Abstract
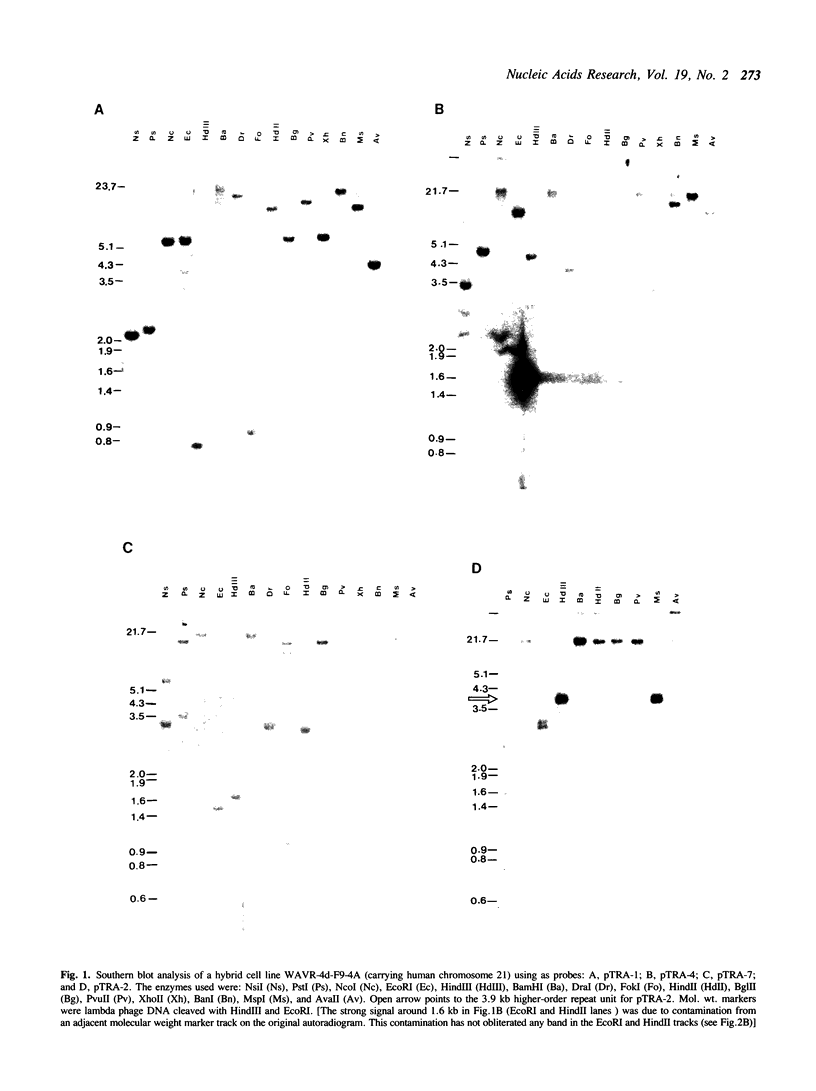
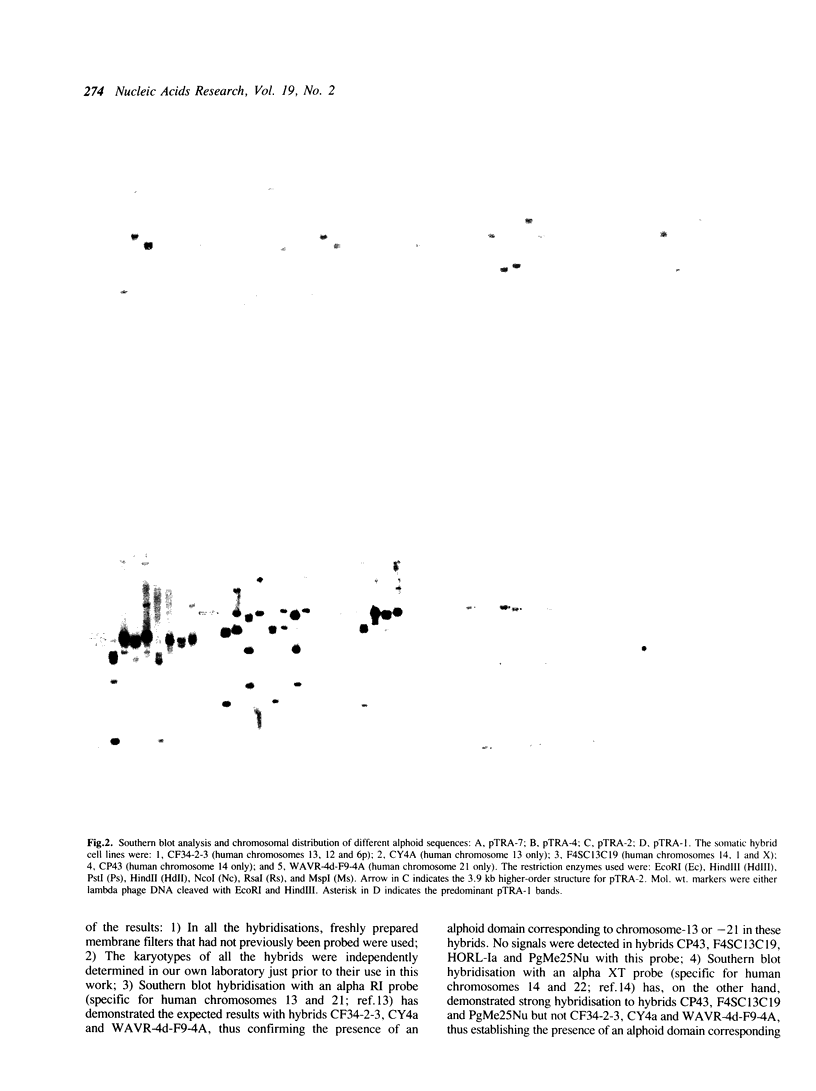
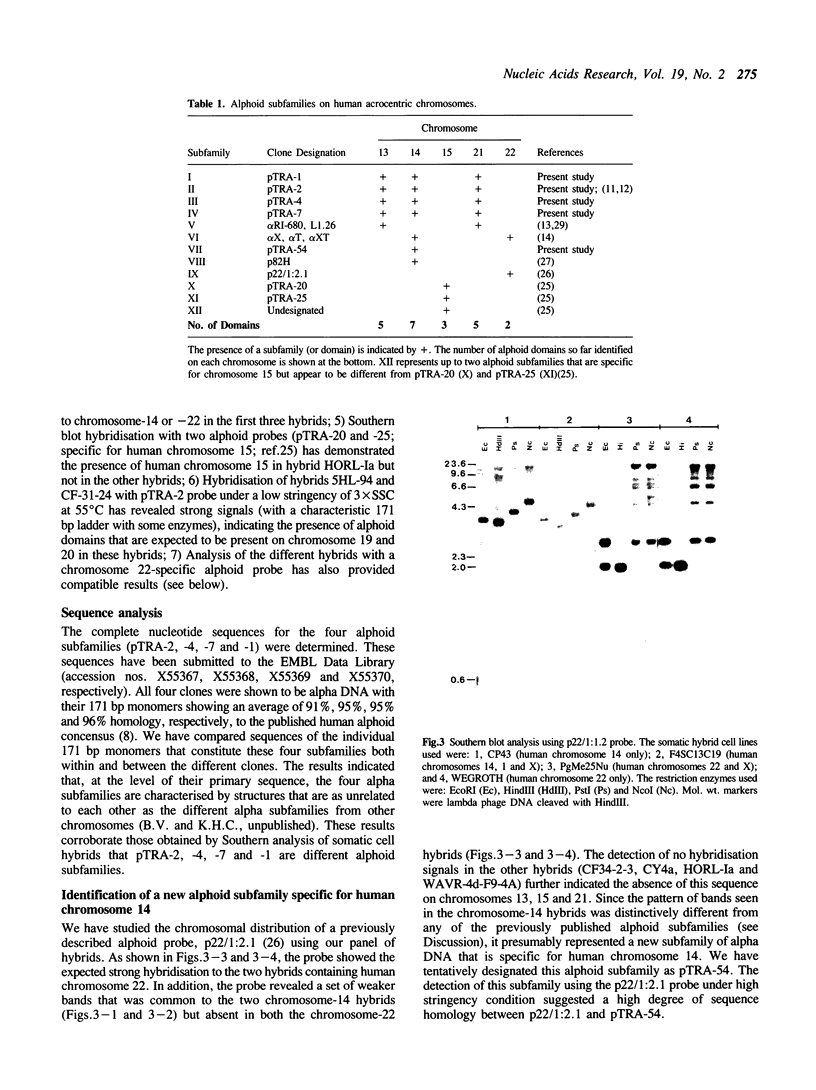
We describe the characterisation of four alpha satellite sequences which are found on a subset of the human acrocentric chromosomes. Direct sequence study, and analysis of somatic cell hybrids carrying specific human chromosomes indicate a unique 'higher-order structure' for each of the four sequences, suggesting that they belong to different subfamilies of alpha DNA. Under very high stringency of Southern hybridisation conditions, all four subfamilies were detected on chromosomes 13, 14 and 21, with 13 and 21 showing a slightly greater sequence homology in comparison to chromosome 14. None of these subfamilies were detected on chromosomes 15 and 22. In addition, we report preliminary evidence for a new alphoid subfamily that is specific for human chromosome 14. These results, together with those of earlier published work, indicate that the centromeres of the five acrocentric chromosomes are characterised by a number of clearly defined alphoid subfamilies or microdomains (with at least 5, 7, 3, 5 and 2 different ones on chromosomes 13, 14, 15, 21 and 22, respectively). These microdomains must impose a relatively stringent subregional pairing of the centromeres of two homologous chromosomes. The different alphoid subfamilies reported should serve as useful markers to allow further 'dissection' of the structure of the human centromere as well as the investigation of how the different nonhomologous chromosomes may interact in the aetiology of aberrations involving these chromosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choo K. H., Earle E., Vissel B., Filby R. G. Identification of two distinct subfamilies of alpha satellite DNA that are highly specific for human chromosome 15. Genomics. 1990 Jun;7(2):143–151. doi: 10.1016/0888-7543(90)90534-2. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Vissel B., Brown R., Filby R. G., Earle E. Homologous alpha satellite sequences on human acrocentric chromosomes with selectivity for chromosomes 13, 14 and 21: implications for recombination between nonhomologues and Robertsonian translocations. Nucleic Acids Res. 1988 Feb 25;16(4):1273–1284. doi: 10.1093/nar/16.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Vissel B., Earle E. Evolution of alpha-satellite DNA on human acrocentric chromosomes. Genomics. 1989 Aug;5(2):332–344. doi: 10.1016/0888-7543(89)90066-9. [DOI] [PubMed] [Google Scholar]
- Clarke L., Baum M. P. Functional analysis of a centromere from fission yeast: a role for centromere-specific repeated DNA sequences. Mol Cell Biol. 1990 May;10(5):1863–1872. doi: 10.1128/mcb.10.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L. Centromeres of budding and fission yeasts. Trends Genet. 1990 May;6(5):150–154. doi: 10.1016/0168-9525(90)90149-z. [DOI] [PubMed] [Google Scholar]
- Devilee P., Cremer T., Slagboom P., Bakker E., Scholl H. P., Hager H. D., Stevenson A. F., Cornelisse C. J., Pearson P. L. Two subsets of human alphoid repetitive DNA show distinct preferential localization in the pericentric regions of chromosomes 13, 18, and 21. Cytogenet Cell Genet. 1986;41(4):193–201. doi: 10.1159/000132229. [DOI] [PubMed] [Google Scholar]
- Geurts van Kessel A. H., den Boer W. C., van Agthoven A. J., Hagemeijer A. Decreased tumorigenicity of rodent cells after fusion with leukocytes from normal and leukemic donors. Somatic Cell Genet. 1981 Nov;7(6):645–656. doi: 10.1007/BF01538754. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R., Spurr N. K., Goodfellow P. N., Solomon E., Carritt B., Bodmer W. F. Chromosomal localization of human cellular homologues of two viral oncogenes. Nature. 1982 Oct 21;299(5885):747–749. doi: 10.1038/299747a0. [DOI] [PubMed] [Google Scholar]
- John B., Miklos G. L. Functional aspects of satellite DNA and heterochromatin. Int Rev Cytol. 1979;58:1–114. doi: 10.1016/s0074-7696(08)61473-4. [DOI] [PubMed] [Google Scholar]
