Abstract
Perfluoroalkyl acids (PFAAs) have attracted attention in recent years for their environmental ubiquity, as well as their toxicity. Several PFAAs are found in human tissues globally, as humans are exposed on a daily basis through intake of contaminated food, water, and air, irrespective of proximity to industry. Perfluorooctanoic acid (PFOA) is a PFAA shown to be developmentally toxic in mice, with broad and varied health consequences that may include long-lasting effects in reproductive tissues and metabolic reprogramming. To date, the only demonstrated mode of action by which the health effects of PFOA are mediated is via the activation of the peroxisome proliferator-activated receptor alpha (PPARα). The endogenous roles for this receptor, as well as the adverse outcomes of activation by exogenous agents during development, are currently under investigation. Recent studies suggest that PFOA may alter steroid hormone production or act indirectly, via ovarian effects, as a novel means of endocrine disruption. Here we review the existing literature on the known health effects of PFOA in animal models, focusing on sensitive developmental periods. To complement this, we also present epidemiologic health data, with the caveat that these studies largely address only associations between adult exposures and outcomes, rarely focusing on endocrine-specific endpoints, susceptible subpopulations, or windows of sensitivity. Further research in these areas is needed.
Keywords: PFOA, Mammary gland, PPAR, Exposure, Developmental effects
1. Background
In recent years perfluoroalkyl acids (PFAAs) – a sub-class of fluorochemicals with fully fluorinated carbon chains – have gained increasing attention as an emerging category of pollutants. As such, they have become the target for risk evaluation and reduced production [1]. These synthetic fluorinated organic chemicals have extensive industrial applications, including as surfactants and emulsifiers often in the production of other fluorinated chemicals, as well as grease and stain-repellents, friction reducers (wiring, computers), water-proofing and insulating agents, and in fire extinguishing foam [2]. Perfluorooctanoic acid (PFOA; sometimes referred to as its ammonia salt, ammonium perfluorooctanoate, APFO, PFO or C8) is a well-studied and environmentally ubiquitous PFAA. PFOA is a persistent, non-lipophilic, protein-binding PFAA, with a serum half-life in mice of 16–22 days [3] and of 2–4 years in humans [4–6]. In addition to its frequent presence in the ambient environment, PFOA is consistently detectable in the serum of wildlife animals and humans, and has been shown to be developmentally toxic at both high and low doses in rodents. Some animal studies of exposure to PFOA have suggested the potential for the compound to disrupt endocrine signaling [7,8].
The precise mode of action responsible for the health effects of PFOA is unclear, particularly under low-dose conditions. However, the target tissues and health outcomes of PFOA exposure discussed below are frequently endocrine-regulated, further suggesting a potential endocrine-disrupting capacity of PFOA. Furthermore, the primary identifiable mode of action for PFOA-induced toxicity is agonism of the peroxisome proliferator-activated receptor alpha (PPAR-α), which is a nuclear hormone receptor, although activation of the nuclear receptors, constitutive androstane receptor and pregnane X receptor, also occurs [9,10]. The goal of this article is to review the reproductive and other potentially endocrine-related health effects of PFAAs, specifically PFOA.
2. PFOA exposure in human populations
PFOA has been measured worldwide in human blood, with median serum levels in the adult US population of about 4 ng/ml (measured in 2003–2004) [11], and slightly higher levels in samples from children (6.1–7.6 ng/ml; 2001–2002) [12]. After the discovery that industrial releases of PFOA to the environment at DuPont’s Washington Works fluorochemical-manufacturing plant in Parkersburg, WV, resulted in elevated exposures of surrounding residents to PFOA, a court-mandated settlement included the creation of the C8 Science Panel, a collaborative scientific effort to study health effects in the exposed population [13]. Since that time, the C8 health project has measured serum PFOA concentrations in over 69,000 residents in six water districts exposed to PFOA levels of ≥0.05 µg/l (or 50 ng/l) in drinking water from public water supplies or private wells, which subsequently resulted in higher human exposure to the fluorochemical in this geographical area than in other areas of the US [10,13]. Of these districts, the Little Hocking, OH, drinking water supply possessed the highest measured PFOA levels (above 3 ng/ml) and that community exhibited the highest average serum levels. The mean serum PFOA concentration was 424 ng/ml in adults living in the affected water districts who drank tap water, with a range of 0.25–22,412 ng/ml (during 2005–2006) [4,14,15], compared to a mean serum PFOA concentration of 83 ng/ml in the entire study population, including residents of more distant regions of the Mid-Ohio Valley.
Although pathways for human exposure to PFOA and other PFAAs have not been well characterized, it is currently thought that adult humans receive their primary PFOA exposure via the food and water supply, and children may have varied exposure routes [16]. A study employing the Danish National Birth Cohort data addressed potential dietary sources of PFOA to pregnant women, and found, as with other PFAAs, a positive correlation between serum PFOA concentrations and intake of red meat and packed snack foods, as compared to an inverse correlation with vegetable and poultry intake [17]. Other dietary studies have found shellfish [18], meat, and eggs [19] to be associated with PFAA exposure. Currently there is no federal drinking water standard (Maximum Contaminant Level, MCL) set for PFOA. Some US states, including Minnesota, New Jersey, and others, have moved forward with establishing and implementing their own guidelines for safety limits, in order to address local situations of drinking water contamination. Another potential source of exposure is indoor dust, which may be ingested orally or by inhalation. Indeed, PFOA has been detected in indoor dust from all countries tested to date, including Canada, Japan, Sweden, the United Kingdom, and the United States, and is frequently found in concentrations at or exceeding 100 ng/g [20]. The confirmed presence of PFOA in treated sewage sludge applied to agricultural land may also contribute to human exposure if the compound is taken up by plant tissues to be sold as food for human consumption, or ingested by grazing livestock [21,22].
Another possible source of PFAA exposure is breast milk. Numerous studies have been published reporting various concentrations of PFOA in breast milk ranging from below the limit of detection to concentrations near 1–3 ng/ml [23,24]. The details of these studies reporting PFOA in milk and maternal/cord blood are shown in Table 1. A Massachusetts study reported PFOA levels in milk samples from volunteers and by comparing these data to the National Health and Nutrition Evaluation Survey (NHANES) values for serum PFOA, this group determined that there is an estimated 1% milk to serum partition ratio for PFOA in the US population [25]; others were unable to measure such a partition factor for PFOA because the majority of their PFOA milk data were below the limit of detection [26,27].
Table 1.
