Abstract
Background
Despite funding constraints for treatment programmes in Africa, the costs and economic consequences of routine laboratory monitoring for efficacy and toxicity of antiretroviral therapy (ART) have rarely been evaluated.
Methods
Cost-effectiveness analysis was conducted in the DART trial (ISRCTN13968779). Adults in Uganda/Zimbabwe starting ART were randomised to clinically-driven monitoring (CDM) or laboratory and clinical monitoring (LCM); individual patient data on healthcare resource utilisation and outcomes were valued with primary economic costs and utilities. Total costs of first/second-line ART, routine 12-weekly CD4 and biochemistry/haematology tests, additional diagnostic investigations, clinic visits, concomitant medications and hospitalisations were considered from the public healthcare sector perspective. A Markov model was used to extrapolate costs and benefits 20 years beyond the trial.
Results
3316 (1660LCM;1656CDM) symptomatic, immunosuppressed ART-naive adults (median (IQR) age 37 (32,42); CD4 86 (31,139) cells/mm3) were followed for median 4.9 years. LCM had a mean 0.112 year (41 days) survival benefit at an additional mean cost of $765 [95%CI:685,845], translating into an adjusted incremental cost of $7386 [3277,dominated] per life-year gained and $7793 [4442,39179] per quality-adjusted life year gained. Routine toxicity tests were prominent cost-drivers and had no benefit. With 12-weekly CD4 monitoring from year 2 on ART, low-cost second-line ART, but without toxicity monitoring, CD4 test costs need to fall below $3.78 to become cost-effective (<3xper-capita GDP, following WHO benchmarks). CD4 monitoring at current costs as undertaken in DART was not cost-effective in the long-term.
Conclusions
There is no rationale for routine toxicity monitoring, which did not affect outcomes and was costly. Even though beneficial, there is little justification for routine 12-weekly CD4 monitoring of ART at current test costs in low-income African countries. CD4 monitoring, restricted to the second year on ART onwards, could be cost-effective with lower cost second-line therapy and development of a cheaper, ideally point-of-care, CD4 test.
Introduction
It is essential to evaluate the economic impact of antiretroviral therapy (ART) programmes using a public health approach [1], to guide policymakers how best to prioritise scarce resources for HIV/AIDS care and treatment in the public sector. This is particularly important with the current financial crisis, threats to sustained HIV programme funding and the many individuals still in urgent need of first and increasingly second-line treatment, particularly in South and Eastern Africa [2]–[4].
Several studies have evaluated the clinical benefit [5]–[11] and cost-effectiveness [12]–[18] of different strategies for monitoring the safety and efficacy of ART. Results have been contradictory; for example, in one modelling study of ART in resource-limited settings [15], monitoring with viral loads or CD4 cell counts compared with clinical assessment alone led to only modest benefits in patient survival and drug resistance. Conversely, a recent modelling study for Côte d’Ivoire, a lower-middle income country with a GDP per capita of $1071, found both monitoring strategies cost-effective [17]. A recently published trial from Uganda concluded that, compared with clinical monitoring alone, routine CD4 count monitoring is considerably more cost effective alone than combined with viral load monitoring [18].
Here we present a cost-effectiveness analysis, from the public healthcare perspective, of Laboratory and Clinical Monitoring (LCM) compared with Clinically-Driven Monitoring (CDM) of ART alongside a randomised controlled trial conducted in Uganda and Zimbabwe [11].
Methods
Ethics Statement
The DART trial was approved by Research Ethics Committees in Uganda, Zimbabwe and the UK, and all enrolled participants gave individual informed consent.
Effectiveness and resource utilisation data were collected in the Development of Antiretroviral Therapy (DART) trial, conducted from 2003–2008 at 3 centres in Africa: Entebbe and Kampala (plus satellite), Uganda; Harare, Zimbabwe. The trial compared LCM (routine 12-weekly laboratory monitoring: CD4 counts for efficacy; haematology and biochemistry for toxicity) with CDM (CD4 counts never available to clinicians) in ART-naïve adults (≥18 years) starting therapy with symptomatic HIV disease and CD4<200 cells/mm3. Additional diagnostic investigations and monitoring tests (except CD4 in CDM) could be requested according to clinical judgement at routine visits, patient-initiated visits or hospital admission. Concomitant medicines were provided. The trial demonstrated no impact of routine toxicity tests on any adverse event outcome; and a small, statistically significant benefit of CD4 monitoring on HIV disease progression and death from the second year onwards [11]. DART also showed that regular fixed duration ART interruptions to reduce drug costs were harmful [19]; and that cotrimoxazole prophylaxis on ART significantly reduced mortality [20].
Mean total cost per patient by group was estimated using intention-to-treat. Research component costs were separated from those for ART delivery/monitoring and excluded from analysis. Individual patient data on healthcare resource utilisation and health outcomes of all DART participants were analysed, except for concomitant medications which were analysed by group. Because of the large number of different medications used over the 6 year trial, only those used by >30% of patients, or where there was difference of >3% in the proportion using a medication between the two randomised groups were accounted for. Healthcare utilisation outside of the trial (health clinic visits, hospitalisations and concomitant medications) were elicited at every 4-weekly visit and included. Unit costs of CD4 counts, biochemistry, haematology, clinic and health centre visits were estimated in primary economic costing analyses [21] for each centre, using costs from the main Kampala centre for its satellite. Staff costs per routine and extra patient-initiated visits at DART clinics were estimated in a time and motion study at the centres (patient visits: Entebbe, n = 152; Kampala, n = 171; Harare n = 175). Financial information was obtained from trial centre records. ART costs were based on preferential prices of non-generic drugs for sub-Saharan Africa in 2008 [22]. Country-specific costs for hospital bed-days were obtained from WHO [23].
All local prices were converted to 2008 US$ using exchange rates over the trial period (e.g. $1US$ = 1,900 Uganda Shillings; 1US$ = 777,500,000 Zimbabwean dollars in 2008) and the US Consumer Price Index [24]. Official daily Zimbabwean rates were applied to essential medical equipment and supplies, and market rates from independent sources to staff salaries and locally acquired items. No adjustment for purchasing power parity was attempted given the lack of comparable standards in price level information sources.
