculating their reciprocal blast scores from a Salmonella protein and its closest homologue in a particular species. CS values range from 0 when no homologue or orthologue is detected in another species to 100% amino acid identity. Proteins with amino acid identity below the median ∼ 55% identity are unlikely to be true orthologues (Fig. 6A, grey colour). CSs are arranged in matrices where the x-axis corresponds to different species and y-axis corresponds to the Salmonella genes investigated in this study. Then, we grouped genes present in similar species (CS > 55%) and calculated the rank order in which genes were incorporated into the corresponding groups (i.e. order among conserved ORF sequences across species, see Experimental procedures) (Fig. 6A). The cross-correlation between this rank order and the GC content of the genes reveals a highly linear relation between them (F statistics, P-value < 0.0005, Fig. 6B). This model suggests that those genes with a rank order < 10 and GC content > 0.5 correspond to ancestral genes (Fig. 6B, top left). By contrast, genes with a rank order > 10 (Fig. 6B) and GC content < 0.5 correspond to horizontally acquired genes (Fig. 6B, bottom right).
Fig. 6.

Relation between promoter architecture and the ancestral and horizontally acquired nature of PhoP-activated genes. A. Hierarchical clustering (Statistical Toolbox, Matlab 2007b, complete linkage method and correlation similarity were applied) of the Conservation Scores (CS) across 10 species of proteins encoded by 23 PhoP-activated genes in Salmonella. CS is calculated as in Janky and van Helden (2007) (e-value < 1E-5, expected number of false positives in a reciprocal blast search). CS values (represented by colours) can range from 55% to 100% when the closest homologue exhibits that percentage of amino acid identity (grey colour corresponds to CS < 55%). (Note that the phylogenetic relationships shown at the top of the figures do not represent phylogenetic distances.) Top cluster groups genes exhibiting the highest CSs among the evaluated bacterial genomes (Salmonella enterica serovar Typhimurium 14028s; Shigella flexneri 2a; Escherichia coli K12; Citrobacter koseri ATCC BAA-895; Enterobacter sakazakii ATCC BAA-894; Klebsiella pneumoniae MGH 78578; Sodalis glossinidius morsitans; Erwinia carotovora atroseptica SCRI1043; Serratia proteamaculans 568; and Yersinia pestis KIM). Bottom cluster groups genes exhibiting the lowest CSs along the evaluated bacterial genomes. B. Scatter plot combining the ranking of ancestry with the CG-content (Bioinformatics Toolbox, Matlab 2007b) of the 23 proteins in Salmonella (F statistic P-value < 0.0005). The ranking of ancestry is defined as the order in which genes were incorporated into the clusters plotted in (A) (i.e. order among conserved ORF sequences across species, see Supporting information). Colours correspond to promoter architectures (yellow: I, blue: II, brown: III, magenta: IV, cyan: V. The empty square indicates a reclassification of the orgB promoter).
We then examined whether ancestral and horizontally acquired genes harboured distinct promoter architectures. We determined that ancestral genes are primarily driven by promoters with architectures I and III (Figs 2 and 6B), whereas horizontally acquired genes are transcribed from promoters with architectures II, IV and V (Figs 2 and 6B). The orgB promoter with architecture I (Figs 2 and 6B), rank order > 10 and GC content < 0.5 (Fig. 6B) constitutes an exception within the present data distribution. Because a leave-one-out analysis points to orgB as an outlier (see Experimental procedures), we investigated its regulatory region with position weight matrices resulting from the sequences footprinted by PhoP-P (Fig. 1 and Fig. S3). We identified a putative second PhoP box (i.e. CTGTTGAGGGGATACTGAT) resembling submotif S3 (Fig. 1C), in the direct orientation and located 56 nt upstream of the transcription start site, which is similar to the location of PhoP boxes in the pipD and mgtC promoters (Table 1). Therefore, orgB appears to be driven by an architecture V promoter. Our analysis shows that the promoters of PhoP-activated ancestral genes typically have simpler architecture (e.g. a single PhoP box at discrete locations), whereas those activating transcription of horizontally acquired genes are more varied and complex (Fig. 6B, see colour distributions along the linear model).
Discussion
The PhoP-P protein utilizes a variety of promoter architectures to activate gene transcription in S. enterica serovar Typhimurium. Our analysis is based on the experimentally validated cis-acting features of 23 PhoP-activated natural promoters and mutational analysis of these features. Therefore, it differs from approaches that focused on a single promoter or that used artificial promoters (Mayo et al., 2006; Lu et al., 2009), which has led others to propose forms of PhoP activation that either were incomplete (Lejona et al., 2003) or could not be reconciled with our present understanding of activators functioning with the σ70 form of RNAP (Gal-Mor et al., 2011). We propose that the architectures identified in this work are critical contributors to the regulatory programme carried out by the PhoP protein.
The main conclusions of our investigations are as follows: first, PhoP-P uses only particular combinations of cis-regulatory features (i.e. promoter architectures) to activate transcription from its promoters. This is in contrast to the suggestion that PhoP-activated promoters can ‘tolerate’ different orientations and locations of the PhoP box(es) (Zhao et al., 2008). In fact, we demonstrated that specific, non-arbitrary combinations of cis-acting sequences comprise these architectures because: (i) each class of architecture is shared by multiple promoters, indicating that each architecture was adopted due to functional constraints, and (ii) only a few of the potential arrangements of the cis-acting features are found. Unlike regulators such as the cAMP receptor (CRP) (Barnard et al., 2004; Browning and Busby, 2004), PhoP seems to promote transcription not only from promoters having PhoP boxes located at integral turns away from the predicted −10 hexamer (e.g. Architecture III promoters; Fig. 2), but also from promoters with PhoP boxes in the reverse orientation and located at half-integral turns away from the predicted −10 hexamer (e.g. Architecture V promoters; Fig. 2). In addition, we established that the PhoP box can function from more than one location up to a certain distance from the predicted −10 hexamer, and spaced by integral and half-integral turns of the DNA helix from this sequence (Fig. 4C and D). At any given position, the PhoP box is active only in one of the two possible orientations as the PhoP box conferred high levels of expression when present 48 nt upstream of the predicted −10 hexamer in the reverse orientation but no expression when in the direct orientation at the same location (Fig. 4C and D). The converse was true when the PhoP box was present 12 nt upstream of the predicted −10 hexamer sequence (Fig. 4C and D).