PFOA concentrations in milk and other tissues from global studies of lactating women and neonates.
| Location, sampling date | Population (n) | Matrix | PFOA concentration | Author |
|---|---|---|---|---|
| Japan, 2003 | - Pregnant women, Sapporo hospital convenience sample: 3rd trimester maternal plasma (15), cord blood at delivery (15) |
Plasma, cord blood | Maternal plasma (range): <LOQ–2.3 ng/ml; Cord blood: <LOQ LOQ = 0.5 ng/ml |
[94] |
| Atlanta, GA, USA, 2003 | - Lactating women, Atlanta convenience sample (20) |
Milk | Milk: <LOQ LOQ = 0.2 mg/ml |
[95] |
| Shenyang, China (date unknown) |
- Pregnant women, China: cord blood at delivery |
Cord blood | Cord blood geometric mean 0.264 µg/l | [96] |
| Zhousan, China, 2004 | - Lactating women, Zhoushan hospital convenience sample (19) |
Milk | PFOA range: 47–210 ng/l LOQ = 21–27 ng/l |
[97] |
| Baltimore, MD, USA, 2004–2005 |
- Baltimore THREE Study: cord blood at delivery (299) |
Cord blood | Median: 1.6 ng/ml Range: 0.3–7.1 ng/ml LOD = 0.1–0.2 ng/ml |
[32] |
| Denmark, 1996–2004 | - Danish National Birth Cohort, pregnant women: 1st trimester maternal plasma (1400), 2nd trimester plasma (200), cord blood at delivery (50) |
Plasma, cord blood | Mean ± SEM ng/ml: 1st trimester: 5.6 ± 2.5, 2nd trimester 4.5 ± 1.9, Cord blood: 3.7 ± 3.4 (LOQ = 1 ng/ml) |
[34] |
| Sweden, 2004; Sweden (pooled composite milk samples), 1996–2004 |
- Primiparous lactating women, Sweden, 3 weeks postpartum (12); - Annual composite, Swedish (25–90 women/yr) |
Milk, serum (matched) | Milk (range): <0.209–0.492 ng/ml, Serum (mean ± SD): 3.8 ± 1.0 ng/ml; Pooled milk: <0.209; (LOD = 0.01, blank level = 0.209) |
[26] |
| Germany, 2003 | - Pregnant women, Germany hospital convenience sample: maternal plasma during labor (11), matched cord blood at delivery (11) |
Plasma, cord blood | Plasma median (range): 2.6 µg/l (1.5–4.0) µg/l, Cord blood median (range): 3.4 µg/l (1.5–4.6) µg/l LOD = 0.5 µg/l |
[33] |
| Massachusetts, USA, 2004 | - Lactating women, Massachusetts hospital convenience sample (42) |
Milk | Mean ± SD: 43.8(33.1) pg/ml, Median: 36.1 pg/ml, Range: (<LOD–161 pg/ml) (LOQ = 30.1 pg/ml) |
[25] |
| Leipzig/Munich Germany, 2006; Hungary, 1996–1997 |
- Lactating women, Munich hospital convenience sample (19); - Leipzig Milk bank (38); - Lactating women, Hungary with preterm infants 3–7 weeks postpartum (13) |
Milk | Median for locations combined: <LOQ; Range: <LOQ–460 ng/l (LOQ = 200 ng/l) |
[98] |
| Chapel Hill, NC, USA, 2004–2005 |
- US EPA MAMA study, lactating women: serum and milk at two collections (34 – serum, 18 – milk, visit 1; 30 – serum, 20 – milk, visit 2) |
Milk, serum | Serum – visit 1 median, IQR: 3.5 ng/ml, 2.4 ng/ml, Serum – visit 2 median, IQR: 2.9 ng/ml, 1.3 ng/ml; Milk: all <LOQ Serum LOD = 0.1 ng/ml Milk LOQ = 0.3 ng/ml |
[27] |
| Denmark, 1996–2004 | - Danish National Birth Cohort, pregnant women: maternal serum collected during week 4–14 of pregnancy (1400). |
Serum | Maternal serum: <LOQ–6.97 ng/ml LOQ = 1 ng/ml |
[29] |
| Germany, 2007–2008 | - Pregnant women, Munich birthing class participants: maternal serum 2× during pregnancy (40, 38), 6 months postpartum (47), monthly breast milk, cord blood (33) |
Milk, serum, cord blood | Milk: <0.15–0.25 µg/l Maternal serum median (range): 1.9 (0.7–8.7) µg/l Cord blood median (range): 1.4 (0.5–4.2) µg/l |
[30] |
| Catalonia, Spain. 2007–8 | - Lactating primiparous women (10), Tarragona county, 41–60 d postpartum |
Milk | All samples <LOD LOD = 0.5 ng/ml |
[99] |
| USA Standard Reference Material (SRM), serum 2004, milk 2006 |
- Human (non-maternal) serum and milk collected from several US states and multiple milk banks, used to generate standard reference material (2 SRM/tissue) |
Milk, serum | Milk range across 4 labs: <LOD–0.149 ng/g SRM 1953 Serum, range across 6 labs: (4.43–5.86 ng/g) SRM 1957 Milk LOD ≈ 0.8–0.9 ng/g SRM 1953 |
[28] |
| 12 Chinese Provinces, 2007 | - Lactating women, China, across diverse geographies (24, pooled milk samples, either rural or urban for each province; 1237 individuals). |
Milk | Geographic mean, median: 46 pg/ml, 34.5 pg/ml; Mean, rural Shanghai samples: 814 pg/ml; Mean, urban Shanghai samples: 616 pg/ml LOD = 14.15 pg/ml |
[100] |
| Barcelona, Spain, 2008 | - Lactating women, Barcelona hospital convenience sample: milk 40 d postpartum (20) |
Milk | Range: <LOQ–907 ng/l LOQ = 15.2 ng/l |
[23] |
| Belgium, cord blood 2002–2005, milk 2006 |
- Fourth WHO-coordinated survey of milk pollutants, Belgium: lactating women from all 11 provinces (22 pooled samples); - Cord blood at delivery (not paired), Belgian hospital convenience sample, 8 regions of Belgium (735) |
Milk, cord blood | Milk median, range: 0.3 (<0.3–3.5 ng/ml) Cord blood median (range): 0.6 ng/ml (ND – 9.5 ng/ml); LOD = 0.3 ng/ml |
[24] |
LOD, limit of detection; LOQ, limit of quantitation; IQR, inter-quartile range.
It is possible that variation between labs in limits of detection or quantitation may contribute to some reported differences in milk-borne PFOA concentrations. Also, analytical method differences or artifactual measurements of PFOA from accidental introduction by laboratory equipment, storage containers, or chemicals containing PFOA may contribute to inaccurate measurements. Until more inter-lab analytical comparisons on a single milk sample are completed (e.g., Keller et al.’s work using a standard reference material) [28], milk PFOA concentration measurements will remain challenging to compare between labs. It is possible that the differences seen between studies may also lie in the lactational stage or the mother’s parity at sample collection. These variables should be carefully considered when comparing study results. In fact, another recent study indicated that serum PFOA concentration is inversely related to duration of breastfeeding only in multiparous women [29], suggesting that milk is an excretory route for PFOA. Among those multiparous women [29], the proportion who stopped breastfeeding before the child reached 6 months of age increased with each 10 ng/ml increase in serum PFOS concentrations (OR 1.20, 95% CI 1.06–1.37), and 1 ng/ml increase in serum PFOA concentrations (OR 1.23, 95% CI 1.13–1.33).
A comparison of serum PFOA concentrations in mothers and cord blood at birth, and in corresponding infant blood at 6 and 19 months has been recently conducted [30]. The majority of the infants in this study were exclusively breast-fed (74%). Although the authors could not detect PFOA in most breast milk samples due to assay sensitivity, longitudinal serum PFOA data in mother:infant pairs at birth, 6 months, and 19 months strongly suggest that infant serum PFOA levels are significantly increased by exposure through breast feeding. By 6 months of age, infant serum PFOA levels were elevated to a median of 6.9 ng/ml (range 0.96–26.9 ng/ml). However, at 19 months of age, infant serum PFOA levels declined to a median of 4.6 ng/ml, and individual levels decreased for most infants tested at both time points. The authors suggested that the statistically increased PFOA exposures at 6 months of age was due to exposure through breast milk, and the decrease between 6 and 19 months was triggered by decreased breast feeding and weaning in infants over 6 months of age [30]. As a toddler’s intake is decreasing, we suggest that the fairly rapid increase in the body size of the child (and increased blood volume) could also lead to a decrease in serum PFOA concentrations over time. In rodent studies where PFOA was administered orally and measured in serum and milk, partition ratios were substantially higher than those reported for humans (~1% [25]) and varied by stage of lactation (15–50%) [8], with the beginning and weaning stages of lactation (lower milk volumes) demonstrating highest milk:serum ratios. Further studies measuring PFOA in the milk and blood of the same individual at multiple stages of lactation are necessary to accurately assess the milk:serum relationship in women.
