Abstract
Purpose
To identify the genetic defect in an autosomal dominant isolated ectopia lentis (EL) family.
Methods
Detailed family history and clinical data were collected from the family including sixteen patients with isolated EL. Blood samples of nine patients, one normal person and two unknown children’s were collected. Genomic DNA was extracted from leukocytes of peripheral blood. Genotyping was performed by microsatellite markers and logarithm-of-odds (LOD) scores were calculated using the LINKAGE Programs. Mutation screening in the candidate gene, fibrillin-1 (FBN1), was performed by direct sequencing.
Results
Linkage to the FBN1 locus is verified. Mutation screening in FBN1 identified a C>T transition at nucleotide position c.2920. This nucleotide change results in the cysteine substitution for highly conserved arginine at codon 974 (p.R974C). This mutation is identified in all affected individuals but is not found in 50 control healthy people.
Conclusions
A novel mutation of FBN1 results in an arginine to cysteine residue (p.R974C) substitution, which is responsible for the patients with isolated EL in this Chinese family.
Introduction
Ectopia lentis (EL) is an inherited connective disorder characterized by lens dislocation. Most cases of EL are associated with Marfan syndrome, a genetic autosomal dominant disorder that is characterized by manifestations involving the cardiovascular, skeletal, and ocular systems [1].
Mutations of the fibrillin-1 (FBN1) gene on chromosome 15q have been described in patients with classical Marfan syndrome, neonatal MFS, aortic aneurysms, ectopia lentis, Marfanoid skeletal features, familial arachnodactyly, Shprintzen-Goldberg syndrome, Weill-Marchesani syndrome, and severe progressive kyphoscoliosis [2-6].
An isolated EL pedigree has been reported several times in different races [7-10]. This kind of phenotype may be an independent subtype caused by specific FBN1 mutations and other regulatory factors.
In this study, we recruited a Chinese family affected with isolated EL. Linkage to the FBN1 locus is identified. Mutation screening in FBN1 identified a C>T transition at nucleotide position c.2920. This nucleotide change results in the substitution of highly conserved arginine by cysteine at codon 974 (p.R974C). The mutation is cosegregated in the patients but not found in the 50 control subjects from the same ethnic background.
Methods
Patients and clinical data
The five-generation family enrolled in this study was found in a Northwestern province of China. Clinical examination, peripheral blood collection, and DNA extraction were performed in the Department of Ophthalmology, Peking Union Medical College Hospital, Beijing, China. Informed consent in accordance with the Declaration of Helsinki and the Institutional Review Board and Ethics Committee of Peking city was obtained from all participants. The family includes sixteen confirmed patients with isolated EL. Blood samples of nine patients, one normal person, and two younger children (the symptom of ectopia lentis occurs from 17 to 20 in this family) were collected (Figure 1). Clinical data of these subjects was ascertained by case records of surgeries and detailed ocular examinations.
Figure 1.
Pedigree and haplotype of the family. A five-generation pedigree with twelve available members is shown. Four markers adjacent to FBN1 were selected. The disease haplotype (represented by the black bar) cosegregated in all affected members but was not shared by unaffected ones.
Genotyping and linkage analysis
With fluorescently labeled microsatellite markers (Sangon Biotech Co,. Ltd, Shanghai, China), linkage analysis of candidate gene region at 15q was done on the DNA samples from 12 ophthalmologically examined individuals. Two-point linkage analysis was performed by MLINK from LINKAGE Program Package.
Mutation analysis
All coding exons of FBN1 were amplified by polymerase chain reaction (PCR) using a set of sixty-five pairs of primers (Appendix 1) [11]. The standard PCR reaction (50 μl) contained 80 ng of genomic DNA, 10 pM each primer, 25 μl TaqMix and water. The thermal profile included denaturation for 5 min, followed by 36 cycles of denaturation (30 s at 95 °C), annealing (30 s at 54 °C-60 °C), and extension (45 s at 72 °C), followed by a final step of extension (10 min at 72 °C). The PCR products were sequenced on an ABI3730 Automated Sequencer (PE Biosystems, Foster City, CA).