All 23 experimentally verified PhoP-activated promoters have at least one PhoP box upstream of the predicted −10 hexamer (Table 1; Fig. 2). Yet, it has been proposed that the PhoP-activated sseL gene is driven by a promoter harbouring two PhoP boxes: one overlapping the transcription start site and the other one downstream of the start site (Gal-Mor et al., 2011). Thus, it is not clear how PhoP can initiate transcription from the sseL promoter rather than interfere with RNAP binding and/or transcription elongation. Moreover, we could not identify a single instance of a promoter harbouring a PhoP box in the reverse orientation, located < 24 nt upstream of the predicted −10 hexamer, and separated by half-integral turns of the DNA helix from that hexamer in the 23 PhoP-activated promoters analysed in this study (Fig. 2).
None of the natural PhoP-activated promoters described to date harbours a single PhoP box > 30 nt upstream of the predicted −10 hexamer, in the direct orientation and/or separated by integral turns of the DNA helix from that hexamer (Fig. 2). Yet, others have proposed an unusual promoter architecture for several PhoP-activated genes (Zhao et al., 2008). For instance, the pagD promoter was proposed to have a single PhoP box in the direct orientation located 144 nt away from the transcription start site (Zhao et al., 2008). Thus, it is unclear how PhoP-P would interact with RNAP from the proposed location. By contrast, our investigations revealed the presence of a second PhoP box in the pagD promoter, one that is footprinted by PhoP-P. The identified PhoP box is present in the direct orientation 22 nt upstream of the predicted −10 hexamer (Table 1; Fig. 2). The different promoter architectures driving transcription of the pagD (Architecture IV; Fig. 2) and the divergent pagC (Architecture V; Fig. 2) genes suggest they are likely to be independently activated by the PhoP protein [as opposed to them being co-ordinately transcribed by PhoP-P binding to a single PhoP box as suggested by others (Zhao et al., 2008)].
Second, promoter architectures are associated with the ancestral or horizontally acquired character of the corresponding genes rather than the function of the encoded gene products. For instance, both the ancestral mgtA gene and the horizontally acquired mgtB gene specify Mg2+ transporters (Groisman, 2001), yet, they are transcribed from promoters with different architectures (Fig. 2).
Third, the length of the 5′ leader regions of PhoP-activated transcripts, which can be quite long, is not correlated with promoter architecture (Fig. S2B) or gene ancestry (Fig. S2C). For example, the mgtA and the mgtC transcripts have similarly long leader regions, but differ both in the architectures of their respective promoters and in their gene ancestries (Table 1; Figs 2 and 6; and Fig. S2B and C).
Fourth, PhoP-P plays at least four distinct roles when promoting transcription directly. For architecture I and III promoters, which have the PhoP box overlapping and upstream of the proposed −35 hexamer, respectively, PhoP-P is predicted to establish interactions with different RNAP subunits (Fig. 3) (Perez and Groisman, 2009). Most transcription factors activate transcription by making contact with either the α-CTD or the σ70 subunit of RNAP (Hochschild and Dove, 1998). The PhoP protein appears to interact with the α-CTD in promoter architectures having a PhoP box that does not overlap with the −35 hexamer. In addition, PhoP, like the PhoB and VanR proteins (Blanco et al., 2002; Depardieu et al., 2005), is predicted to contact the σ subunit of RNAP in promoter architectures having a PhoP box completely overlapping the −35 hexamer. Unlike the λ cII protein, which requires the α-CTD when cII-specific direct repeats straddle the promoter −35 element (Jain et al., 2005), the PhoP protein does not require contacting the α-CTD when the PhoP box overlaps the −35 in the mgtA promoter (Yamamoto et al., 2002; Perez and Groisman, 2009). Moreover, like the CRP protein (Salles and Weinstock, 1989; Kolb et al., 1993; Busby and Ebright, 1999; Tebbutt et al., 2002), PhoP activates promoters harbouring more than one binding site located upstream from the predicted −10 hexamer, which may require PhoP-P to interact with different RNAP subunits at the same promoter. This suggests that the PhoP protein can interact with RNAP in multiple ways due to the varied architectures of its promoters.
In addition, PhoP can promote transcription via mechanisms that do not involve interactions with RNAP. For instance, PhoP also activates transcription from the ugd promoter in a manner dependent on the response regulator RcsB (Mouslim et al., 2003). PhoP binds to a single site located 145 nt upstream from the RcsB-dependent transcription start site where it may interact with RcsB and/or alter the ugd promoter in a way that aids RcsB binding and/or recruitment of RNAP (Mouslim et al., 2003).
And fifth, promoter architectures correlate relatively well (Table 1, F statistics, P-value < 0.01) with promoter strength as measured by expression microarray analysis of wild-type versus phoP mutant strains (Harari et al., 2010). The yrbL, pmrD and mgtA genes are an exception to this correlation. This could reflect that the pmrD and yrbL promoters are directly repressed by the response regulator PmrA, which is activated post-translationally by the PhoP-dependent PmrD protein (Kato et al., 2003; Zwir et al., 2005; Harari et al., 2010). And in the case of mgtA, the transcript levels depend on its 5′ leader region (Cromie et al., 2006; Park et al., 2011).
In those PhoP-activated promoters that harbour two PhoP boxes, one of the PhoP boxes is required for activation whereas the second PhoP box plays a variety of functions depending on its location. PhoP boxes located upstream of the predicted −10 proximal PhoP box stimulate gene transcription (Fig. 5C). In the case of the ybjX promoter, mutation of the PhoP box present at the site normally corresponding to the −35 hexamer abolishes transcription whereas mutation of the second PhoP box, which is located upstream of the first PhoP box, decreases but does not eliminate transcription (Fig. 5B). Like the architecture II ybjX promoter (Fig. 2), the virK promoter harbours two PhoP boxes: one where the −35 hexamer is normally present, which is also required for transcription (Fig. 5D), and one further upstream that serves to overcome H-NS-mediated silencing (Fig. 5D).