3. PFOA exposures and developmental outcomes
Considerations in determining the effects of environmental exposures include not only the accumulation, but also the timing of exposure. Developmental windows of exposure to environmental chemicals are increasingly being recognized as important contributors to long-term adult health. Pregnancy, lactation, and other periods in early life (i.e., puberty) often represent windows of sensitivity for environmental exposures in developing organisms [31]. PFAAs represent one class of environmental chemicals that has been shown to act during these developmental windows.
A number of epidemiologic studies have tried to characterize human developmental exposures to PFAAs, and determine potential correlations between maternal internal doses and developmental outcomes in their children. PFAAs in human cord blood from Baltimore mothers were assessed [32], and PFOA was detected in 100% of the samples. This was the first study to demonstrate that PFOA is capable of traversing the human placental barrier, thus exposing the fetus in utero. At approximately the same time, a pilot study was undertaken in Germany with a cohort of 11 pregnant women supporting the findings of the previously described study as well as determining that levels of the chemical in umbilical cord plasma mirrored the PFOA concentration from the paired maternal serum [33]. A study of the Danish National Birth Cohort on pregnant women and their subsequent offspring also found a significant inverse association of maternal PFOA concentrations and birth weight, birth length, and abdominal circumference [34,35], indicating that higher serum PFOA levels were associated with decreased infant indices. More recently, a Canadian study of a smaller cohort of pregnant women assessing birth outcomes, found no association between maternal serum PFOA and either birth weight or length of gestation [36]. A similar study in a Japanese cohort also found no correlation between maternal PFOA and birth weight, but did find birth weight to be inversely associated with maternal perfluorooctane sulfonate, another eight carbon perfluorinated compound (PFOS) [37]. Nolan et al. [38] found no association between PFOA-contaminated water services (PFOA measured in the water supply) and birth weight in the highly exposed Ohio River valley population. The aforementioned studies showing decreased birth weight did not produce clinically significant birth weight reductions (e.g., that would lead to small for gestational age indication) [32,34].
One study of the C8 population reported significantly increased odds ratios of adverse pregnancy related outcomes associated with water-borne PFOA exposure including maternal anemia and dysfunctional labor, but the authors point to the small number of reported cases as possibly making these data artifactual [39]. Although a significant association between PFOA concentrations surpassing the 90th percentile and birth defects has been reported in the highly exposed C8 Science Panel cohort [40], no such association with birth defects was seen in another study on women from the same geographical area [39]. Developmental milestone delays in children <2 years of age were not associated with higher PFOA serum concentrations [41]. The differences in reported correlations in these studies may be related to the range of potential exposures in the various countries or regions, including typical length of breastfeeding practices, and the fact that water proxies of exposure are utilized instead of serum PFOA levels in some studies. Nonetheless, regional variances in human serum concentrations of PFOA do exist. This was detailed in Hines et al. [42], where women with longer duration of residence in North Carolina (the home to another manufacturing site) had significantly increased levels of PFAAs.
Some of the earliest studies of the developmental toxicity of PFOA were performed in the rat. Rats are not an ideal species in which to study PFOA-induced developmental effects, given the unique characteristic of the female rat to rapidly eliminate PFOA, resulting in a half-life of several hours [10]. This is a consequence of high renal expression of certain organic anion transporters (OAT). Further, the dosing regimen, in the case of standard developmental toxicology screens, entails daily oral gavage administration of a compound. Because of the rapid elimination in the rat, steady state is not reached with daily dosing, resulting in episodic exposure of the fetus, rather than continuous exposure. However, the high expression of this OAT involved in urinary elimination is specific to the rat, and neither the mouse nor the human exhibit similar sex-specific differences [10]. Studies of developmental toxicity in rats and rabbits found little significant effect of the compound, though one study in rats observed diminished postnatal weight gain and post-weaning survival among those in the highest dose group, as well as pubertal delays in both male and female offspring (30 mg PFOA/kg body weight/d; mg/kg/d); [43]. Later rodent studies were performed in the mouse, which, as described above, is more representative of female human toxicokinetics. These studies found the mouse to be more sensitive than the rat. While no gross malformations were observed, full litter resorptions were notable at doses of 5 mg/kg/d and above. Postnatal survival of off-spring was profoundly diminished among the highest dose groups of 10–40 mg/kg/d, and slightly diminished at the intermediate dose of 5 mg/kg/d (no effect at 3 mg/kg/d for reduced survival) [7]. Furthermore, growth deficits were also observed in a dose-dependent manner (no effect level of 1 mg/kg/d), and this finding was the first suggestion that lactation in dams might be compromised at ≥5 mg/kg/d gestational exposures, contributing to the postnatal growth deficits and increased mortality observed in offspring. Eye opening was significantly delayed at ≥5 mg/kg/d. Interestingly, puberty was slightly delayed in female offspring at ≥20 mg/kg/d, while the onset of puberty was accelerated in male offspring, even in the lowest dose tested (1 mg/kg/d). Other studies confirmed the findings of developmental deficits through further assessment of endpoints of interest (full litter resorptions, delayed eye opening, mammary growth deficits, etc.), and isolated their origins to transplacental and milk-borne exposures [44,45].
The relationship between developmental health effects and PFOA exposure in rodents vs humans is still unclear, but similarities may exist. Some epidemiological studies reported reduced birth weight and other developmental effects associated with PFOA exposure similar to those reported in mice [32,34,35]. In a British cohort, prenatal exposure to PFAAs was not associated with age at menarche [46]. However, the most recent report from the C8 Science Panel [47] suggests that delayed puberty (measured as either serum estradiol >20 pg/ml or self-reported menarche) in girls in their study was associated with the highest levels of serum PFOA. However, these two studies addressing pubertal outcomes should not be directly compared, because the former evaluated PFOA exposure of mothers during pregnancy, while the latter measured exposure of children ages 8–18. These two studies also had vastly different PFOA exposure ranges, with the former having much lower average exposure levels than did the latter.
4. Developmental PFOA exposure and adult disease onset
The Barker hypothesis [48–50] suggests that developmental exposures or nutritional conditions have adverse adult outcomes most notably when there is a mismatch between the in utero and adult metabolic environments. Barker’s classic epidemiology study [48,51] reported that children born during the Dutch famine became adults who were overweight and had an increased incidence of cardiovascular disease, non-insulin dependent diabetes, and kidney disease vs those born before or after the famine [50]. Early life exposures with associated changes in adult health outcomes have become known as the ‘developmental origins of adult disease’. There are numerous diseases that are now recognized as having a potential developmental origin.
While endocrine disruption is usually equated with adverse outcomes in reproductive tissue development or function, though not necessarily mediated directly through hormone receptors, it is important to recall that the endocrine system plays a broader role in regulating homeostasis. As novel endocrine disruptors are identified and explored, non-reproductive endpoints, such as metabolic effects, may be revealed as primary targets of environmental endocrine disruptors. Developmental exposure to certain environmental chemicals has been associated with adverse adult metabolic outcomes including overweight and obesity, pointing to programmatic changes in early life metabolic set-points [52]. The possibility that metabolic programming of offspring may be permanently affected by early, developmental exposure to PFOA is explored here.