Survival benefits were estimated from the difference in the area under the Kaplan-Meier [25] survival curve between the two groups up to the trial end. Quality-adjusted life years (QALYs) were calculated as the weighted total time spent in four possible health states during the trial, with each state’s utility as its weight. Health states were defined by performance scale assessment (1 = asymptomatic; 2 = symptomatic; 3 = bed-ridden <50% in the previous month; 4 = bed-ridden >50% in the previous month) during 12-weekly routine doctor visits and currently-ongoing WHO stage 3/4 events. Hence, health states, unlike WHO staging, may improve from baseline. Utilities for estimating QALYs were obtained from a longitudinal sub-study (n = 275) conducted at the DART Entebbe centre (see Supporting Information S1), relative to the utility of the asymptomatic HIV-infected state. This state was assumed to be optimal, thus having a value of 1, to HIV-infected patients, and 0.81, to the general population [26].
Incremental cost effectiveness ratios (ICERs) defined as the ratio of incremental mean cost to mean survival or QALY gained, were estimated. Costs and health benefits occurring after 12 months were discounted at 3% per annum, and adjusted for censoring due to drop-out using the method of Li [27]. 95% confidence intervals for differences in means and ICERs were estimated using bootstrap percentile methods [28]. Sensitivity analyses included generic drug prices (WHO [29]), reduced prices for second-line therapy [22], laboratory monitoring costs from the literature [30], and National Referral Laboratories prices.
A Markov model of transitions between discrete CD4 states and death was used to extrapolate outcomes post-DART, from 6 to 25 years after ART initiation. An individual alive at trial closure would be in one of three states, defined by CD4 <100, 100–200, and >200 cells/mm3, qualified by first-line or second-line ART due to expected differences in costs and benefits. After every 12-week period, the participant would have moved to a different state, remained in the initial state or died. Overall 97.7% of expected CD4 counts before end of trial/death/loss to follow-up were available. For routine CD4 tests, clinic visits and ART use, full compliance with the DART follow-up schedule was assumed. Other costs and QALYs were analysed as random events, divided into 12-week periods and matched to latest CD4 count range to derive estimates of cost and benefit (‘pay-offs’) for Markov states as a function of latest CD4 count and group. In this extrapolation, only cotrimoxazole prophylaxis and treatment and antimalarial drugs, were included as concomitant medications; the former were the only concomitant medications whose use varied significantly over years on ART and the latter were the only medications whose use varied significantly across centres. The analysis accounted for background mortality risks derived from counterfactual life tables for Uganda that exclude the effect of HIV/AIDS (see Supporting Information S2).
ICERs <3x per capita GDP were used as an indication of cost-effectiveness, following benchmarks suggested by WHO [31]. Under a fixed, limited healthcare budget, an alternative threshold applies, equal to the ICER of CDM versus the ‘no ART’ option (see Supporting Information S2). The probability of cost-effectiveness over different threshold values, i.e., the cost-effectiveness acceptability curve, was derived from probabilistic sensitivity analysis.
Results
In DART, 3316 (1660 LCM; 1656 CDM) HIV-infected, treatment-naïve, symptomatic African adults started ART (65% female; median (IQR) age 37 (32–42); CD4 86 (31–139) cells/mm3) and were followed for median 4.9 (4.5–5.3) years. Unit costs are presented in Table 1. The annual cost of the first-line regimens used in DART was US$309–585; switching to second-line regimens resulted in approximately three-fold higher ART costs.
Table 1. Unit costs in 2008 US$.
| Item | Observed | Source and Date | Sensitivity Analysis | Source and Date | ||
| First line therapy annual cost* | ||||||
| ZDV+3TC+TDF | $349.12 | Midpoint of MSF prices, 2009 [18] | $290.72 | Generic prices (from WHO)2008 [25] | ||
| ZDV+3TC+NVP | $308.79 | $165.64 | ||||
| ZDV+3TC+ABC | $585.46 | $441.80 | ||||
| Second-line therapy annual cost* | ||||||
| ddI+EFV+KAL | $1032.59 | Midpoint of MSF prices, 2009 [18] | $874.18 | Lower bound of MSF prices,2009 [19] | ||
| ddI+NVP+KAL | $967.98 | $763.22 | ||||
| ddI+ABC+KAL | $1244.65 | $1062.52 | ||||
| CD4 cell counts | $15.79 | Entebbe | Micro-costing– centre specific, 2008 | $8.82 | Rosen, Long and Sane,2008 [26] | |
| $10.07 | JCRC | |||||
| $18.82 | UZCRC | |||||
| Haematology panel tests | $6.46 | Entebbe | $5.30 | National Referral Laboratories, 2008 | ||
| $7.13 | JCRC | |||||
| $12.32 | UZCRC | |||||
| Biochemistry panel tests | $11.66 | Entebbe | $29.50 | |||
| $10.67 | JCRC | |||||
| $12.23 | UZCRC | |||||
| DART visits | $4.08 | Entebbe | N/A | N/A | ||
| $3.30 | JCRC | |||||
| $8.38 | UZCRC | |||||
| Health Centre visits | $3.26 | Entebbe | N/A | N/A | ||
| $8.84 | JCRC | |||||
| $8.73 | UZCRC | |||||
| Per diem hospital cost† | $25.57 | Adam, Evans & Murray, 2003 [17] | N/A | N/A | ||
| Other diagnostic investigations and tests‡ | $6.26 | National Referral Laboratories, 2008 | N/A | N/A | ||
Annual drug costs of keeping a patient on this specific combination of drugs continuously for 365 days.
Per diem hospital costs from Adam T, Evans DB and Murray CJL, 2003 were reflated to 2008 USD($).
Other unit costs for diagnostic investigations (including X rays, TB smears, CSF analysis etc) and non-routine biochemistry and haematology tests are not listed here but were obtained from National Referral Laboratories prices list.
Healthcare resources consumed by patients, overall costs of resource utilisation and cost differences between the groups are shown in Table 2. The overall mean incremental cost of LCM is US$765 [95%CI 685,845] per patient, a 31% increase over CDM. The drivers of cost differences between LCM and CDM are, in order of magnitude, routine 12-weekly toxicity monitoring, CD4 monitoring, and second-line drugs. There are small offsets with lower costs of clinically-indicated diagnostic investigations, first-line therapy and fewer days in hospital in LCM, likely consequences of earlier switch to second-line therapy in LCM [11].