Results
Clinical findings
We identified a five-generation family with sixteen confirmed individuals affected with isolated EL (Figure 2). Eleven patients’ blood samples were collected. All participated patients have received surgery. The onset age of patients with EL was around 15 to 30 years. Patient II-1 was a seventy-eight-year old woman who, except for the EL, had no other Mafan-related disorders. The stature of all the patients in this family was shorter than 1.70 m. We did not find the cardiovascular system, the skeleton system, and other Marfan-related syndromes in the collected ten patients.
Figure 2.
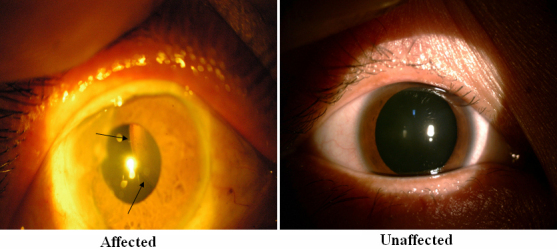
Slit lamp photograph showing dislocation of the patient’s lens (III:14 in Figure 1). Arrows indicate the edge of the lens.
Linkage and haplotype analysis
We obtained positive logarithm-of-odds (LOD) scores with markers at 15q (Table 1). A maximum positive LOD score of 2.61 at θ=0.00 was obtained by marker D15S126. The haplotype shows complete cosegregation in all the 9 affected individuals who were studied (Figure 1).
Table 1. Result of linkage analysis.
|
|
|
LOD scores at θ= |
|||||
|---|---|---|---|---|---|---|---|
| Marker | Physical distance (M) | 0.00 | 0.01 | 0.10 | 0.20 | 0.30 | 0.40 |
| D15S992 |
48.8 |
2.23 |
2.19 |
1.82 |
1.38 |
0.89 |
0.37 |
| MTS-1 |
48.9 |
0.12 |
0.12 |
0.10 |
0.08 |
0.05 |
0.03 |
|
FBN1 |
48.7–48.9 |
|
|
|
|
|
|
| D15S1082 |
49.0 |
2.06 |
2.02 |
1.67 |
1.26 |
0.80 |
0.33 |
| D15S126 | 49.3 | 2.61 | 2.57 | 2.16 | 1.66 | 1.10 | 0.48 |
Mutation analysis
Sequencing of FBN1 shows a heterozygous C>T change in the affected individuals at nucleotide position c.2920 (Figure 3). This nucleotide change results in a cysteine substitution for a highly conserved arginine at codon 974 (p.R974C; Figure 4). All affected family members presented this nucleotide change. Haplotyping shows individual IV-15 was affected, while individual V-1 was unaffected. Mutation detection is consistent with haplotyping of those two members. This nucleotide substitution was not observed in 50 control subjects from the same ethnic background.
Figure 3.
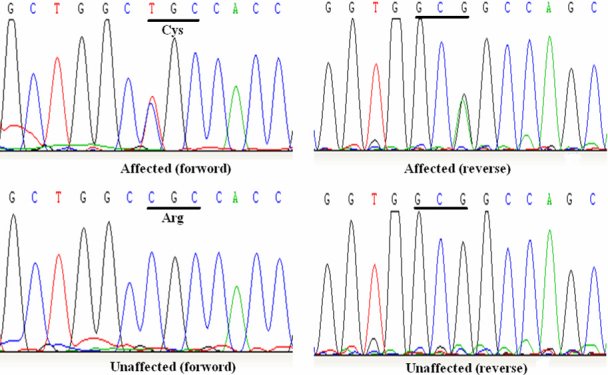
DNA sequence analysis of FBN1 in unaffected and affected individuals. A heterozygous change C>T at codon 974 (CGC-C/TGC), resulting in the substitution of arginine to cysteine (p.R974C) in the affected individuals.
Figure 4.
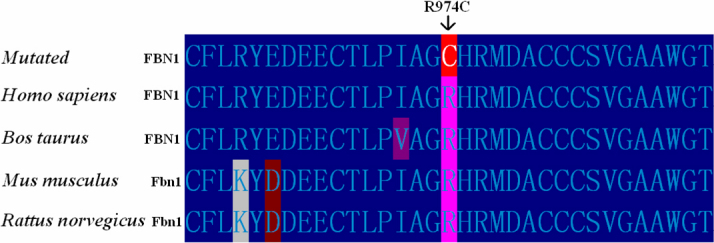
The alignment of amino acids of FBN1 from Bos taurus, Rattus norvegicus, Mus musculus, and Homo sapiens. The Arg974 residue is highly conserved during evolution.