4.1. PFOA and metabolic end points
Epidemiological data relating diabetes and PFOA exposure were collected at two time periods from highly exposed individuals living or working near a known point source for PFOA exposure [53,54]. Earlier cohort studies reported an increased mortality risk for workers with diabetes mellitus at this manufacturing plant [53]. More recent cross-sectional studies from residents in six water districts participating in the C8 Health Study surrounding this plant, found no significant associations between serum PFOA and fasting serum glucose or self-reported type II diabetes [15]. However, a more specific nested case–control analysis [15] of this population (1) restricting enrollees to 20 years of continuous residence in the high PFOA contaminated water district and (2) restricting data collection to medical records with diabetes verification in the 10 years before 2005, found there was a negative association between diabetes and serum PFOA, by PFOA decile. However, Lundin et al. [55] found significantly increased diabetes mortality in occupationally exposed groups vs non-exposed workers. Yet another study, evaluating a cross-sectional US population, found no association of serum PFOA levels with self-reported diabetes [56]. A publication using NHANES data from the US population found that serum PFOA concentration in adults was significantly associated with beta cell function as measured with the homeostasis model of insulin resistance (HOMA2), which allows for input of values for plasma glucose, insulin, specific insulin and C-peptide. In this study, other PFAAs which are often part of a person’s PFAA mixture exposure, also had aberrant HOMA or insulin related associations [57]. Data from studies related to fluorochemical exposure and diabetes have inconsistent outcomes in both the human and animal toxicology literature, and further study is needed to discern the role of exposure level and timing of exposure in these disparate outcomes.
In studies of the offspring of CD-1 mice orally exposed to PFOA throughout pregnancy (0.01–5 mg/kg/d) and to residual PFOA through nursing [58], the dose-related effects of PFOA exposure on metabolic end points manifested with split or dichotomous outcomes. Low dose exposures (0.01–0.3 mg/kg/d) induced elevated leptin, insulin and body weight beginning after 10 weeks of age, while higher (≥1 mg/kg) PFOA exposures resulted in mixed effects including significantly decreased body weight and spleen weight, and significantly increased brown adipose weight in adulthood. Ovary removal in animals before puberty halted the body weight related effects of low dose PFOA exposure, indicating the ovarian axis as an important contributor to the PFOA-dependent metabolic change. Also, an age-matched adult-only exposure to a separate group of animals, for an identical amount of time, produced no effect on body weight, indicating early life as a critical window of exposure for these low dose PFOA effects in female mice.
Body weight can be regulated through pathways including the thyroid, the hypothalamic pituitary adrenal axis, and the PPARs, with all of these pathways converging on leptin as a common mediator. Leptin is a hormone produced by white adipose tissue that regulates food intake, metabolism, and puberty progression [59]. Aberrant leptin concentrations or leptin resistance have been reported to induce adult overweight or obesity in multiple animals models of developmental exposure to environmental chemicals including PFOA, diethylstilbestrol, tributyltin, lead, monosodium glutamate, bisphenol A and polychlorinated biphenyls [52,58,60]. The aforementioned chemicals have also been reported to act as endocrine disruptors in animal toxicology models. Multiple endocrine disrupting chemicals have been termed ‘obesogens’ [60] because of their effect on adult body weight following early life exposures. Often, these effects appear to be sex-specific, manifesting in either exclusively male or female offspring. These effects are often seen at low, environmentally relevant doses with higher doses producing distinct, dichotomous effects, as was the case for PFOA [58].
Alterations in lipid metabolism are another area of interest in PFAA research because of laboratory animal and epidemiological data reporting cholesterol changes correlated with serum PFAA concentrations [10,61]. Interestingly, in the epidemiological data, serum PFAA levels are correlated with serum cholesterol. NHANES data demonstrated a positive association of PFOS, PFOA, and per-fluorononanoic acid (PFNA) concentrations with total and non-high density lipoprotein (HDL) cholesterol [62]. Linear regression analysis of serum lipids and cholesterol in children (age 1–17.9 yrs) with high PFOA exposure, living in the aforementioned Washington Works communities, revealed that PFOA was positively associated with total cholesterol and low density lipoprotein (LDL)-cholesterol, and that PFOS was positively associated with total, LDL-, and HDL-cholesterols [63]. The difference in mean cholesterol values between the first and fifth quintiles for PFOA was 4.6 mg/dl (total cholesterol) and 3.8 mg/dl (LDL); the difference between these quintiles in PFOS was 8.5 mg/dl (total cholesterol) and 5.8 mg/dl (LDL). Animal toxicology studies, which often monitor serum cholesterol alterations after acute high dose PFOA exposures, have found serum PFOA concentration and serum cholesterol concentration in laboratory rodents to be inversely correlated, which does not corroborate the human data. It is possible that PFOA works by varying mechanisms to affect lipid metabolism in humans and rodents (likely also affected by the exposure levels); as fibrates lower cholesterol levels in both rodents and humans, it is possible that PFOA is acting in a manner similar to the fibrates in rodents, but not humans. It is not clear that the animal studies in which the cholesterol data are reported have recapitulated human exposure scenarios (i.e., chronic low dose), therefore early life, low dose rodent data are still needed.
One population that is often overlooked in risk assessment and developmental toxicology is lactating women. A convenience sampling of 34 lactating North Carolina women in the US EPA Methods Advancement in Milk Analysis (MAMA) study [27] found serum PFOA concentrations were not significantly associated with serum cholesterol; regression analysis also yielded no significant associations between PFAA concentration and cholesterol or lipids. In the MAMA study, serum, saliva, urine and milk samples were collected from healthy lactating women between 4–7 weeks (visit 1) and 3–4 months (visit 2) postpartum. Previously reported data on PFAAs from the MAMA study were further analyzed looking for differences between serum lipids or cholesterol and serum PFAA concentration comparing the 1st and 4th quartile of PFAAs. No significant statistical differences were found at either visit. Total serum cholesterol (p = 0.006) and lipids (p = 0.04) significantly decreased between visits in the lactating women of the MAMA study (see Table 2); serum triglycerides were not significantly different between visits (109 ± 10 visit 1 vs 101 ± 11 visit 2). This differs from the data reported above in non-lactating adults, where PFOA concentrations have been reported to be positively associated with cholesterol concentration. The difference between lactating women and the general population with regard to cholesterol and PFOA may be related to altered partitioning of PFOA during lactation, to the small sample size in the MAMA study [42], metabolic differences between lactating and non-lactating individuals, or exposure levels between studies.
Table 2.
Serum cholesterol, triglycerides, and total lipids in lactating women from the MAMA study.
| Total cholesterol* | Triglycerides | Total lipids# | Sample size (n) | Visit |
|---|---|---|---|---|
| 206.0 ± 5.9 | 112.4 ± 9.5 | 642.3 ± 19.7 | 34 | 1 |
| 188.5 ± 7.0 | 103.0 ± 11.2 | 593.1 ± 22.8 | 30 | 2 |
Values expressed as means ± standard (mg/dl). Significant between visit differences as measured with Wilcoxon signed ranks test for cholesterol.
p = 0.0055.
Lipids p = 0.0363.