Table 2. Healthcare resource utilisation (US$2008).
| Healthcare resource utilisation | Observed total over trial period per patient (median 4.9 years) | Cost difference LCM – CDM * | |
| LCM | CDM | ||
| First-line therapyNumber of days Mean (SD)Costs Mean (SD) | 1464.96 (555)1451 (603) | 1481.90 (545)1470 (603) | −19 |
| Second-line therapyNumber of days Mean (SD)Costs Mean (SD) | 152.99 (355)406 (964) | 102.89 (270)265 (718) | +141 |
| CD4 monitoringNumber of CD4s Mean (SD)Costs Mean (SD) | 19.81 (6)288 (121) | 0 (0)0 (0) | +288 |
| Routine 12-weekly haematology toxicity monitoringNumber of haematology tests Mean (SD)Costs Mean (SD) | 21.58 (7)183 (81) | 0 (0)0 (0) | +183 |
| Routine 12-weekly biochemistry toxicity monitoringNumber of CD4s biochemistry tests Mean (SD)Costs Mean (SD) | 20.93 (6)227 (69) | 0 (0)0 (0) | +227 |
| Clinically indicated diagnostic investigations, other tests outsideroutine visits (both groups) or monitoring tests (except CD4)requested for clinical reasons at routine visits (CDM)Number of diagnostic investigationsCost Mean (SD) | 6.56 (11)41 (81) | 10.49 (18) †66 (170) | −25 |
| DART clinic visitsNumber of visits Mean (SD)Costs Mean (SD) | 61.18 (19)414 (195) | 60.12 (20)405 (197) | +9 |
| Health centre visitsNumber of visits Mean (SD)Costs Mean (SD) | 7.73 (7)53 (56) | 7.88 (8)55 (64) | +2 |
| Nights in hospitalNumber of visits Mean (SD)Costs Mean (SD) | 5.51 (13)141 (347) | 6.77 (16)177 (444) | −36 |
| Concomitant medications | 46 | 48 | −2 |
| Overall mean total costs (SD)[95% confidence interval] | 3249 (1246) | 2485 (1095) | 765[685,845] ‡ |
Discrepancies in totals and differences are due to rounding.
1.47 (3) and 0.86 (3) of the total were standard haematology or biochemistry tests respectively performed at routine doctor visits as part of the trial for CDM but requested for clinical management.
95% CIs were estimated with bootstrapping percentile method.
Mean survival benefit with LCM was 0.112 life-years (41 days). The unadjusted incremental cost per life year gained (LYG) was US$6819, with 95% CI from US$3282 to the dominated outcome where it produces lower survival benefits alongside higher costs than CDM (Table 3a). After adjusting for censoring due to drop-out and discounting costs and benefits occurring after year one, the ICER per LYG was $7386 [95%CI 3277,dominated]). The incremental cost per QALY gained was $7793 [4442,39179] using patients’ utility values, and $9621 [5484,48359] adjusting utilities to the general population’s values. Across DART centres, total mean costs of LCM and CDM ranged from $2647–3358 and $1930–2490 respectively, with adjusted discounted incremental costs of $8420–12340 per QALY gained adjusting utilities to the general population’s values (data not shown).
Table 3. Main and sensitivity analyses: life years gained, QALYs, costs and ICERs.
| LCMN = 1656 | CDMN = 1660 | DifferenceLCM – CDM | |
| (a) MAIN RESULTS | |||
| Overall survival days* [95% CI] (undiscounted) | 2000 | 1959 | +41 [−15,+95] |
| Overall survival years* [95% CI] (undiscounted) | 5.48 | 5.36 | +0.112 [−0.04,+0.26] |
| Overall mean total costs in US$ [95% CI] (unadjusted) | 3249 | 2485 | +765 [685,845] |
| Overall QALYs – patients’ values | 3.351 | 3.255 | +0.096 |
| Overall QALYs – general population’s values | 2.714 | 2.636 | +0.078 |
| Overall mean total costs in US$ [95% CI] | 3146 | 2398 | +748 [+679,+818] |
| Incremental Cost per Year Gained in US$ (unadjusted) [95% CI] | 6819 [3282, Dominated] | ||
| Incremental Cost per Life Year Gained in US$ [95% CI] | 7386 [3277, Dominated] | ||
| Incremental Cost per QALY Gained in US$ – patients’ values [95% CI] | 7793 [4442,39179] | ||
| Incremental Cost per QALY Gained – in US$ general population’s values [95% CI] | 9621 [5484,48359] | ||
| (b) SENSITIVITY ANALYSIS | |||
| Lower bound prices second-line therapy * | |||
| Mean second-line therapy costs in US$ (SD) | 357 (845) | 236 (637) | +121 |
| Overall mean total costs in US$ (unadjusted) [95% CI] | 3194 | 2449 | +745 [669,821] |
| Incremental Cost per Life Year Gained in US$ (unadjusted) [95% CI] | 6643 [3307, Dominated] | ||
| Incremental Cost per Life Year Gained in US$ [95% CI] | 6443 [2891, Dominated] | ||
| Incremental Cost per QALY Gained in US$ – patients’ values [95% CI] | 6798 [3917, 30501] | ||
| Incremental Cost per QALY Gained in US$ – general population’s values [95% CI] | 8392 [4836,37656] | ||
| National referral laboratory prices * | |||
| Mean CD4 monitoring costs in US$ (SD) | 175 (57) | 0 (0) | +175 |
| Standard 12-weekly haematology biochemistry toxicity monitoring (SD) | 699 (216) | 23 (65) | +676 |
| Overall mean total costs in US$ (unadjusted) [95% CI] | 3425 | 2493 | +932 [+851,+1013] |
| Incremental Cost per Life Year Gained in US$ (unadjusted) [95% CI] | 8318 [3876, Dominated] | ||
| Incremental Cost per Life Year Gained in US$ [95% CI] | 8990 [4160, Dominated] | ||
| Incremental Cost per QALY Gained in US$ – patients’ values [95% CI] | 9485 [5334, 47957] | ||
| Incremental Cost per QALY Gained in US$ – general population’s values [95% CI] | 11710 [6586, 59206] | ||
See column sensitivity analysis in Table 1.
In sensitivity analyses with prices for low-cost second-line drugs, ICERs reduced: the adjusted incremental cost per LYG was $6443 [2891, Dominated]; and per QALY was $6798 [3917,30501] using patients’ values and $8392 [4836,37656] using general population values (Table 3b). Generic drug prices would reduce ICERs by 0.3%, since the resulting rise in incremental LCM costs of first-line consumption is offset by reduced additional costs of second line therapy (results not shown). Incremental costs per LYG and QALY increased in the sensitivity analysis of laboratory costs because the National Referral Laboratory biochemistry panel cost considerably more than in DART, outweighing lower CD4 and haematology costs. All these results are above the (weighted average) threshold of cost-effectiveness for Uganda and Zimbabwe of $1,200 for 2008 [24].