Promoters with architecture V also harbour at least two PhoP boxes (Fig. 2). The location of the first PhoP box corresponds to an activation site (Fig. 2), whereas the location of the second PhoP box – either overlapping the predicted −10 hexamer or downstream of the transcription start site (Fig. 2) – is anticipated to repress or silence gene expression. Our data suggest that PhoP-P, apparently acting on its own, binds at one site to activate transcription and at another site to repress transcription in the same promoter (Fig. S4) when Salmonella experiences inducing conditions for the PhoP/PhoQ system. This distinguishes PhoP-P from other forms of regulation where distinct activators and repressors bind to different sites in the same promoter (Valentin-Hansen et al., 1996; Roy et al., 1998; Browning et al., 2000; 2002; Barnard et al., 2004; Guido et al., 2006), or where the same protein can function as an activator in one context and as a repressor in another (Ansari et al., 1995; Li et al., 1997; Roy et al., 1998; 2004).
The PhoP-P protein has less affinity for the second PhoP box than for the first PhoP box (Fig. S3), suggesting that the PhoP-P protein occupies the second PhoP box after the first one. This arrangement could give rise to an expression behaviour whereby a gene is expressed only when Salmonella experiences a discrete range of inducing conditions that produce intermediate levels of PhoP-P. This is because at high PhoP-P concentrations, PhoP-P would bind to the second PhoP box, silencing expression. Therefore, as PhoP-P positively regulates its own transcription (Soncini et al., 1995), which results in a continuous increase of PhoP-P levels during the first 30 min of the bacterium experiencing inducing conditions (Shin et al., 2006), promoters having two PhoP boxes, one of which located downstream of or overlapping the predicted −10 region, may give rise to a time-dependent expression over a particular time period. The proposed scenario is analogous to that previously demonstrated for the bipA gene from Bordetella bronchiseptica, which is expressed at intermediate activation levels for the BvgA/BvgS regulatory system (Stockbauer et al., 2001), and is controlled by a promoter with a high-affinity BvgA binding site involved in activation and a low-affinity binding site involved in repression (Williams et al., 2005).
PhoP-activated promoters can also harbour sites for other DNA-binding proteins. For example, the SlyA protein binds the pagC and ugtL promoters to overcome silencing by the H-NS protein (Perez et al., 2008). In agreement with previous results (Kong et al., 2008), we showed that the PhoP protein, like the SlyA protein (Perez et al., 2008), can function to overcome silencing by H-NS by using a second PhoP box such as that of the H-NS silenced virK promoter. It has been proposed that the role of the PhoP protein at these complex promoters is limited to antagonizing H-NS (Kong et al., 2008; Zhao et al., 2008). However, we demonstrated that the PhoP protein is required to promote activation from the pagC promoter in vitro, even when H-NS was not included in the reaction (Perez et al., 2008). In addition, the PmrA protein binds the pmrD promoter (Kato et al., 2003) to repress its transcription, and it is predicted to bind and repress the Salmonella yrbL promoter as it does for the E. coli yrbL promoter (Zwir et al., 2005). The ugd promoter has binding sites for the PmrA, PhoP and RcsB proteins, and the latter two proteins are necessary to promote ugd transcription under certain inducing conditions (Mouslim and Groisman, 2003).
It has been postulated that the PhoP and SlyA proteins control expression of a group of divergently transcribed genes (Zhao et al., 2008). Because the proposed location of the PhoP boxes was excessively far upstream from the mapped transcription start sites, the authors proposed that the PhoP box was acting to overcome H-NS rather than to recruit RNAP in these promoters (Zhao et al., 2008). However, this does not appear to be the case, at least for some of the proposed genes, because we could identify other PhoP boxes at locations matching those of known promoter architectures (Fig. 2). For example, we demonstrated that the PhoP box identified in the virK and pipB2 promoter region (Zhao et al., 2008) (virK-mut-up-rev; Fig. 5D), located 55 nt and 121 nt upstream of the predicted −10 hexamer in the virK and pipB2, respectively, has no effect on the virK transcription (Fig. 5D). By contrast, a PhoP box located 12 nt upstream of the predicted −10 hexamer is required for virK transcription (Fig. 5D). Likewise, the location of the PhoP box proposed for the pipB2 promoter (Zhao et al., 2008) is too far away from the RNAP binding site to be functional. Indeed, we identified a PhoP box in the direct orientation located ∼ 20 nt upstream of the predicted −10 hexamer (Harari et al., 2010) like that present in the rstA and ompX promoters with architecture III (Fig. 2). This may be the case of the STM1632 promoter, where we identified a PhoP box in the direct orientation and located 13 nt upstream of the predicted −10 hexamer, which is typical of architecture I promoters and in contrast to the proposal of others (Zhao et al., 2008). In addition, PhoP boxes consistent with promoter architecture V have been identified in the pepN, STM2585 (Harari et al., 2010), STM3124 and ppiA promoters, located 59 nt, 48 nt, 47 nt and 36 nt upstream of the predicted −10 sequence respectively.
Finally, certain promoter architectures appear to be species specific. For instance, the PhoP-activated mgtC promoter from Yersinia pestis harbours a PhoP box at a location/orientation that has yet to be identified among PhoP-activated promoters in Salmonella, and similarly, the architecture of the Salmonella ugtL promoter has not been encountered in the Yersinia PhoP regulon (Perez et al., 2008; Perez and Groisman, 2009). Notably, the PhoP proteins from Salmonella and Yersinia have diverged, and the Salmonella protein is unable to promote transcription from the Yersinia mgtC promoter; yet, it can bind to this promoter just like the Yersinia PhoP protein and promote transcription from other promoters having conserved architectures (Perez et al., 2008; Perez and Groisman, 2009). Likewise, the Yersinia PhoP protein could not transcribe the Salmonella ugtL promoter (Perez et al., 2008; Perez and Groisman, 2009). This suggests that caution must be exercised when trying to deduce whether a gene is under the control of a given regulator because a binding site for such regulator is present in the identified promoter region.