4.2. Reproductive tissue outcomes following PFOA exposures
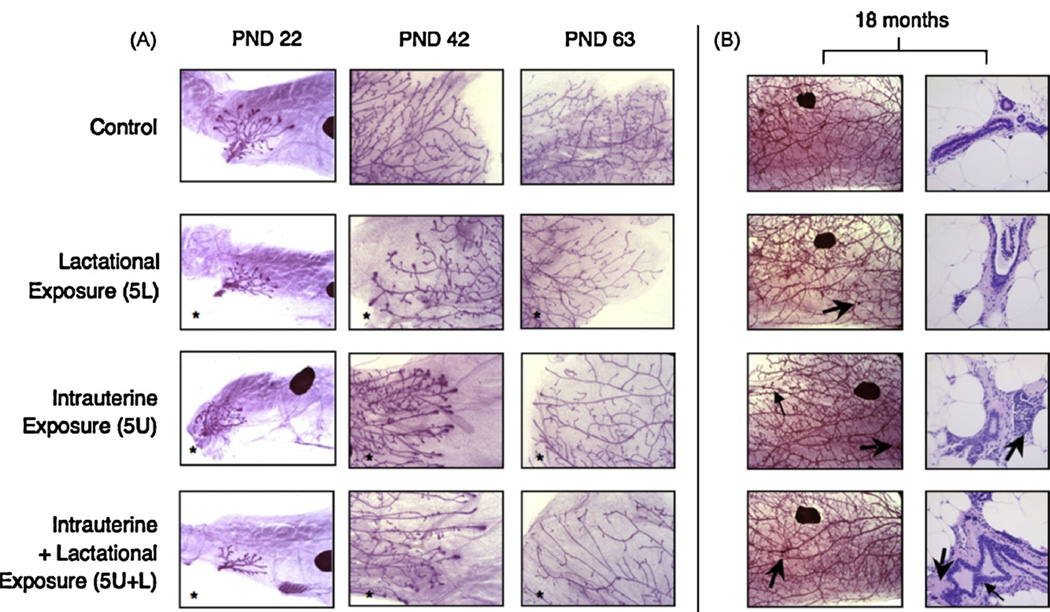
In addition to its metabolic effects, developmental exposure to PFOA seems to target reproductive tissues, in animal studies. Notably, our own laboratory has worked extensively, documenting the consequences in the mammary gland following developmental PFOA exposures in CD-1 mice. At exposure levels sufficient to diminish growth in offspring (5 mg/kg/d, maternal dose), development of mammary epithelium is significantly stunted in female offspring, independent of body weight effects [44]. Furthermore, the tissue remains permanently stunted into maturity, at 63 days of age, even among offspring exposed at lower doses (3 mg/kg/d) and those exposed only via lactational transfer [64]. At 18 months of age, gestationally-exposed offspring exhibit distinct histopathologic differences from controls, which may arise as a consequence of the delayed timing of certain mammary maturation events (Fig. 1). For example, while longitudinal growth of the epithelium is delayed in treated animals, it does eventually grow to the length of the gland, as compared to branching morphogenesis, the reduction in which was not overcome. On the other hand, the appearance of decreased branching may provide clues to the mode of action by which PFOA exerts its effects on the tissue. As judged by whole mount morphological evaluation, developmentally PFOA-exposed animals exhibited a higher density of darkly-staining foci in the mammary epithelium in late-life, which may represent inflammation, increased stromal density, or potentially hyperplastic regions of ductal epithelium [64]. In this same study, a cross-foster component of the experiments demonstrated that neonatal exposures, via only milk (lactational transfer from dams) was sufficient at both the 5 mg/kg and the 3 mg/kg maternal doses for lasting mammary effects in female offspring. These effects were evident in PND1 pups after only 12–14 h of nursing, suggesting a large amount of PFOA is sequestered into milk during late pregnancy. This finding demonstrated that early postnatal exposures to PFOA also have the potential to permanently alter mammary gland development.
Fig. 1.
MG development of female offspring in a late-life effects cross-foster study. (A) Whole mount preparations of mammary tissue from female offspring are shown at PND 22 (25×; a lymph node appears as a large darkly staining object), PND 42 (50×), and PND 63 (50×). Glands pictured are representative of mean respective scores (N = 10–13 adult females per treatment group at PND 22, N = 9–18 per group at PND 42, N = 9–17 per group at PND 63). *Significant treatment effect by ANOVA, compared to control; p < 0.05. (B) On the left, whole mounts from representative adult female offspring at 18 months of age (16×). Large arrows indicate unusual, darkly staining foci; one small arrow in 5U indicates peripheral, localized increases in epithelial density observed in some PFOA-exposed animals at 18 months. On the right, histopathologic images from contralateral glands in the same animal show ductal areas that account for darkly staining foci observed on whole mounts (400×; N = 5–12 females per group). This figure was reproduced with permission from Elsevier, Inc., and is excerpted from [64].
Because chronic drinking water exposures are reported to represent a primary route of human exposure, we recently performed a multi-generational study addressing the consequences of both developmental (during gestation) and chronic (drinking water) exposures on the lactational function of the mammary gland in the F1 offspring, specifically evaluating the development of their F2 offspring (White et al., submitted, Environ Health Perspect). While lactational morphology in P0 and F1 females was compromised at P0 administered doses of 1 and 5 mg/kg/d, and following chronic drinking water exposure at 5 ppb (serum PFOA concentrations ranged from 60 to 90 ng/ml for most animals), no change in milk volume was detected in the F1 dams. Although the F2 offspring in all PFOA-treatment groups grew at the same rate as controls, they exhibited histological delays in the development of the mammary gland, between 22 and 63 days postnatally, especially at the highest doses. These data suggest that significant morphological effects on the mammary gland can occur at serum concentrations within the reported range for human exposures [62].
Our work has continued in an effort to identify the lowest-and no-observed adverse effect levels (LOAEL and NOAEL, respectively) for the developmental effects of PFOA on the mammary gland. To this end, we followed a similar dosing and experimental paradigm as in our prior work, employing maternal doses ranging from 0.01 to 3.0 mg/kg/d for all or half of gestation, with the lowest dose being two orders of magnitude smaller than our lowest reported dose prior to this study (Macon et al., submitted, Toxicol Sci). Surprisingly, all treatment groups displayed some compromise in histologic mammary gland development, including the lowest dose groups. With respect to mammary gland effects, gestational exposure to the maternal dose of 0.01 mg/kg/d is the reported LOAEL, and a NOAEL for these effects has not been identified. These findings are noteworthy, given that offspring hepatomegaly – historically one of the most sensitive endpoints in developmental and other toxicity studies of PFOA – did not occur at the lowest doses employed in this study (transient effects at 0.3 mg/kg/d in full gestation and 1.0 mg/kg/d in half gestation exposure paradigms), indicating that mammary gland stunting is a more sensitive endpoint than increased liver weight. Importantly, the internal dose of the mice exhibiting low dose mammary gland abnormalities (approximately 50–60 ng/ml) was below the median serum level in Little Hocking, Ohio [65], the most highly exposed of the six water districts in the C8 Health Study.