Scenario analyses were undertaken to explore the cost-effectiveness of monitoring strategies not directly evaluated in DART. In a limited CD4 monitoring scenario without routine toxicity monitoring, 12-weekly CD4 monitoring was restricted to the second year on ART onwards (as outcomes between groups differed only from the third year on ART following differences in rates of switching to second-line ART from the second year [11]). This generated an adjusted discounted incremental cost per LYG of US$2997 [95%CI 1333,dominated], US$3162 [1815,14178] and US$3903 [2241,17504] per QALY gained based on patient and general population values respectively. If low second-line therapy costs are also assumed, the cost per CD4 count needs to fall below $3.78 to become cost-effective in this trial-based analysis.
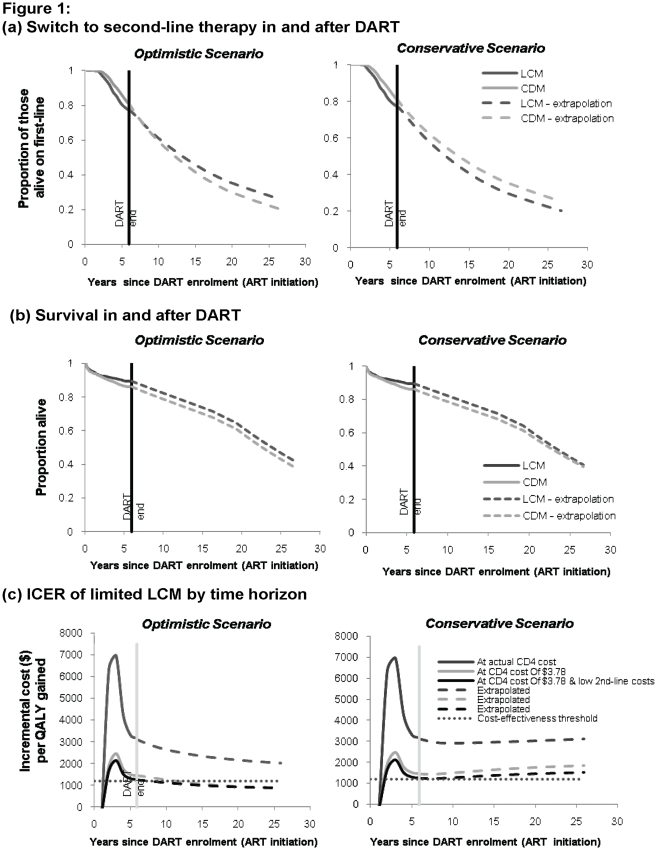
ART outcomes were extrapolated long-term by applying predicted transition probabilities corresponding to the last two years of DART (see Supporting Information S2): at CD4 >200 cells/mm3, LCM has a lower switch rate than CDM, and vice versa at low (<100 cells/mm3) CD4 levels. In our optimistic scenario, this differential was applied; whereas in the conservative scenario both groups were assigned the same probability of switching to second-line at high CD4 counts. Long-term differences emerge: from the third year after DART onwards, a higher proportion of CDM participants would be on second-line therapy in the optimistic scenario because of the greater switch rate at high CD4 levels where most time was spent; whereas in the conservative scenario, a higher proportion of LCM participants continue to have switched to second-line (Figure 1; top panel). After 25 years of ART, 60% of the participants would have died. Only in the optimistic scenario is the survival benefit of LCM maintained (Figure 1; middle panel). This continued survival differential is due to second-line therapy having higher mortality risks than first-line, and more CDM participants on second-line therapy in the long-term optimistic scenario.
Figure 1. Long-term extrapolation of outcomes and ICERs.
The ICER per QALY gained over time is shown for the limited laboratory monitoring (CD4 from the second year on ART only) scenario in Figure 1, bottom panels under three cost conditions: a) CD4 cost observed in DART ($14.46, weighted average across centres); b) hypothetical, CD4 test ($3.78) and c) hypothetical CD4 test and lowest observed second-line costs ($763; see Table 1). The scenarios employ patients’ utility values. In the optimistic scenario, ICERs decline with time on therapy, driven by the cumulative gap in survival and second-line therapy use between groups, although this effect would be dampened under a discount rate higher than 3% (not shown). In the optimistic scenario condition (c), the cost per CD4 count needs to fall to <$6.75 for the ICER to remain under $1200. Under a fixed budget the maximum cost-effective value is $5.81 (see Supporting Information S2).
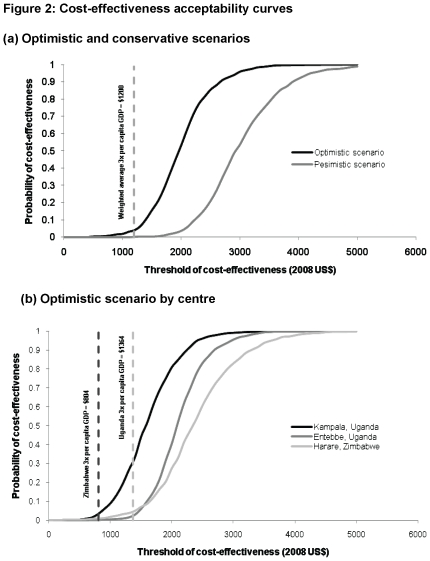
Figure 2 shows the probability of cost-effectiveness as a function of the monetary threshold per QALY gained under condition (a) above. In the conservative scenario, there is zero probability of cost-effectiveness at the US$1200 threshold: in the optimistic scenario this probability increases to less than 5% (Figure 2a). These aggregate results mask variation between centres stemming from cost and monetary threshold differences. In the optimistic scenario, limited CD4 monitoring had a <4% probability of cost-effectiveness in Entebbe and Harare at country-specific thresholds (unit cost of CD4 count tests) of $1364 ($15.79) and $804 ($18.82) respectively, and 30% probability of cost-effectiveness in Kampala with a CD4 unit cost of $10.07 (Figure 2b). The country-specific cost-effectiveness thresholds were achieved at test costs of $7.87, $7.43, and $3.39, respectively, in the three centres over the optimistic extrapolation. Under fixed limited budgets, the respective thresholds ($1044, $1088, and $1169) are met at CD4 test costs of $5.15, $5.50 and $7.45.
Figure 2. Cost-effectiveness acceptability curves.