Experimental procedures
DNase I footprinting assays
Primers A2, B2, C2, D2, E2, F2, G2, H2, I2, J2, K2, L2, M2 and N2 (Table S1) were labelled with 2 units of T4 polynucleotide kinase and 10 pmol of [γ-32P]-ATP using 5 µl of 5 × forward buffer (Gibco/BRL) in a total volume of 25 µl at 37° C for 1 and 2 h and unincorporated 32P was removed using ProbeQuant G-50 microcolumns (GE Healthcare). Then, the labelled primer and its partner unlabelled primer (A1 and A2, B1 and B2, C1 and C2, D1 and D2, E1 and E2, F1 and F2, G1 and G2, H1 and H2, I1 and I2, J1 and J2, K1 and K2, L1 and L2, M1 and M2) were used to generate corresponding PCR fragments using genomic DNA from strain 14028s. DNase I footprinting assays were carried out as described in Kato et al. (2003) using different concentrations of PhoP-P-His6 and 0.02 units of DNase I (Epicentre).
SI nuclease-protection assay
SI nuclease-protection assays were performed as described (Kox et al., 2000) with RNA harvested from early exponential (OD600, 0.3–0.4) phase cultures of wild-type (14028s) and phoP (MS7953s) Salmonella grown in 30 ml of N-minimal medium, pH 7.4, containing either 10 mM MgCl2 or 10 µM MgCl2. Total RNA was isolated using SV Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's specifications. The RNA was hybridized to fragments generated by PCR using chromosomal DNA was a substrate. Probes used for mig-14, pagK, yobG, pagP, ybjX, slyB, pcgL, pagD and virK were the same as those used for DNase I footprinting (Table S1). Probes for yrbL, ompX, pipD and mgtC were generated by labelling C2, J4, K3, M4 and using PCR as described above with labelled and unlabelled primers C3 and C2, J3 and J4, K1 and K3 and M3 and M4 respectively (Table S2).
Bacterial strains, plasmids and growth conditions
Bacterial strains, plasmids, and primers used in the GFP assay are listed in Table S3 and S4. S. enterica serovar Typhimurium strains used in this study were derived from strain 14028s. Bacteria were grown at 37°C with aeration in N-minimal media (pH 7.7), supplemented with 0.1% casamino acids, 38 mM glycerol and 10 µM or 10 mM MgCl2.
Construction of plasmids harbouring fusions between PhoP-activated promoters and a promoterless gfp gene
Plasmid pMS-virK, encoding the virK promoter region fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9224 and 9225 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-virK-mut-up-for, encoding the virK promoter region with the mutated upstream PhoP box in the direct orientation fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9228 and 9229 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-virK-mut-up-rev, encoding the virK promoter region with the mutated upstream PhoP box in the reverse orientation fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9226 and 9227 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-virK-mut-down, encoding the virK promoter region with the mutated downstream PhoP box fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9231 and 9230 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4, encoding a shortened derivative of the phoP promoter region including the PhoP box and the predicted binding site fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8142 and 8143 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev, encoding the phoP4 promoter region where the PhoP box in the direct orientation was placed in the opposite orientation and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8233 and 8234 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-rstA4, encoding a shortened derivative of the rstA promoter region including the PhoP box and the predicted binding site fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8144 and 8145 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-rstA4-rev, encoding the rstA4 promoter region where the PhoP box in the direct orientation was placed in the opposite orientation and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8235 and 8236 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-K1, encoding the phoP4 promoter region where the PhoP box was placed in the opposite orientation and moved further upstream at the position normally occupied by the PhoP box in the pagK promoter and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8604 and 8592 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-C, encoding the phoP4 promoter region where the PhoP box was placed in the opposite orientation and moved further upstream at the position normally occupied by the PhoP box in the mgtC promoter and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8489 and 8490 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP, encoding the phoP promoter region fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 4811 and 4432 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-rstA, encoding the rstA promoter region fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 4842 and 5231 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-7nt, encoding the phoP4 promoter region where the PhoP box was located 7 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8694 and 8693 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-17nt, encoding the phoP4 promoter region where the PhoP box was located 17 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8691 and 8692 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-12nt, encoding the phoP4 promoter region where the PhoP box was located 12 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8142 and 8143 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-21nt, encoding the phoP4 promoter region where the PhoP box was located 21 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8736 and 8749 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-22nt, encoding the phoP4 promoter region where the PhoP box was located 22 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8737 and 8750 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-23nt, encoding the phoP4 promoter region where the PhoP box was located 23 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8545 and 8548 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-24nt, encoding the phoP4 promoter region where the PhoP box was located 24 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8738 and 8751 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-25nt, encoding the phoP4 promoter region where the PhoP box was located 25 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8739 and 8752 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-26nt, encoding the phoP4 promoter region where the PhoP box was located 26 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8740 and 8753 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-27nt, encoding the phoP4 promoter region where the PhoP box was located 27 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8741 and 8754 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-28nt, encoding the phoP4 promoter region where the PhoP box was located 28 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8613 and 8593 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-33nt, encoding the phoP4 promoter region where the PhoP box was located 33 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8597 and 8602 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-48nt, encoding the phoP4 promoter region where the PhoP box was located 48 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8487 and 8488 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-28nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 28 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8732 and 8745 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-29nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 29 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8731 and 8744 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-30nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 30 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8730 and 8743 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-31nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 31 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8729 and 8742 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-36nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 36 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8441 and 8438 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-47nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 47 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8734 and 8747 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-26nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 26 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8733 and 8746 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-27nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 27 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8608 and 8601 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-32nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 32 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8598 and 8606 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-37nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 37 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8604 and 8592 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-48nt, encoding the phoP4 promoter region where the PhoP box in the opposite orientation was located 48 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8489 and 8490 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-ybjX, encoding the ybjX promoter region fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9253 and 9255 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-ybjX-mut-up, encoding the ybjX promoter region with the mutated upstream PhoP box in the reverse orientation fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9272 and 9273 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-ybjX-mut-down, encoding the ybjX promoter region with the mutated downstream PhoP box fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9293 and 9295 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-virK-phoP4, encoding a shortened derivative of the phoP promoter region including the PhoP box and the predicted binding site fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9436 and 9437 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-23nt, encoding the rstA4 promoter region where the PhoP box in the opposite orientation was located 23 nt upstream of the predicted −10 hexamer and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8235 and 8236 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-K1-TTTAAT, encoding the phoP4 promoter region where the PhoP box was placed in the opposite orientation and moved further upstream at the position normally occupied by the PhoP box in the pagK promoter and the original predicted −10 sequence was replaced by the ‘TTTAAT’ sequence and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9183 and 9184 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-K1-TAATAT, encoding the phoP4 promoter region where the PhoP box was placed in the opposite orientation and moved further upstream at the position normally occupied by the PhoP box in the pagK promoter and the original predicted −10 sequence was replaced by the ‘TAATAT’ sequence and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9189 and 9186 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-C-TATAAT, encoding the phoP4 promoter region where the PhoP box was placed in the opposite orientation and moved further upstream at the position normally occupied by the PhoP box in the mgtC promoter and the original predicted −10 sequence was replaced by the ‘TATAAT’ sequence and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8521 and 8524 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4-rev-K1-TTGACA, encoding the phoP4 promoter region where the PhoP box was placed in the opposite orientation and moved further upstream at the position normally occupied by the PhoP box in the pagK promoter and the original predicted −35 sequence was replaced by the ‘TTGACA’ sequence and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 8607 and 8610 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
Plasmid pMS-phoP4+11nt-TATGTT, encoding the phoP4 promoter region where the PhoP box was located 23 nt upstream of the predicted −10 hexamer and replaced by the ‘TATGTT’ sequence and fused to a promoterless gfp gene, was constructed by cloning a PCR fragment generated using primers 9181 and 9188 between the BamHI and XhoI sites of plasmid pMS201 (Mangan and Alon, 2003).