Discussion
In the present study, we identified a novel mutation (R974C) in FBN1 from a Chinese family with isolated EL. This missense variation is found in all the affected family members. The mutation leads to a cysteine substitution for highly conserved arginine in the 8-Cys repeat latent transforming growth factor-beta binding protein (LTBP) motif encoded by FBN1 exon 25.
To date, over 600 mutations in FBN1 have been reported. The relationship between the genotype and the phenotype is still not clear [12]. Some information is concluded from reported materials by researchers [13]. For instance, mutations in exons 24–32 of FBN1 are usually associated with a severe form of Marfan syndrome (MFS), called neonatal MFS [13]. Mutations of cysteine substitution are usually associated with isolated EL [14]. On the another hand, some facts obstruct the conclusion, including mutations in the exons 24–32 of FBN1 caused MFS and isolated EL, and the same mutation can cause MFS and isolated EL in different families. Since these conclusions were obtained from the clinical materials, but not from the pathogenic mechanism, we can not correlate the genotype and the phenotype from clinical symptoms only. So, we need to explain the pathogenic mechanism on the molecular biologic level.
Fibrillin-1 is a large (320 kDa) multidomain glycoprotein that is a main component of the 10 to 12 nm sized extracellular microfibrils found in a wide range of tissues. Fibrillin-1 is considered to be important for elastogenesis, elasticity, and homeostasis of elastic fibers [15,16].
In the previous experiments, researchers found that fibrillin-1 was not only a structural component of extracellular matrix but also was a mediator of the transforming growth factor-beta (TGFβ) signaling pathway [17,18]. Mutations that influence the TGFβ signaling pathway are believed to cause severe multiple defects [19].
Through a literature search, eighteen mutations of FBN1 causing EL were found [8-10,14,20-23]. In these mutations, three mutations of FBN1 can cause EL and MFS in different families. Mutations of cysteine substitution were found in fifteen families [8-10].
In this study, we found the extra created cysteine in the LTBP motif only results in isolated EL. This suggests that the correct cysteine localization and disulfide binding play an important role in the structural integrity of the lens suspensory ligaments. The change has no or slight effect in the extracellular matrix of other organs.
It is reported that some specified mutation can cause MFS and isolated EL in different families. This may be influenced by genetic or environmental modifiers.
In summary, we report a novel conserved arginine to cysteine substitution in exon 25 of FBN1 which is associated with isolated EL. Our results further expanded the mutation spectrum of FBN1 and provided useful materials to the further researches.
Appendix 1. Table 1. FBNI primers and conditions for PCR.
To access the data, click or select the words “Appendix 1.” This will initiate the download of a compressed (pdf) archive that contains the file.