Studies were also undertaken to address the impact of peripubertal exposures to PFOA on the mammary gland, another sensitive window of susceptibility. Chemical exposures during this developmental window have been shown to alter cancer risk or functional outcomes [31]. Yang et al. [66] assessed the effects of peripubertal PFOA exposure on the mammary gland development of two inbred mouse strains, BALB/c and C57BL/6. Immediately post-weaning, these animals received 5 daily treatments/week, for 4 weeks, without indication of overt toxicity. The findings of these studies highlight the substantial role played by genetic variability and exposure timing in the response to, and outcome of, a PFOA exposure. While BALB/c mice exhibited a similar inhibitory response to that of gestationally-exposed CD-1 mice, at doses of 5 and 10 mg/kg/d PFOA, C57BL/6 mice instead exhibited an increased number of terminal end buds and terminal duct diameter only at the mid-range dose of 5 mg/kg/d. At the higher dose of 10 mg/kg/d, however, C57BL/6 mice displayed inhibition of growth in a manner similar to the other two strains studied. The mechanisms for the delayed or stunted growth is not clear, but the increase in stimulated terminal duct development seen in C57BL6 was associated with a 3-fold increase in serum progesterone and was also evident in PPAR-α knock out animals, suggesting that PPAR-α was not the underlying mechanism for increased TEB number and stimulated terminal ducts [67]. This physiological change was not evident in ovariectomized animals, indicating that the ovary may have an indirect role.
Human reproductive tissue and functional outcomes have been evaluated in the PFAA literature. In men, sperm quality is affected, with higher exposures to the combination of both PFOA and PFOS being associated with the greatest effects [68]. Couple subfecundity and increased time to pregnancy associated with serum PFOA concentration has also been reported [69], along with an increased risk of irregular menstrual cycles in the 3 higher quartiles of PFOA exposure vs the lowest quartile. Stein et al. [40] studied pregnancy, including self-reported pre-eclampsia and miscarriage in the highly exposed WV/Ohio population and found no association between serum PFOA and miscarriage, but a small, yet significant association between PFOA and PFOS and pre-eclampsia.
Estrogenic activity or possibly estradiol (E2) levels may be part of the mode of action for PFOA. In male rats (known to have a PFOA half-life of 4–6 days) provided 13.6 mg/kg/d PFOA in their diet, elevated serum E2 levels were noted from 1 to 12 months of exposure [9]. PFOA does not bind to or activate the E2 receptor, but elevated serum E2 has been reported in Japanese men occupationally exposed to the PFAAs [70]. As discussed earlier [58], the removal of the ovary ablated the effects of PFOA-induced weight gain in CD-1 mice, suggesting an important role of this endocrine organ. Also, ovarian hormones (including progesterone) were aberrant following peri-pubertal PFOA exposure in C57BL/6 mice [67]. In the MAMA study, serum levels of PFOSA and MePFOSA were positively correlated with human serum and milk E2 levels (Table 3), while PFNA (a 9-carbon PFAA) was negatively associated with milk E2. When tested in the immature mouse uterotrophic assay (Dixon et al., submitted Reprod Toxicol), low doses of PFOA (0.01 mg/kg/d) increased the uterine wet weight, but minimal estrogenic effects were noted at the cellular level. Finally, PFOA has been reported to alter pubertal timing in female and male offspring of rats and multiple strains of mice [7,66], and has recently been reported to delay pubertal timing in girls, but not boys [47]. These data, along with the animal toxicology studies, indicate reproductive and developmental endpoints, under the control of ovarian steroids, as sensitive to PFOA exposure and deserve further research attention.
Table 3.
Associations of PFAA exposures with endogenous hormone end points in lactating women from the MAMA study.
| Hormonemedia visit | PFCmedia visit | Rho (p-value) |
|---|---|---|
| EstradiolM1 | PFOSAS1 | 0.44 (0.011) |
| EstradiolM2 | MePFOSAS2 | 0.37 (0.060) |
| EstradiolM2 | PFNAS2 | −0.34 (0.045) |
| ProlactinM2 | MePFOSAS2 | −0.38 (0.050) |
| EstradiolS2 | PFOSAS1 | 0.53 (0.0026) |
| EstradiolS2 | MePFOSAS2 | 0.31 (0.093) |
Spearman correlations between serum PFAA concentrations and data on serum or milk immunoglobulins (S is serum; M is milk). Visit number (1 or 2) is shown as a subscript for the matrix analyzed (milk or serum). Bolded associations were significant based on p < 0.05.
4.3. Thyroid as a target tissue for effects of PFOA and other PFAAs
The thyroid has functions that may potentially affect metabolic set points; namely, individuals with diagnosed thyroid disease are likely to have body weight changes related to the disease. Thyroid disease in the US is on the rise and there is a sex-specific difference in the percentage of individuals affected (i.e., 16% of adult women and 3% of adult men have thyroid disease) [56]. A recent publication using NHANES data (collected in 1999–2000, 2003–2004 and 2005–2006) found associations with self-reported diagnosed thyroid disease and PFAA serum concentrations [56]. Adult women in the highest quartile of PFOA exposure had a statistically increased odds ratio (OR) of having thyroid disease vs women in quartile one or two; non-significant, but similar trends were also seen in adult males who are statistically less affected by thyroid disease than women. With PFOS exposure in adult males, the highest quartile PFOS exposure was associated with statistically increased OR of thyroid disease vs quartile one and two PFOS exposure. PFOS had no significant effect on OR for thyroid disease in women [56]. The NHANES data showing an association between serum PFOA and women with thyroid disease are robust and reverse causation is unlikely because this association is still seen in the medicated population of those with the disease. Another recent study examining thyroid tissue from surgical resection reported that PFOA does not concentrate in the thyroid. The serum concentration of PFOA was significantly higher than the thyroid tissue concentration. Also, PFOA concentration was not associated with underlying thyroid disease (n = 28, 8 males and 20 females) when compared to control thyroid tissues from autopsy [71]; this study has limited power and repeating it with a larger cohort and including thyroid hormone data may give more clarity to the clinical significance of PFOA concentration or body burden on the thyroid function. Other epidemiological studies show no associations between PFOA and thyroid status including thyroid stimulating hormone (TSH) in highly exposed Ohio/West Virginia residents [65], TSH and free thyroxine in New York state fisherman (n = 31, 27 men) [72], and thyroid hormone status in repeated measurements of occupationally exposed workers [73]. However, other studies in the animal toxicology literature are inconclusive. In separate studies, serum thyroid hormone concentrations were depressed in rodents [10] or monkeys [74] exposed to PFOA, but a lack of TSH elevation and analytical problems may contribute to these data being considered artifactual [10,75]. Thus, the epidemiological data indicate that the thyroid may be one axis significantly affected by PFAA exposure while the animal toxicology literature is less certain due to technical issues.
In another epidemiological study [69], PFAA serum concentration was related to increased time to pregnancy in women within the Danish National Birth Cohort study. Fertility issues can be related to inadequate thyroid function via hypothyroidism (subfecundity) or hyperthyroidism (i.e., Graves disease and increased miscarriage rate; [76]); unfortunately, this theory was not explored in the large cohort study. Also, it is not clear that this theory could be properly tested on multiparous women. Clinically, maternal hypothyroidism (or subclinical hypothyroidism) is a concern during pregnancy because of its affect on fetal brain development, i.e., potential contribution to decreased IQ and altered behavior of the offspring. The relationship between parentally-reported attention deficit hyperactivity disorder (ADHD) among 12–15 years old children and serum PFAAs in NHANES data has recently been evaluated [77]. In examining the 48 cases among 571 participants, the calculated OR for PFOA was 1.12 (95% CI 1.01–1.23). However, PFOA was weakly correlated with blood lead and data on maternal alcohol consumption (other risk factors for ADHD) during pregnancy were not collected. A recent study [78] showed low level developmental exposures to PFOA produced behavioral effects in mice that extended into adulthood. These behavioral changes were supported by a recent report [79] that prenatal exposure to 0.3 mg/kg/day affected activity levels in mice independent of brain weight changes. Neither of these studies evaluated the thyroid hormone levels of the mice, so further work is needed to examine the relationship between endocrine disruption and behavioral or learning effects.