DNA sequencing was used to verify that the promoter regions had the predicted sequences.
GFP reporter assay
Single colonies were used to inoculate 2 ml of cultures and grown for 16 h in N-minimal media at pH 7.7 (Snavely et al., 1991) with 10 mM Mg2+ and kanamycin (25 µg ml−1) at 37°C in a shaking incubator. The cultures were diluted 1:50 into 2 ml of the same media described above and grown an additional 4 h at 37°C in a shaking incubator. One millilitre of media cells was then harvested, washed once with 1 ml of N-minimal media containing 2.5 mM Mg2+ and resuspended in 200 µl of N-minimal media with 2.5 mM Mg2+. The concentrated cultures were diluted 1:50 in 150 µl of N-minimal media containing no Mg2+ or containing 10 mM Mg2+ in flat-bottomed 96-well plates (PerkinElmer 6005225, Waltham, MA). The final Mg2+ concentrations in these two conditions were 50 µM and 10 mM, which induce and repress the PhoP/PhoQ system respectively. The cultures were overlaid with 50 µl of mineral oil (Sigma M-3516, St. Louis, MO) to prevent evaporation. The GFP assay was performed as described (Kalir et al., 2001). The cultures were grown in a Wallac Victor3 multiwell fluorimeter (PerkinElmer) set at 37°C and assayed with an automatically repeating protocol of shaking (1 mm orbital, fast speed, 30 s), fluorescence readings (filters F485, F535, 0.5 s, CW lamp energy 10 000), and absorbance (OD) measurements (600 nm, P600 filter, 0.1 s) at 6 min intervals. Background fluorescence of cells bearing a promotorless GFP vector was subtracted from the values obtained with the cells harbouring plasmids with PhoP-activated promoters (or the engineered derivatives) fused to a promoterless gfp gene.
GFP data pre-processing
The raw data corresponding to the GFP and OD signals were used to calculate the promoter activity as [dGi(t)/dt]/ODi(t)] (Ronen et al., 2002). The activity signal, ODs and background were smoothed by a shape-preserving interpolant fitting algorithm (Piecewise Cubic Hermite Interpolating Polynomial, Matlab 7.1) that finds values of an underlying interpolating function at intermediate points not described in the experimental assays. Then, we applied a polynomial fit (sixth order) on each expression signal. This smoothing procedure captured the dynamic well, while removing the noise inherent in the differentiation of noisy signals (Ronen et al., 2002). Observed ODs were standardized (linear regressions R2 > 0.99) by using India ink from actual ODs measured by spectrophotometer. Observed fluorescence was standardized using dilutions of fluorescein and expressed as fluorescein concentrations.
In vitro transcription assays
Linear DNA templates for in vitro transcription assays were generated by PCR using primer pair 7817 and 8302 and plasmid as a template: pMS-virK, pMS-virK-mut-up-for and pMS-virK-mut-down. In vitro single round transcription assays were performed as in Perez et al. (2008) and Perez and Groisman (2009). Briefly, a mixture of template DNA (0.15 pmol), purified His-tagged proteins, and RNA polymerase holoenzyme (Epicentre, Madison, WI) were incubated in 15 µl of transcription buffer [50 mM Tris-HCl (pH 8.0), 50 mM NaCl, 3 mM MgCl2, 0.1 mM EDTA, 0.1 mM DTT and 25 µg ml−1 BSA] for 30 min at 37°C to form open complexes. A 5 µl mixture of substrate and heparin was then added to make a final concentration of 160 µM each of ATP, GTP and CTP; 50 µM UTP; 2 µCi of [α-32P]-UTP; and 200 µg ml−1 heparin. After 10 min of incubation at 37°C, reactions were stopped by adding TBE-urea loading buffer (Invitrogen) and resolved in 10% TBE-Urea gels (Invitrogen). Overproduction and purification of proteins used in this assay were performed as in Perez et al. (2008) and Perez and Groisman (2009).
Analysis of the PhoP submotifs and RNAP sequences
PhoP motifs were analysed as in Harari et al. (2010), and RNAP binding sites were identified based on methods described in Harari et al. (2009).
Calculation of the ranking of ancestry
We applied a hierarchical agglomerative clustering (Statistical Toolbox, Matlab 2007b) with a complete linkage method and correlation similarity measurement to group both: gene products with similar CSs across 10 species (rows) as well as species harbouring similar gene products (columns). The function that controls the vertical order in which a row is plotted in the hierarchical clustering (Spotfire Decision Site 9.1.2) is defined as follows. Given two subclusters within a cluster (there are always exactly two subclusters), both subclusters are weighted and the subcluster with the highest weight is placed above the other subcluster. This function is systematically applied until a single cluster containing all rows is obtained. To calculate the weight w3 of a new cluster C3 formed from two subclusters C1 and C2 with a weight of w1 and w2, and each containing n1 and n2 rows, the following expression is used:
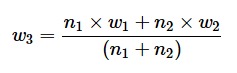 |
The weight of a subcluster with a single row is calculated as the average value of its columns.