Discussion
Here we report the costs and economic outcomes associated with routine laboratory monitoring of ART using individual patient data from Uganda and Zimbabwe in the DART trial. Routine toxicity monitoring had no additional benefits on any adverse event outcome and was more costly than clinically driven toxicity monitoring. While effective, routine (12-weekly) laboratory CD4 count monitoring was more costly than monitoring without CD4 counts. Even ignoring routine toxicity monitoring, based on the ICERs derived for life-years or QALYs gained, the CD4 monitoring strategy used in DART is beyond the cost-effective threshold of <3x per capita GDP, which is the WHO benchmark used for assessing cost-effectiveness of interventions in low-income sub-Saharan African countries [30].
Since DART publication, three other randomised trials comparing different ways to monitor adults on ART in low and middle income countries have been presented or published: the HBAC trial in Uganda [11], [18], the PHPT-3 trial in Thailand [32] and the ANRS/ESTHER trial in Cameroon [33]. Although different in design, the two trials that included a clinical monitoring arm (Uganda, Cameroon) both show small but important benefits for CD4 laboratory monitoring and thus support the key observation from DART on which this cost-effectiveness analysis is predicated. Interestingly, HBAC and PHPT-3, the two studies that compared viral load with CD4 monitoring, have both failed to show any additional benefit on clinical outcome from virological monitoring, either in place of or additional to CD4 monitoring [32], [33].
According to our primary costing studies and models, we estimated that the current costs of CD4 tests need to drop to below USD$3.78 for 12-weekly CD4 monitoring to be cost-effective. Extrapolating over the longer-term under the fixed budget rule (instead of the arbitrary, conventional threshold used to approximate the value of a life year in optimal health or without disability [31]) that the option with most total benefits be selected suggested laboratory monitoring at unit costs of USD$5-7 would be cost-effective in a population starting ART with relatively severe immunodeficiency as in DART. In patients starting ART at higher CD4 counts, more CD4 counts would need to be done to identify failure, and so incremental cost effectiveness ratios would likely be higher, requiring lower unit cost of CD4 counts to achieve cost-effectiveness. Costs could reduce either through direct kit costs falling, or through efficiency gains for example with new technologies such as point-of-care (POC) tests. Such technology is now being developed and evaluated: if costs fall below $3–4 per test and the devices can be used at health-centre level then POC could become a very attractive option for routine ART efficacy monitoring [34]. Another option would be to perform CD4 monitoring less frequently than 12-weekly. However, as excess morbidity/mortality in CDM appeared to mainly be a consequence of later switch to second-line, less frequent CD4 monitoring could also be less effective, reducing cost savings.
The long-term economic value of routine CD4 monitoring is inversely related to the costs of second-line therapy: the lower the costs of second-line therapy, the greater the likelihood of CD4 monitoring being cost-effective. Of the three other trials of ART monitoring strategies,only the Uganda HBAC study so far has conducted cost-effectiveness analysis [18]. It reports an ICER of $174 per disability-adjusted-life-year (DALY) for CD4/clinical vs clinical monitoring alone of ART [18], an order of magnitude lower than the ICER reported here. This difference between our findings is initially perplexing, particularly as participants receiving CD4 or viral load monitoring also received full blood counts; of note, toxicity outcomes were not reported in HBAC [9]. However, the substantially increased cost of second-line versus first-line ART is likely to have contributed to their finding, as, in contrast to DART, more participants (n = 17) in the clinical monitoring alone group switched to second-line, compared to those receiving CD4 (n = 4) or CD4 and viral load (n = 7) monitoring.
DART and HBAC used different clinical thresholds to determine first-line failure and thus trigger switch to second-line ART. The most important difference is the inclusion of single WHO 3 events in the HBAC clinical criteria for switch (weight loss, unexplained fever, diarrhoea, oral candidiasis), which were not included in WHO guidelines [35], tend to be non-specific and are most associated with high CD4 counts/suppressed viral load [36]; in contrast clinical switch in DART was based on WHO 4 events alone (as per WHO guidelines [35]). Thus the cost differential between CD4 and clinical monitored groups will be exaggerated in HBAC by increased unnecessary premature switching in the clinical monitoring group, thus reducing the ICER. Given the influence of second-line costs on cost-effectiveness, and given that ART will be given life-long, it is unfortunate that HBAC was relatively underpowered to address this critical issue, with only 28 switches in total (as opposed to 675 in DART with more in the LCM arm from the second year of the trial).
Furthermore, the median of only 3 years of follow-up in HBAC, compared to 5 years in DART, inevitably has implications for the accuracy of longer term predictions based on observed data. Although our model did not adjust for the effect of past or ongoing opportunistic infections on the risk of death over and above that captured by current CD4 count, any resulting bias favouring clinical monitoring is likely to be reversed by the assumption of higher switch rates with CDM to second-line therapy at high CD4 counts throughout the period after DART.
To be eligible for DART, all potential participants underwent CD4 testing and needed to have CD4<200 cells/mm3 to enter the trial. Our analysis was therefore not able to consider the costs and benefits of CD4 testing for ART eligibility [4], [13], [16]. Initiating ART earlier, with higher CD4 counts, would make LCM less cost-effective, as fewer lives would potentially be saved by laboratory monitoring; subgroup analysis showed that the ICER among patients with CD4<100 cells/mm3 was 30% smaller than for the whole trial population (USD$6764 vs. USD$9621). As commented above, this would also lower the unit cost thresholds at which routine CD4 testing becomes cost-effective.
Our study is unique in its prospective valuation of patient preferences for health states in a sub-sample of DART patients. Interestingly, applying utility weights to health states in DART defined according to performance status and any ongoing WHO stage 3 or 4 events, as assessed by a doctor, showed that the estimated survival difference (0.101 life years) was associated with an almost equal gain in asymptomatic survival (0.097 life years).
DART used a simple and easy to interpret switch criterion of CD4<100 cells/mm3 which does not require longitudinal measurement of CD4 counts, or pre-ART counts. More complex failure criteria based on prior values (CD4 fall <pre-ART, >50% decline from peak) may increase the proportions switching to second-line without necessarily improving outcomes, thus reducing the likelihood of CD4 monitoring being cost-effective; these criteria are also more challenging to implement. Interestingly the HBAC study used persistently declining CD4 cell counts on two consecutive measurements to indicate treatment failure: this has been demonstrated to have the lowest sensitivity of the three failure criteria [37].