4.4. PFOA as an immunomodulator
In addition to thyroid axis perturbation, immune modulation is also a known effect of PFAA exposure. Immunosuppression has been reported in adult animal models of PFOA exposure manifesting with B and T cell depletions, thymus atrophy, splenic atrophy, suppression of inflammatory responses, and decreased de novo antibody production, albeit at relatively high doses of PFOA exposure [80–84]. The lowest dose at which immune effects were reported was a drinking water study in male ICR mice, with a LOAEL for changes in mature splenic lymphocyte populations of 0.49 mg/kg/day, and no NOAEL was identified [85]. Sensitivity of the immune system to PFAAs appears to differ based on mouse strain. In the human literature, a cross sectional study comparing adult residents living in a contaminated water district to non-exposed volunteers found no association between serum PFOA and lymphocytes, neutrophils, eoisionophils or basophils, but there was a significant association between PFOA and absolute monocyte counts [65]. In another small adult study (n = 34), elevated serum IgA was reported in PFOA production workers with non-significant effects on serum IgG or IgM [86]. Within participants of the Danish National Birth Cohort Study, prenatal PFOA or PFOS exposure was not associated with any increased risk of hospitalization for infectious disease in early childhood [87]. Because the human studies are few and often lack power or consistency, and indicators in the animal toxicology literature point to immunomodulation, more research on the effect of PFAAs on immune function is warranted. However, it is not yet clear from the animal studies if developmental exposure is required for immunological effects.
Lactation is an important time for evaluation of the immune system, as maternal influences significantly contribute to the immature infant immune system. Little information is known and few studies have been conducted in lactating women detailing PFAA exposures. Data from the MAMA study on lactating North Carolina women (Table 4) report a significant negative correlation between serum IgE and serum PFOS at two distinct times during lactation; the majority of the IgE data were within normal physiological ranges [42]. Another 6-carbon polyfluorinated compound, perfluorohexanesulfonate (PFHxS) was also negatively associated with maternal IgE, albeit at only one of two visits, and it was also negatively correlated with secretory IgA in milk (Table 4). Another study interested in the associations between PFAAs (PFOA specifically) and immune markers is the C8 Science Panel project. A preliminary status report listed on the C8 Science Panel project website highlights immune related indicators that will soon be reported in peer reviewed literature; “For IgA the pattern of association indicated a significant decreasing trend with increasing PFOA; this was also apparent for IgE but only in females” [88]. They also reported a downward relationship between C reactive protein and increasing PFOA in men and women. The authors indicate that these changes were small in magnitude and that most of the serum immune parameters remained within the physiologically normal range, but the changes were apparent at the lowest deciles. In earlier studies of production workers (n = 31), significant elevations in serum IgA were associated with increasing PFOA exposure; these earlier data, while lacking power, are incongruent with the current findings of the C8 panel and those in the MAMA study for other PFAA (Table 4), possibly due to the high exposure in occupational settings. The C8 panel mentions that its future studies will speak to the associations or lack thereof of PFAAs with known diseases of the immune system. Influenza immunization titers and PFOA serum concentrations are an area that is proposed to be followed in their upcoming studies. Thus, PFOA exposure and immune function appear to be inversely associated in multiple human and animal models, albeit with mild changes in immune values.
Table 4.
Associations of PFAA exposures with immune end points in lactating women from the MAMA study.
| Immunoglobulinmedia visit | PFAAmedia visit | Rho (p-value) |
|---|---|---|
| IgES1 | PFHxSS1 | −0.29 (0.0954) |
| IgES1 | PFOSS1 | −0.33 (0.0543) |
| IgES1 | Total PFCS1 | −0.31 (0.0777) |
| IgES2 | PFHxSS2 | −0.40 (0.0266) |
| IgES2 | PFOSS2 | −0.48 (0.0072) |
| IgES2 | Total PFCS2 | −0.46 (0.0098) |
| Secretory IgAM1 | PFHxSS1 | −0.47 (0.0059) |
| Secretory IgAM2 | PFNAS2 | −0.388 (0.045) |
| IgMS2 | PFOSAS2 | −0.36(0.050) |
Spearman correlations between serum PFAA concentrations and data on serum or milk PFAA concentrations and data on serum or milk immunoglobulins (S is serum; M is milk). Visit number (1 or 2) is shown as a subscript for the matrix analyzed (milk or serum). Bolded associations were significant based on p < 0.05.
4.5. PFOA effects in the liver
One of the most well documented, though not necessarily endocrine-mediated effects of PFOA observed in animal models is hepatomegaly. This and other liver effects may contribute to the hepatocarcinogenicity of PFOA in rodents. The molecular target of PFOA for liver effects was originally defined as PPAR-α [45]. Expressed at high levels in the rodent liver, this nuclear recap tor is involved primarily in metabolism, but also in growth and development, cell regulation, and carcinogenesis. In its hepatic role in metabolism, PPAR-α contributes to the regulation of fatty acid oxidation, as well as ketogenesis, lipid transport, and gluconeogenesis [89]. It has more recently been established that the effects of PFOA on the liver are mainly PPARα-mediated, but PPARα-independent mechanisms also exist. PFOA increased liver weight in PPARα-knockout mice (KO), as well as their wild type (WT) counterparts, but the archetypal PPARα activator (Wyeth 14643) elevated liver weight only in WT mice [83,90,91]. However, histological differences between the livers of WT and KO mice treated with PFOA were evident [91]. Increased peroxisomes were common in the treated WT mice, while KO mice had few peroxisomes and increased vacuoles.
Hepatomegaly appears to be a transient effect in developmentally PFOA-exposed mice. Interestingly, the liver weight increases reported in studies of developmentally PFOA-exposed animals had dissipated by 18 months, when the Hines et al. [58] study was terminated and serum PFOA concentrations had returned to control, background levels. In more recent studies (Macon et al., submitted, Toxicol Sci), it appeared that liver hypertrophy induced by full gestational exposure to PFOA was ameliorated 4 weeks after exposure ceased, even though serum PFOA levels remain elevated for up to 12 weeks. This reversibility of effect has also been reported in adult-dosed rats and mice.
The basic endogenous functions of PPARs are still being assessed. A number of dietary fatty acids are believed to bind and activate PPAR-α, which is consistent with the role the receptor is thought to play in metabolism of dietary fats and the regulation of blood lipids. PPAR-α has also been the intentional target of pharmaceutical agents, in the case of fibrate drugs for the treatment of hyperlipidemia, a class of drugs which raise HDL cholesterol and lower triglycerides and VLDL cholesterol. PFOA is structurally homologous to dietary fatty acids to which PPAR-α is known to respond. Based on information to date, PFOA seems to affect cholesterol and triglycerides in humans differently than in rodents, as opposed to fibrates and Wyeth 14643, which affect lipids similarly in humans and rodents. The reason for this difference may be that fibrates and Wyeth 14643 are pure PPAR-α activators, while PFOA also activates other nuclear receptors. Furthermore, activation of PPAR-α and other nuclear receptors by PFOA may prove to be a novel means of endocrine disruption, especially as it pertains to metabolism.