- Jørgensen A. L., Bostock C. J., Bak A. L. Homologous subfamilies of human alphoid repetitive DNA on different nucleolus organizing chromosomes. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1075–1079. doi: 10.1073/pnas.84.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A. L., Kølvraa S., Jones C., Bak A. L. A subfamily of alphoid repetitive DNA shared by the NOR-bearing human chromosomes 14 and 22. Genomics. 1988 Aug;3(2):100–109. doi: 10.1016/0888-7543(88)90139-5. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Kao F. T., Law M. L., Woo S. L. Assignment of the alpha 1-antitrypsin gene and a sequence-related gene to human chromosome 14 by molecular hybridization. Am J Hum Genet. 1983 May;35(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- Mahtani M. M., Willard H. F. A primary genetic map of the pericentromeric region of the human X chromosome. Genomics. 1988 May;2(4):294–301. doi: 10.1016/0888-7543(88)90017-1. [DOI] [PubMed] [Google Scholar]
- McDermid H. E., Duncan A. M., Higgins M. J., Hamerton J. L., Rector E., Brasch K. R., White B. N. Isolation and characterization of an alpha-satellite repeated sequence from human chromosome 22. Chromosoma. 1986;94(3):228–234. doi: 10.1007/BF00288497. [DOI] [PubMed] [Google Scholar]
- Miklos G. L., John B. Heterochromatin and satellite DNA in man: properties and prospects. Am J Hum Genet. 1979 May;31(3):264–280. [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shulkin J. D., Sparkes M. C., Moedjono S. Assignment of PGM3 to the long arm of human chromosome 6. Studies using Chinese hamster X human cell hybrids containing a human 6/15 translocation. Cytogenet Cell Genet. 1980;28(1-2):116–120. doi: 10.1159/000131519. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Suh E. J., Hershfield M. S. Regional localization of the human genes for S-adenosylhomocysteine hydrolase (cen----q131) and adenosine deaminase (q131----qter) on chromosome 20. Hum Genet. 1984;66(4):292–295. doi: 10.1007/BF00287630. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. Highly repeated sequences in mammalian genomes. Int Rev Cytol. 1982;76:67–112. doi: 10.1016/s0074-7696(08)61789-1. [DOI] [PubMed] [Google Scholar]