DART did not evaluate routine viral load testing. However, the difference between DART groups receiving and not receiving routine CD4 monitoring trial based was similar to one modelling study [15] and the HBAC randomised trial of laboratory monitoring in Uganda [9]. Both suggest limited additional benefits with viral load monitoring, highlighting the importance of defining the optimal threshold for virologic failure and submitting routine viral load monitoring to a definitive evaluation [38]. On the basis of a recent review [39] and the ICER of $5181 per DALY for viral load/CD4/clinical monitoring vs. CD4/clinical monitoring reported by the HBAC study [18], at current costs and with current technologies, there is less evidence of cost-effectiveness for viral load monitoring than there is for CD4 count monitoring.
A further limitation of the cost-effectiveness analysis presented here is that it has only considered the patients on ART, and in particular has not considered other possible impacts such as increased HIV transmission, including of drug resistant virus, from unsuppressed patients on ART or, in contrast, reduced transmission from having been able to put more patients on ART without providing routine laboratory monitoring for the same fixed budget. Modelling studies, when they have examined the evolution and transmission of drug resistance under a public health approach to ART, have been relatively reassuring [15], [40]. A modelling component within the Lablite project will further model these aspects.
Our findings have implications for public sector ART programmes in Africa, given the unmet demand for treatment, the limited availability of laboratory services and stagnant or declining international funding for health. Strengthening laboratory services is a priority: CD4 testing should focus on ART initiation; toxicity testing can be clinically guided rather than routine; access to quality-controlled diagnostic testing must be widened. With fixed and constrained budgets, relative to no ART provision [41], the number of lives/life-years saved with current costs and approaches is greater for a clinically-driven rather than laboratory monitoring strategy. Further, it is an equitable option as its lower cost allows more patients to access treatment and for follow up to be decentralised to health centres [42]. Resources could therefore better be allocated for untreated patients to start ART, and for laboratory diagnostic investigation of clinical events, rather than for routine laboratory monitoring [43]. The recently started LabLite project aims to demonstrate that such decentralised ART delivery (outside research clinics) is safe and effective in rural areas in Malawi, Uganda and Zimbabwe.
Results from the DART trial and the cost-effectiveness analysis also raise challenging issues about how to act on research findings when effectiveness is demonstrated but cost-effectiveness is not, as profound underlying tensions between patients, healthcare workers, funders and policy makers are exposed [44]. This paradox is a challenge for the public sector even in high-income countries (e.g. in the UK with the potential provision of very expensive cancer drugs with significant toxicity but also proven, limited benefits in the NHS), as resources universally are finite. It is even more difficult when interventions that are routinely provided in better resourced settings with higher GDPs, like CD4 testing, produce benefit but are not cost-effective in low-income settings. Particularly in resource-constrained settings, spending money on one healthcare component such as ART monitoring necessarily means fewer resources can be spent on other competing priorities (patients still in need of ART, bed nets to prevent malaria, vaccination programmes and so on) and we would argue our research helps policy makers to choose how to maximise health benefit.
In conclusion, the DART trial has clearly shown that routine laboratory monitoring of ART is not currently cost-effective in low-income African countries. There is no rationale for toxicity monitoring which does not affect outcomes and is costly. Routine CD4 monitoring has small but measurable benefits on survival; for it to be cost-effective the costs of second-line ART and CD4 testing need to fall substantially. Laboratories will remain important for quality of care especially for the diagnosis of intercurrent events on ART. Realising the survival benefits of CD4 monitoring widely in Africa is likely to be dependent on the development of a cheap, ideally point-of-care CD4 test. In the meantime, given competing priorities, HIV programmes in Africa may best spend limited resources on increasing access to first and second-line ART.
Supporting Information
Modelling Utility Estimation in DART patients.
(DOC)
Modelling the long term survival and costs of patients in DART.
(DOC)
Acknowledgments
We thank all the patients and staff from all the centres participating in the DART trial.
MRC/UVRI Uganda Research Unit on AIDS, Entebbe, Uganda: H Grosskurth, P Munderi, G Kabuye, D Nsibambi, R Kasirye, E Zalwango, M Nakazibwe, B Kikaire, G Nassuna, R Massa, K Fadhiru, M Namyalo, A Zalwango, L Generous, P Khauka, N Rutikarayo, W Nakahima, A Mugisha, J Todd, J Levin, S Muyingo, A Ruberantwari, P Kaleebu, D Yirrell, N Ndembi, F Lyagoba, P Hughes, M Aber, A Medina Lara, S Foster, J Amurwon, B Nyanzi Wakholi, K Wangati, B Amuron, D Kajungu, J Nakiyingi, W Omony, K Fadhiru, D Nsibambi, P Khauka.
Joint Clinical Research Centre, Kampala, Uganda: P Mugyenyi, C Kityo, F Ssali, D Tumukunde, T Otim, J Kabanda, H Musana, J Akao, H Kyomugisha, A Byamukama, J Sabiiti, J Komugyena, P Wavamunno, S Mukiibi, A Drasiku, R Byaruhanga, O Labeja, P Katundu, S Tugume, P Awio, A Namazzi, GT Bakeinyaga, H Katabira, D Abaine, J Tukamushaba, W Anywar, W Ojiambo, E Angweng, S Murungi, W Haguma, S Atwiine, J Kigozi, L Namale. A Mukose, G Mulindwa, D Atwiine, A Muhwezi, E Nimwesiga, G Barungi, J Takubwa, S Murungi, D Mwebesa, G Kagina, M Mulindwa, F Ahimbisibwe, P Mwesigwa, S Akuma, C Zawedde, D Nyiraguhirwa, C Tumusiime, L Bagaya, W Namara, J Kigozi, J Karungi, R Kankunda, R Enzama.
University of Zimbabwe, Harare, Zimbabwe: A Latif, J Hakim, V Robertson, A Reid, E Chidziva, R Bulaya-Tembo, G Musoro, F Taziwa, C Chimbetete, L Chakonza, A Mawora, C Muvirimi, G Tinago, P Svovanapasis, M Simango, O Chirema, J Machingura, S Mutsai, M Phiri, T Bafana, M Chirara, L Muchabaiwa, M Muzambi, E Chigwedere, M Pascoe, C Warambwa, E Zengeza, F Mapinge, S Makota, A Jamu, N Ngorima, H Chirairo, S Chitsungo, J Chimanzi, C Maweni, R Warara, M Matongo, S Mudzingwa, M Jangano, K Moyo, L Vere, I Machingura.