References
- 1.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–26. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Kainulainen K, Pulkkinen L, Savolainen A, Kaitila I, Peltonen L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N Engl J Med. 1990;323:935–9. doi: 10.1056/NEJM199010043231402. [DOI] [PubMed] [Google Scholar]
- 3.Dietz HC, Pyeritz RE, Hall BD, Cadle RG, Hamosh A, Schwartz J, Meyers DA, Francomano CA. The Marfan syndrome locus: confirmation of assignment to chromosome 15 and identification of tightly linked markers at 15q15-q21.3. Genomics. 1991;9:355–61. doi: 10.1016/0888-7543(91)90264-f. [DOI] [PubMed] [Google Scholar]
- 4.Magenis RE, Maslen CL, Smith L, Allen L, Sakai LY. Localization of the fibrillin (FBN) gene to chromosome 15, band q21.1. Genomics. 1991;11:346–51. doi: 10.1016/0888-7543(91)90142-2. [DOI] [PubMed] [Google Scholar]
- 5.Lee B, Godfrey M, Hori H, Mattei MG, Sarfarazi M, Tsipouras P, Ramirez F, Hollister DW. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991;352:330–4. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- 6.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, Stetten G, Meyers DA, Francomano CA. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–9. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 7.Lönnqvist L, Child A, Kainulainen K, Davidson R, Puhakka L, Peltonen L. A novel mutation of the fibrillin gene causing ectopia lentis. Genomics. 1994;19:573–6. doi: 10.1006/geno.1994.1110. [DOI] [PubMed] [Google Scholar]
- 8.Yu R, Lai Z, Zhou W, Ti DD, Zhang XN. Recurrent FBN1 mutation (R62C) in a Chinese family with isolated ectopia lentis. Am J Ophthalmol. 2006;141:1136–8. doi: 10.1016/j.ajo.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 9.Deng T, Dong B, Zhang X, Dai H, Li Y. Late-onset bilateral lens dislocation and glaucoma associated with a novel mutation in FBN1. Mol Vis. 2008;14:1229–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Vanita V, Singh JR, Singh D, Varon R, Robinson PN, Sperling K. A recurrent FBN1 mutation in an autosomal dominant ectopia lentis family of Indian origin. Mol Vis. 2007;13:2035–40. [PubMed] [Google Scholar]
- 11.Nijbroek G, Sood S, McIntosh I, Francomano CA, Bull E, Pereira L, Ramirez F, Pyeritz RE, Dietz HC. Fifteen novel FBN1 mutations causing Marfan syndrome detected by heteroduplex analysis of genomic amplicons. Am J Hum Genet. 1995;57:8–21. [PMC free article] [PubMed] [Google Scholar]
- 12.The FBN1 Gene Mutations Database http://www.umd.be/FBN1/
- 13.Faivre L, Collod-Beroud G, Loeys BL, Child A, Binquet C, Gautier E, Callewaert B, Arbustini E, Mayer K, Arslan-Kirchner M, Kiotsekoglou A, Comeglio P, Marziliano N, Dietz HC, Halliday D, Beroud C, Bonithon-Kopp C, Claustres M, Muti C, Plauchu H, Robinson PN, Adès LC, Biggin A, Benetts B, Brett M, Holman KJ, De Backer J, Coucke P, Francke U, De Paepe A, Jondeau G, Boileau C. Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet. 2007;81:454–66. doi: 10.1086/520125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adès LC, Holman KJ, Brett MS, Edwards MJ, Bennetts B. Ectopia lentis phenotypes and the FBN1 gene. Am J Med Genet. 2004;126A:284–9. doi: 10.1002/ajmg.a.20605. [DOI] [PubMed] [Google Scholar]
- 15.Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai LY, Keene DR, Glanville RW, Bächinger HP. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991;266:14763–70. [PubMed] [Google Scholar]
- 17.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-b activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–11. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGFb1. J Cell Biol. 2007;176:355–67. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, Ihara M, Kinoshita A, Yoshiura K, Junien C, Kajii T, Jondeau G, Ohta T, Kishino T, Furukawa Y, Nakamura Y, Niikawa N, Boileau C, Matsumoto N. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet. 2004;36:855–60. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin C, Yao K, Jiang J, Tang X, Shentu X, Wu R. Novel FBN1 mutations associated with predominant ectopia lentis and marfanoid habitus in Chinese patients. Mol Vis. 2007;13:1280–4. [PubMed] [Google Scholar]
- 21.Sui RF, Wei HB, Zhao JL, Hu SY, Wang B, Huang SZ, Dong M. Novel mutation of fibrillin 1 gene cause ectopia lentis in a Chinese family. Zhonghua Yan Ke Za Zhi. 2004;40:828–31. [PubMed] [Google Scholar]
- 22.Comeglio P, Johnson P, Arno G, Brice G, Evans A, Aragon-Martin J, da Silva FP, Kiotsekoglou A, Child A. The importance of mutation detection in Marfan syndrome and Marfan-related disorders: report of 193 FBN1 mutations. Hum Mutat. 2007;28:928. doi: 10.1002/humu.9505. [DOI] [PubMed] [Google Scholar]
- 23.Loeys B, Nuytinck L, Delvaux I, De Bie S, De Paepe A. Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med. 2001;161:2447–54. doi: 10.1001/archinte.161.20.2447. [DOI] [PubMed] [Google Scholar]