Correlation analysis
Correlations among submotifs and orientation, activation/repression PhoP boxes and promoter architectures have been performed calculating multiple linear regressions using the stepwise regression method, Statistics Toolbox, Matlab R2007b. Outlier identification has been performed using a univariate statistical approach, where the target distributions correspond to those of the correlation analysis and the outlier regions has been defined based on 0.95 confidence coefficient (Ben-Gal, 2005). A leave-one-out sampling process demonstrated that separating the orgB promoter improves the significances of the associations (P-values) ∼ 300%.
Acknowledgments
We thank Dr Xuanlin Tu for the experiments presented in Figs 3 and 4; Dr Richard Gourse for his generous gift of RNA polymerase, Jennifer Aronson for editing of the manuscript, and three anonymous reviewers for valuable comments. I.Z. is supported in part by grants TIN-13950, TIC-02788 and GREIB-2011. This work was supported, in part, by Grant AI49561 from the National Institutes of Health to E.A.G., who is an Investigator of the Howard Hughes Medical Institute.
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aguirre A, Cabeza ML, Spinelli SV, McClelland M, Garcia Vescovi E, Soncini FC. PhoP-induced genes within Salmonella pathogenicity island 1. J Bacteriol. 2006;188:6889–6898. doi: 10.1128/JB.00804-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari AZ, Bradner JE, O'Halloran TV. DNA-bend modulation in a repressor-to-activator switching mechanism. Nature. 1995;374:371–375. doi: 10.1038/374370a0. [DOI] [PubMed] [Google Scholar]
- Bachhawat P, Stock AM. Crystal structures of the receiver domain of the response regulator PhoP from Escherichia coli in the absence and presence of the phosphoryl analog beryllofluoride. J Bacteriol. 2007;189:5987–5995. doi: 10.1128/JB.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, et al. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Barchiesi J, Castelli ME, Soncini FC, Vescovi EG. mgtA expression is induced by rob overexpression and mediates a Salmonella enterica resistance phenotype. J Bacteriol. 2008;190:4951–4958. doi: 10.1128/JB.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard A, Wolfe A, Busby S. Regulation at complex bacterial promoters: how bacteria use different promoter organizations to produce different regulatory outcomes. Curr Opin Microbiol. 2004;7:102–108. doi: 10.1016/j.mib.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Ben-Gal I. Outlier detection. In: Maimon O, Rockach L, editors. Data Mining and Knowledge Discovery Handbook: A Complete Guide for Practitioners and Researchers. New York: Kluwer Academic Publishers; 2005. pp. 131–146. [Google Scholar]
- Bijlsma JJ, Groisman EA. The PhoP/PhoQ system controls the intramacrophage type three secretion system of Salmonella enterica. Mol Microbiol. 2005;57:85–96. doi: 10.1111/j.1365-2958.2005.04668.x. [DOI] [PubMed] [Google Scholar]
- Blanco AG, Sola M, Gomis-Ruth FX, Coll M. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure. 2002;10:701–713. doi: 10.1016/s0969-2126(02)00761-x. [DOI] [PubMed] [Google Scholar]
- Browning DF, Busby SJ. The regulation of bacterial transcription initiation. Nat Rev Microbiol. 2004;2:57–65. doi: 10.1038/nrmicro787. [DOI] [PubMed] [Google Scholar]
- Browning DF, Cole JA, Busby SJ. Suppression of FNR-dependent transcription activation at the Escherichia coli nir promoter by Fis, IHF and H-NS: modulation of transcription initiation by a complex nucleo-protein assembly. Mol Microbiol. 2000;37:1258–1269. doi: 10.1046/j.1365-2958.2000.02087.x. [DOI] [PubMed] [Google Scholar]
- Browning DF, Beatty CM, Wolfe AJ, Cole JA, Busby SJ. Independent regulation of the divergent Escherichia coli nrfA and acsP1 promoters by a nucleoprotein assembly at a shared regulatory region. Mol Microbiol. 2002;43:687–701. doi: 10.1046/j.1365-2958.2002.02776.x. [DOI] [PubMed] [Google Scholar]
- Busby S, Ebright RH. Transcription activation by catabolite activator protein (CAP) J Mol Biol. 1999;293:199–213. doi: 10.1006/jmbi.1999.3161. [DOI] [PubMed] [Google Scholar]
- Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg2. Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Depardieu F, Courvalin P, Kolb A. Binding sites of VanRB and sigma70 RNA polymerase in the vanB vancomycin resistance operon of Enterococcus faecium BM4524. Mol Microbiol. 2005;57:550–564. doi: 10.1111/j.1365-2958.2005.04706.x. [DOI] [PubMed] [Google Scholar]
- Dorman CJ. H-NS, the genome sentinel. Nat Rev Microbiol. 2007;5:157–161. doi: 10.1038/nrmicro1598. [DOI] [PubMed] [Google Scholar]
- Feng X, Oropeza R, Kenney LJ. Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol Microbiol. 2003;48:1131–1143. doi: 10.1046/j.1365-2958.2003.03502.x. [DOI] [PubMed] [Google Scholar]
- Gal-Mor O, Elhadad D, Deng W, Rahav G, Finlay BB. The Salmonella enterica PhoP directly activates the horizontally acquired SPI-2 gene sseL and is functionally different from a S. bongori ortholog. PLoS ONE. 2011;6:e20024. doi: 10.1371/journal.pone.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Vescovi E, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Gaston K, Bell A, Kolb A, Buc H, Busby S. Stringent spacing requirements for transcription activation by CRP. Cell. 1990;62:733–743. doi: 10.1016/0092-8674(90)90118-x. [DOI] [PubMed] [Google Scholar]
- Groisman EA. The pleiotropic two-component regulatory system PhoP–PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA, Mouslim C. Sensing by bacterial regulatory systems in host and non-host environments. Nat Rev Microbiol. 2006;4:705–709. doi: 10.1038/nrmicro1478. [DOI] [PubMed] [Google Scholar]
- Guido NJ, Wang X, Adalsteinsson D, McMillen D, Hasty J, Cantor CR, et al. A bottom-up approach to gene regulation. Nature. 2006;439:856–860. doi: 10.1038/nature04473. [DOI] [PubMed] [Google Scholar]