Infectious Diseases Institute (formerly the Academic Alliance) Makerere University, Mulago, Uganda: E Katabira, A Ronald, A Kambungu, F Lutwama, I Mambule, A Nanfuka, J Walusimbi, E Nabankema, R Nalumenya, T Namuli, R Kulume, I Namata, L Nyachwo, A Florence, A Kusiima, E Lubwama, R Nairuba, F Oketta, E Buluma, R Waita, H Ojiambo, F Sadik, J Wanyama, P Nabongo, J Oyugi, F Sematala, A Muganzi, C Twijukye, H Byakwaga.
The AIDS Support Organisation (TASO), Uganda: R Ochai, D Muhweezi, A Coutinho, B Etukoit.
Imperial College, London, UK: C Gilks, K Boocock, C Puddephatt, C Grundy, J Bohannon, D Winogron.
MRC Clinical Trials Unit, London, UK: J Darbyshire, DM Gibb, A Burke, D Bray, A Babiker, AS Walker, H Wilkes, M Rauchenberger, S Sheehan, C Spencer-Drake, K Taylor, M Spyer, A Ferrier, B Naidoo, D Dunn, R Goodall.
Independent DART Trial Monitors: R Nanfuka, C Mufuka-Kapuya.
DART Virology Group: P Kaleebu (Co-Chair), D Pillay (Co-Chair), V Robertson, D Yirrell, S Tugume, M Chirara, P Katundu, N Ndembi, F Lyagoba, D Dunn, R Goodall, A McCormick.
DART Health Economics Group: A Medina Lara (Chair), S Foster, J Amurwon, B Nyanzi Wakholi, J Kigozi, L Muchabaiwa, M Muzambi.
Trial Steering Committee: I Weller (Chair), A Babiker (Trial Statistician), S Bahendeka, M Bassett, A Chogo Wapakhabulo, J Darbyshire, B Gazzard, C Gilks, H Grosskurth, J Hakim, A Latif, C Mapuchere, O Mugurungi, P Mugyenyi; Observers: C Burke, M Distel, S Jones, E Loeliger, P Naidoo, C Newland, G Pearce, S Rahim, J Rooney, M Smith, W Snowden, J-M Steens.
Data and Safety Monitoring Committee: A Breckenridge (Chair), A McLaren (Chair-deceased), C Hill, J Matenga, A Pozniak, D Serwadda.
Endpoint Review Committee: T Peto (Chair), A Palfreeman, M Borok, E Katabira.
Conference presentation
Data were presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention, 19–22 July 2009, Cape Town, South Africa.
Footnotes
Competing Interests: GlaxoSmithKline, Gilead Sciences, Boehringer-Ingelheim and Abbott Laboratories donated drugs for DART. There are no patents, products in development or marketed products to declare. This does not alter the authors’ adherence to all the PLoS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Funding: This work was supported by the United Kingdom (UK) Medical Research Council [grant number G0600344], the UK Department for International Development (DFID) and the Rockefeller Foundation. GlaxoSmithKline, Gilead Sciences, Boehringer-Ingelheim and Abbott Laboratories donated drugs for DART. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. GlaxoSmithKline, Gilead Sciences, Boehringer-Ingelheim and Abbott Laboratories donated drugs for DART.
References
- 1.Gilks CF, Crowley S, Ekpini R, Perriens J, Souteyrand Y, et al. The WHO public health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Progress report 2010: 1–150; 2010. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. pp. 1–150. [Google Scholar]
- 3.World Bank. The global economic crisis and HIV prevention and treatment programmes; vulnerabilities and impact. 2009. pp. 1–40.
- 4.Kenyon C, Skordis J, Boulle A, Pillay K. The ART of rationing–the need for a new approach to rationing health interventions. S Afr Med J. 2003;93:56–60. [PubMed] [Google Scholar]
- 5.Kent DM, McGrath D, Ioannidis JPA. Suitable monitoring approaches to antiretroviral therapy in resource-poor settings: setting the research agenda. Clinical Infectious Diseases. 2003;37:S13–24. doi: 10.1086/375368. [DOI] [PubMed] [Google Scholar]
- 6.Schreibman T, Friedland G. Use of total lymphocyte count for monitoring response to antiretroviral therapy. Clinical Infectious Diseases. 2004;38:257–62. doi: 10.1086/380792. [DOI] [PubMed] [Google Scholar]
- 7.Bisson GP, Gross R, Strom JB, Rollins C, Bellamy S, et al. Diagnostic accuracy of CD4 cell count increase for virologic response after initiating highly active antiretroviral therapy. AIDS. 2006;20:1613–19. doi: 10.1097/01.aids.0000238407.00874.dc. [DOI] [PubMed] [Google Scholar]
- 8.Moore DM, Mermin J, Awor A, Yip B, Hogg RS, et al. Performance of immunologic responses in predicting viral load suppression: implications for monitoring patients in resource-limited settings. J Acquir Immune Defic Syndr. 2006;43:436–39. doi: 10.1097/01.qai.0000243105.80393.42. [DOI] [PubMed] [Google Scholar]
- 9.Mermin J, Ekwaru J, Were W, Degerman R, Bunnell R, et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomized trial. BMJ. 2011;343:d6792. doi: 10.1136/bmj.d6792. doi: 10.1136/bmj.d6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weidle PJ, Wamai N, Solberg P, Liechty C, Sendagala S, et al. Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet. 2006;368:1587–1594. doi: 10.1016/S0140-6736(06)69118-6. [DOI] [PubMed] [Google Scholar]
- 11.DART Trial Team. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375:23–31. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deghaye N, Pawinski RA, Desmond C. Financial and economic costs of scaling up the provision of HAART to HIV-infected health care workers in KwaZulu-Natal. S.Afr.Med.J. 2006;96:140–143. [PubMed] [Google Scholar]
- 13.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, et al. Cost-effectiveness of HIV treatment in resource-poor settings – the case of Côte d’Ivoire. N Engl J Med. 2006;355:1141–53. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 14.Bishai D, Colchero MA, Durack D. Modeling the cost effectiveness of antiretroviral treatment strategies in low income settings AIDS. 2007;21:1333–40. doi: 10.1097/QAD.0b013e328137709e. [DOI] [PubMed] [Google Scholar]
- 15.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, et al. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008;371:1443–51. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 16.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, et al. Cost-effectiveness of HIV monitoring strategies in resource-limited settings – a southern African analysis. Arch Intern Med. 2008;168:1910–1918. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimmel AD, Weinstein MC, Anglaret X, Goldie SJ, Losina E, et al. Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr. 2010;54:258–268. doi: 10.1097/QAI.0b013e3181d0db97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn JG, Marseille E, Moore D, Bunnell R, Were W, et al. CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study. BMJ. 2011;343:d6884. doi: 10.1136/bmj.d6884. doi: 10.1136/bmj.d6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The DART Trial Team. Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts <200 cells/ml. AIDS 2008: 2008;22:237–247. doi: 10.1097/QAD.0b013e3282f2d760. [DOI] [PubMed] [Google Scholar]