- Slate D. L., Shulman L., Lawrence J. B., Revel M., Ruddle F. H. Presence of human chromosome 21 alone is sufficient for hybrid cell sensitivity to human interferon. J Virol. 1978 Jan;25(1):319–325. doi: 10.1128/jvi.25.1.319-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Bachinski L. L., Stallings R. L., Dolf G., Weber C. A., Westerveld A., Siciliano M. J. Complementation of repair gene mutations on the hemizygous chromosome 9 in CHO: a third repair gene on human chromosome 19. Genomics. 1989 Nov;5(4):670–679. doi: 10.1016/0888-7543(89)90107-9. [DOI] [PubMed] [Google Scholar]
- Vissel B., Choo K. H. Human alpha satellite DNA--consensus sequence and conserved regions. Nucleic Acids Res. 1987 Aug 25;15(16):6751–6752. doi: 10.1093/nar/15.16.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. Potential genetic functions of tandem repeated DNA sequence blocks in the human genome are based on a highly conserved "chromatin folding code". Hum Genet. 1990 Mar;84(4):301–336. doi: 10.1007/BF00196228. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Mitchell A. R., Willard H. F. Organization and genomic distribution of "82H" alpha satellite DNA. Evidence for a low-copy or single-copy alphoid domain located on human chromosome 14. Hum Genet. 1988 Jan;78(1):27–32. doi: 10.1007/BF00291229. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Nucleotide sequence heterogeneity of alpha satellite repetitive DNA: a survey of alphoid sequences from different human chromosomes. Nucleic Acids Res. 1987 Sep 25;15(18):7549–7569. doi: 10.1093/nar/15.18.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. I., True J. R., Johnson N. Fitness reduction associated with the deletion of a satellite DNA array. Nature. 1989 Sep 21;341(6239):248–251. doi: 10.1038/341248a0. [DOI] [PubMed] [Google Scholar]