- Harari O, del Val C, Romero-Zaliz R, Shin D, Huang H, Groisman EA, Zwir I. Identifying promoter features of co-regulated genes with similar network motifs. BMC Bioinformatics. 2009;10(Suppl. 4):S1. doi: 10.1186/1471-2105-10-S4-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari O, Park SY, Huang H, Groisman EA, Zwir I. Defining the plasticity of transcription factor binding sites by deconstructing DNA consensus sequences: the PhoP-binding sites among gamma/enterobacteria. PLoS Comput Biol. 2010;6:e1000862. doi: 10.1371/journal.pcbi.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochschild A, Dove SL. Protein-protein contacts that activate and repress prokaryotic transcription. Cell. 1998;92:597–600. doi: 10.1016/s0092-8674(00)81126-5. [DOI] [PubMed] [Google Scholar]
- Jain D, Kim Y, Maxwell KL, Beasley S, Zhang R, Gussin GN, et al. Crystal structure of bacteriophage lambda cII and its DNA complex. Mol Cell. 2005;19:259–269. doi: 10.1016/j.molcel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Janky R, van Helden J. Discovery of conserved motifs in promoters of orthologous genes in prokaryotes. Methods Mol Biol. 2007;395:293–308. doi: 10.1007/978-1-59745-514-5_18. [DOI] [PubMed] [Google Scholar]
- Kadener S, Fededa JP, Rosbash M, Kornblihtt AR. Regulation of alternative splicing by a transcriptional enhancer through RNA pol II elongation. Proc Natl Acad Sci USA. 2002;99:8185–8190. doi: 10.1073/pnas.122246099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalir S, McClure J, Pabbaraju K, Southward C, Ronen M, Leibler S, et al. Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science. 2001;292:2080–2083. doi: 10.1126/science.1058758. [DOI] [PubMed] [Google Scholar]
- Kato A, Groisman EA. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 2004;18:2302–2313. doi: 10.1101/gad.1230804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Groisman EA. The PhoQ/PhoP regulatory network of Salmonella enterica. Adv Exp Med Biol. 2008;631:7–21. doi: 10.1007/978-0-387-78885-2_2. [DOI] [PubMed] [Google Scholar]
- Kato A, Tanabe H, Utsumi R. Molecular characterization of the PhoP–PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J Bacteriol. 1999;181:5516–5520. doi: 10.1128/jb.181.17.5516-5520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Latifi T, Groisman EA. Closing the loop: the PmrA/PmrB two-component system negatively controls expression of its posttranscriptional activator PmrD. Proc Natl Acad Sci USA. 2003;100:4706–4711. doi: 10.1073/pnas.0836837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A, Busby S, Buc H, Garges S, Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- Kong W, Weatherspoon N, Shi Y. Molecular mechanism for establishment of signal-dependent regulation in the PhoP/PhoQ system. J Biol Chem. 2008;283:16612–16621. doi: 10.1074/jbc.M800547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox LF, Wosten MM, Groisman EA. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 2000;19:1861–1872. doi: 10.1093/emboj/19.8.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Groisman EA. An antisense RNA that governs the expression kinetics of a multifunctional virulence gene. Mol Microbiol. 2010;76:1020–1033. doi: 10.1111/j.1365-2958.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejona S, Aguirre A, Cabeza ML, Garcia Vescovi E, Soncini FC. Molecular characterization of the Mg2+-responsive PhoP–PhoQ regulon in Salmonella enterica. J Bacteriol. 2003;185:6287–6294. doi: 10.1128/JB.185.21.6287-6294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, McClure WR, Susskind MM. Changing the mechanism of transcriptional activation by phage lambda repressor. Proc Natl Acad Sci USA. 1997;94:3691–3696. doi: 10.1073/pnas.94.8.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Tolstorukov M, Zhurkin V, Garges S, Adhya S. A mutant spacer sequence between −35 and −10 elements makes the Plac promoter hyperactive and cAMP receptor protein-independent. Proc Natl Acad Sci USA. 2004;101:6911–6916. doi: 10.1073/pnas.0401929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TK, Khalil AS, Collins JJ. Next-generation synthetic gene networks. Nat Biotechnol. 2009;27:1139–1150. doi: 10.1038/nbt.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Scofield DG, Hong X. The evolution of transcription-initiation sites. Mol Biol Evol. 2005;22:1137–1146. doi: 10.1093/molbev/msi100. [DOI] [PubMed] [Google Scholar]
- Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci USA. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo AE, Setty Y, Shavit S, Zaslaver A, Alon U. Plasticity of the cis-regulatory input function of a gene. PLoS Biol. 2006;4:e45. doi: 10.1371/journal.pbio.0040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa S, Ogasawara H, Kato A, Yamamoto K, Eguchi Y, Oshima T, et al. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J Bacteriol. 2003;185:3696–3702. doi: 10.1128/JB.185.13.3696-3702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Gottesman S. A PhoQ/P-regulated small RNA regulates sensitivity of Escherichia coli to antimicrobial peptides. Mol Microbiol. 2009;74:1314–1330. doi: 10.1111/j.1365-2958.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouslim C, Groisman EA. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol Microbiol. 2003;47:335–344. doi: 10.1046/j.1365-2958.2003.03318.x. [DOI] [PubMed] [Google Scholar]
- Mouslim C, Latifi T, Groisman EA. Signal-dependent requirement for the co-activator protein RcsA in transcription of the RcsB-regulated ugd gene. J Biol Chem. 2003;278:50558–50595. doi: 10.1074/jbc.M309433200. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- Park SY, Cromie MJ, Lee EJ, Groisman EA. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell. 2011;142:737–748. doi: 10.1016/j.cell.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen H, Sogaard-Andersen L, Holst B, Valentin-Hansen P. Heterologous cooperativity in Escherichia coli. The CytR repressor both contacts DNA and the cAMP receptor protein when binding to the deoP2 promoter. J Biol Chem. 1991;266:17804–17808. [PubMed] [Google Scholar]