- 20.Walker AS, Ford D, Gilks C. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet: 2010;375:1278–86. doi: 10.1016/S0140-6736(10)60057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creese A, Parker D. World Health Organization, Geneva; 1994. Cost analysis in primary health care: a training manual for programme managers. pp. 1–158. [Google Scholar]
- 22.Médicins San Frontières. Untangling the web of antiretroviral price reductions, 11 Edition. 2008;14 Available: http://www.msfaccess.org/main/hiv-aids/untangling-the-web-of-antiretroviral-price-reductions-11th-edition. Accessed 2009 Feb. [Google Scholar]
- 23.Adam T, Evans DB, Murray CJL. Econometric estimation of country-specific hospital costs. cost effectiveness and resource allocation doi:10.1186/1478–7547–1-3. 2003;27 doi: 10.1186/1478-7547-1-3. Available: http://www.ncbi.nlm.nih.gov/pubmed/12773218. Accessed 2009 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Monetary Fund. World economic outlook database 2009. 2009;15 Available: http://imf.org/external/pubs/ft/weo/2009/02/weodata/index.aspx Accessed 2010 Jun. [Google Scholar]
- 25.Hosmer DW, Lemeshow S. New York: Wiley; 1999. Applied Survival Analysis. [Google Scholar]
- 26.Staven K, Frolan SS, Hellum KB. Comparison of preference-based utilities of the 15D, EQ-5D and SF-6D in patients with HIV/AIDS. Quality of Life Research. 2005;14:971–980. doi: 10.1007/s11136-004-3211-7. [DOI] [PubMed] [Google Scholar]
- 27.Lin DY. Linear regression analysis of censored medical costs. Biostatistics. 2000;1:35–47. doi: 10.1093/biostatistics/1.1.35. [DOI] [PubMed] [Google Scholar]
- 28.Efron B, Tibshirani R. New York: Chapman and Hall; 1993. An introduction to the bootstrap. [Google Scholar]
- 29.WHO International Drug Price Indicator Guide (2008). 2008;3 Available: http://www.essentialdrugs.org/edrug/archive/200903/msg00059.php. Accessed 2009 Jun. [Google Scholar]
- 30.Rosen S, Long L, Sanne I. The outcomes and outpatient costs of different models of antiretroviral treatment delivery in South Africa. Trop Med Int Health. 2008;13:1005–1015. doi: 10.1111/j.1365-3156.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 31.Sachs JD. WHO. Geneva; 2001. Report of the commission on macroeconomics and health. [Google Scholar]
- 32.Jourdain G, Ngo-Giang-Huong N, S Le Coeur S. PHPT-3: a randomized clinical trial comparing CD4 vs viral load ART monitoring/switching strategies in Thailand. 18th Conference on Retroviruses and Opportunistic Infections. 2011;44 Boston, 27 February -2 March 2011, Boston USA. Abstract. [Google Scholar]
- 33.Laurent C, Kouanfack C, Laborde-Balen G, Aghokeng AF, Tcchatechueng JB, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. Lancet Infect Dis; 2011;11:825–33. doi: 10.1016/S1473-3099(11)70168-2. [DOI] [PubMed] [Google Scholar]
- 34.Jani IV, Sitoe NE, Alfai ER. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–9. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organisation. Recommendations for a public health approach. WHO Geneva; 2006. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. [Google Scholar]
- 36.Gilks CF, Walker AS, Munderi P, Kityo C, Reid A, et al. A single CD4 test with threshold ≥250 cells/mm3 can markedly reduce switching to second-line ART in African patients managed without CD4 or viral monitoring. 18th Conference on Retroviruses and Opportunistic Infections, 27 February – 2 March 2011, Boston USA. Abstract. 2011;676 [Google Scholar]
- 37.Riwazza HE, Chaplin B, Meloni ST. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53:1283–90. doi: 10.1093/cid/cir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koethe JR, Westfall AO, Luhanga DK, Clarke DM, Goldman JD, et al. A cluster randomized trial of routine HIV-1 viral load monitoring in Zambia: study design, implementation, and baseline cohort characteristics. PLoS ONE. 2010;5:e9680. doi: 10.1371/journal.pone.0009680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walensky RP, Ciaranello AL, Park J-E, Freedberg KS. Cost-effectiveness of laboratory monitoring in sub-Saharan Africa: a review of the current literature. Clin Infect Dis. 2010;51:85–92. doi: 10.1086/653119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips AN, Pillay D, Garnett G, Bennett D, Vitoria M, et al. Effect on transmission of HIV-1 resistance of timing of implementation of viral load monitoring to determine switches from first to second-line antiretroviral regimens in resource-limited settings. AIDS. 2011;25:843–50. doi: 10.1097/QAD.0b013e328344037a. [DOI] [PubMed] [Google Scholar]
- 41.Munderi P, Watera C, Nakiyingi J, Walker S, Gilks CF. Survival and causes of death, 2 years after introduction of antiretroviral therapy in Africa: a historical cohort comparison in Entebbe, Uganda. XVI International AIDS Conference. Toronto, Canada; Aug 13–18, 2006. Abstract. 2006;THLB0208 [Google Scholar]
- 42.Ubel P, DeKay ML, Baron J, Asch DA. Cost-effectiveness analysis in a setting of budget constraints - is it equitable? N Engl J Med. 1996;334:1174–1177. doi: 10.1056/NEJM199605023341807. [DOI] [PubMed] [Google Scholar]
- 43.Phillips AN, Gilks C, Lundgren JD. Cost-effectiveness of strategies for monitoring the response to antiretroviral therapy in resource-limited settings. Arch Intern Med. 2009;169:904. doi: 10.1001/archinternmed.2009.88. [DOI] [PubMed] [Google Scholar]
- 44.Nunes EP, Grinsztejn B, Schechter M, The DART trial. The doctor’s dilemma revisited. J Antimicrob Chemother. 2011;66(5):964–7. doi: 10.1093/jac/dkr020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Modelling Utility Estimation in DART patients.
(DOC)
Modelling the long term survival and costs of patients in DART.
(DOC)