- Perez JC, Groisman EA. Transcription factor function and promoter architecture govern the evolution of bacterial regulons. Proc Natl Acad Sci USA. 2009;106:4319–4324. doi: 10.1073/pnas.0810343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JC, Latifi T, Groisman EA. Overcoming H-NS-mediated transcriptional silencing of horizontally acquired genes by the PhoP and SlyA proteins in Salmonella enterica. J Biol Chem. 2008;283:10773–10783. doi: 10.1074/jbc.M709843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JC, Shin D, Zwir I, Latifi T, Groisman EA. Evolution of a bacterial regulon controlling virulence and Mg2+ homeostasis. PLoS Genet. 2009;5:e1000428. doi: 10.1371/journal.pgen.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AC, Melo-Barbosa HP, Miyoshi A, Silva A, Azevedo V. Application of RNA-seq to reveal the transcript profile in bacteria. Genet Mol Res. 2011;10:1707–1718. doi: 10.4238/vol10-3gmr1554. [DOI] [PubMed] [Google Scholar]
- Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Ronen M, Rosenberg R, Shraiman BI, Alon U. Assigning numbers to the arrows: parameterizing a gene regulation network by using accurate expression kinetics. Proc Natl Acad Sci USA. 2002;99:10555–10560. doi: 10.1073/pnas.152046799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Garges S, Adhya S. Activation and repression of transcription by differential contact: two sides of a coin. J Biol Chem. 1998;273:14059–14062. doi: 10.1074/jbc.273.23.14059. [DOI] [PubMed] [Google Scholar]
- Roy S, Semsey S, Liu M, Gussin GN, Adhya S. GalR represses galP1 by inhibiting the rate-determining open complex formation through RNA polymerase contact: a GalR negative control mutant. J Mol Biol. 2004;344:609–618. doi: 10.1016/j.jmb.2004.09.070. [DOI] [PubMed] [Google Scholar]
- Salles B, Weinstock GM. Interaction of the CRP–cAMP complex with the cea regulatory region. Mol Gen Genet. 1989;215:537–542. doi: 10.1007/BF00427053. [DOI] [PubMed] [Google Scholar]
- Shi Y, Latifi T, Cromie MJ, Groisman EA. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J Biol Chem. 2004;279:38618–38625. doi: 10.1074/jbc.M406149200. [DOI] [PubMed] [Google Scholar]
- Shin D, Groisman EA. Signal-dependent binding of the response regulators PhoP and PmrA to their target promoters in vivo. J Biol Chem. 2005;280:4089–4094. doi: 10.1074/jbc.M412741200. [DOI] [PubMed] [Google Scholar]
- Shin D, Lee EJ, Huang H, Groisman EA. A positive feedback loop promotes transcription surge that jump-starts Salmonella virulence circuit. Science. 2006;314:1607–1609. doi: 10.1126/science.1134930. [DOI] [PubMed] [Google Scholar]
- Shultzaberger RK, Chen Z, Lewis KA, Schneider TD. Anatomy of Escherichia coli sigma70 promoters. Nucleic Acids Res. 2007;35:771–788. doi: 10.1093/nar/gkl956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittka A, Lucchini S, Papenfort K, Sharma CM, Rolle K, Binnewies TT, et al. Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLoS Genet. 2008;4:e1000163. doi: 10.1371/journal.pgen.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely MD, Gravina SA, Cheung TT, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium. Regulation of mgtA and mgtB expression. J Biol Chem. 1991;266:824–829. [PubMed] [Google Scholar]
- Soncini FC, Vescovi EG, Groisman EA. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol. 1995;177:4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Kong W, Weatherspoon N, Qin G, Tyler W, Turk J, et al. Modulation of the regulatory activity of bacterial two-component systems by SlyA. J Biol Chem. 2008;283:28158–28168. doi: 10.1074/jbc.M801058200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli SV, Pontel LB, Garcia Vescovi E, Soncini FC. Regulation of magnesium homeostasis in Salmonella: Mg2+ targets the mgtA transcript for degradation by RNase E. FEMS Microbiol Lett. 2008;280:226–234. doi: 10.1111/j.1574-6968.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- Stockbauer KE, Fuchslocher B, Miller JF, Cotter PA. Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein. Mol Microbiol. 2001;39:65–78. doi: 10.1046/j.1365-2958.2001.02191.x. [DOI] [PubMed] [Google Scholar]
- Tebbutt J, Rhodius VA, Webster CL, Busby SJ. Architectural requirements for optimal activation by tandem CRP molecules at a class I CRP-dependent promoter. FEMS Microbiol Lett. 2002;210:55–60. doi: 10.1111/j.1574-6968.2002.tb11159.x. [DOI] [PubMed] [Google Scholar]
- Tu X, Latifi T, Bougdour A, Gottesman S, Groisman EA. The PhoP/PhoQ two-component system stabilizes the alternative sigma factor RpoS in Salmonella enterica. Proc Natl Acad Sci USA. 2006;130:13503–13508. doi: 10.1073/pnas.0606026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P, Sogaard-Andersen L, Pedersen H. A flexible partnership: the CytR anti-activator and the cAMP-CRP activator protein, comrades in transcription control. Mol Microbiol. 1996;20:461–466. doi: 10.1046/j.1365-2958.1996.5341056.x. [DOI] [PubMed] [Google Scholar]
- Williams CL, Boucher PE, Stibitz S, Cotter PA. BvgA functions as both an activator and a repressor to control Bvg phase expression of bipA in Bordetella pertussis. Mol Microbiol. 2005;56:175–188. doi: 10.1111/j.1365-2958.2004.04526.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ogasawara H, Fujita N, Utsumi R, Ishihama A. Novel mode of transcription regulation of divergently overlapping promoters by PhoP, the regulator of two-component system sensing external magnesium availability. Mol Microbiol. 2002;45:423–438. doi: 10.1046/j.1365-2958.2002.03017.x. [DOI] [PubMed] [Google Scholar]
- Zhao G, Weatherspoon N, Kong W, Curtiss R, 3rd, Shi Y. A dual-signal regulatory circuit activates transcription of a set of divergent operons in Salmonella typhimurium. Proc Natl Acad Sci USA. 2008;105:20924–20929. doi: 10.1073/pnas.0807071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwir I, Shin D, Kato A, Nishino K, Latifi T, Solomon F, et al. Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica. Proc Natl Acad Sci USA. 2005;102:2862–2867. doi: 10.1073/pnas.0408238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