In rodents, hepatic expression of PPAR-α is thought to be much higher than in humans. This historically held thought was based on a small number of expression studies, primarily reporting RNA as opposed to protein. More recent studies have tried to carefully assess inter-species differences in expression of PPAR-α across tissues [92,93]. Rodent studies of PFOA toxicity have primarily utilized high dose adult exposures, known effects of which include hepatotoxicity in the form of hepatomegaly and liver tumors, effects thought to be mediated by high PPAR-α and thus not considered a significant health concern for humans (specifically for tumor outcomes) [2]. Hepatomegaly also occurs in PFOA-exposed neonates though, and fetal murine PPAR-α expression first appears at, and persists from, approximately GD 11 [93]. Though incompletely characterized over life-stages and populations, human PPAR-α is expressed in fetal hepatic tissue, and at increasing levels with age [93]. The identification of human hepatic effects is limited to evaluation of liver enzymes, as it is otherwise difficult due to the invasiveness of procedures to identify effects, such as liver biopsy. Also, the potential differential dose response in human vs model organisms may be related not only to hepatic or other tissue expression, but also differential receptor sensitivity. The potential differences in human sensitivity should contribute to concern over fluorochemical-mediated effects, including endocrine disruption.
5. Conclusions
PFOA is a known developmental toxicant. Exposure during pregnancy has induced both early and later life adverse health outcomes in rodents and associations between PFOA exposures (occupational/environmental) and human health effects have been reported. The dose-responsivity and magnitude of endocrine disrupting effects is not identical across species in all cases, in fact for some outcomes, such as correlations of cholesterol compared to PFOA internal dose, the effects are opposing. However, this fluorinated compound has a short investigative history and in the coming years, there should be enough data to definitively say whether or not developmental exposure to PFOA could contribute to adverse human health outcomes.
The mechanism(s) by which PFOA affects various tissues or organ systems is the subject of on-going research. The use of PPAR-α KO mice has helped to identify which effects are reliant on this mechanistic pathway. We now know that PPAR-α is not the only mechanism involved in transmission of some of the PFOA-induced liver, immune, and developmental effects in mice [58,67,91,92]. Further studies are needed to identify the novel pathways regulating the endocrine-disrupting effects of PFOA and the doses at which they are active and relevant. Defining these pathways could lead to safer development of polyfluorinated applications in the future, as tests could be designed to avoid activation of the signaling mechanisms known to trigger certain systems such as branching and growth of the mammary epithelium. Currently, shorter chain compounds (ex., C6) are being substituted for PFOA. These are anticipated to be less toxic than PFOA, but there are limited toxicity data thus far, and concerns remain about widespread use without adequate testing.
Footnotes
Article submitted for the special issue on Endocrine Disruptors.
Publisher's Disclaimer: Disclaimer: The information in this document has been subjected to review by the US EPA’s National Center for Environmental Assessment and the National Institute for Environmental Health Sciences and approved for publication. The findings and conclusions in this review are those of the authors. Approval does not signify that the contents reflect the views of the Agencies or Institutes, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- 1.USEPA, OPPT. Long-chain Perfluorinated Chemicals (PFCs) Action Plan. 2009 [Google Scholar]
- 2.USEPA. Provisional Health Advisories for Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) 2009 [Google Scholar]
- 3.Lou I, Wambaugh JF, Lau C, Hanson RG, Lindstrom AB, Strynar MJ, Zehr RD, Setzer RW, Barton HA. Modeling single and repeated dose pharmacokinetics of PFOA in mice. Toxicol. Sci. 2009;107(2):331–341. doi: 10.1093/toxsci/kfn234. [DOI] [PubMed] [Google Scholar]
- 4.Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ. Health Perspect. 2010;118(2):222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seals R, Bartell SM, Steenland K. Accumulation and clearance of PFOA in current and former residents of an exposed community. Environ. Health Perspect. 2010 doi: 10.1289/ehp.1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 2006;90(2):510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 8.Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, White SS, Lindstrom AB, Strynar MJ, Petropoulou SS. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 2009;27(3–4):365–372. doi: 10.1016/j.reprotox.2009.02.012. Elmsford, N.Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biegel LB, Hurtt ME, Frame SR, O’Connor JC, Cook JC. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol. Sci. 2001;60:44–55. doi: 10.1093/toxsci/60.1.44. [DOI] [PubMed] [Google Scholar]
- 10.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 2007;99(2):366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 11.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007;115(11):1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato K, Calafat AM, Wong LY, Wanigatunga AA, Caudill SP, Needham LL. Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002. Environ. Sci. Technol. 2009;43(7):2641–2647. doi: 10.1021/es803156p. [DOI] [PubMed] [Google Scholar]
- 13.Frisbee SJ, Brooks J, Maher APA, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, Halverson JA, Vieira VM, Jin C, Leyden KM, Ducatman AM. The C8 health project: design, methods, and participants. Environ. Health Perspect. 2009;117:1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisbee SJ, Brooks AP, Jr., Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, Halverson JA, Vieira VM, Jin C, Leyden KM, Ducatman AM. The C8 health project: design, methods, and participants. Environ. Health Perspect. 2009;117(12):1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacNeil J, Steenland NK, Shankar A, Ducatman A. A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA) Environ. Res. 2009;109(8):997–1003. doi: 10.1016/j.envres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuheler K. Estimating consumer exposure to PFOS and PFOA. Risk Anal. 2008;28:251–269. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 17.Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environ. Sci. Technol. 2008;42(23):8971–8977. doi: 10.1021/es801907r. [DOI] [PubMed] [Google Scholar]
- 18.Haug LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM, Knutsen HK. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int. 2010;36(7):772–778. doi: 10.1016/j.envint.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Sun HW, Wu Q, Zhang XZ, Yun SH, Kannan K. Perfluorochemicals in meat, eggs and indoor dust in China: assessment of sources and pathways of human exposure to perfluorochemicals. Environ. Sci. Technol. 2010;44(9):3572–3579. doi: 10.1021/es1000159. [DOI] [PubMed] [Google Scholar]
- 20.Bjorklund JA, Thuresson K, De Wit CA. Perfluoroalkyl compounds (PFCs) in indoor dust: concentrations, human exposure estimates, and sources. Environ. Sci. Technol. 2009;43(7):2276–2281. doi: 10.1021/es803201a. [DOI] [PubMed] [Google Scholar]
- 21.Clarke BO, Smith SR. Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int. 2011;37(1):226–247. doi: 10.1016/j.envint.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Stahl T, Heyn J, Thiele H, Huther J, Failing K, Georgii S, Brunn H. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plants. Arch. Environ. Contam. Toxicol. 2009;57(2):289–298. doi: 10.1007/s00244-008-9272-9. [DOI] [PubMed] [Google Scholar]
- 23.Llorca M, Farre M, Pico Y, Teijon ML, Alvarez JG, Barcelo D. Infant exposure of perfluorinated compounds: levels in breast milk and commercial baby food. Environ. Int. 2010;36(6):584–592. doi: 10.1016/j.envint.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Roosens L, D’Hollander W, Bervoets L, Reynders H, Van Campenhout K, Cornelis C, Van Den Heuvel R, Koppen G, Covaci A. Brominated flame retardants and perfluorinated chemicals, two groups of persistent contaminants in Belgian human blood and milk. Environ. Pollut. 2010;158(8):2546–2552. doi: 10.1016/j.envpol.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Tao L, Kannan K, Wong CM, Arcaro KF, Butenhoff JL. Perfluorinated compounds in human milk from Massachusetts, U.S.A. Environ. Sci. Technol. 2008;42(8):3096–3101. doi: 10.1021/es702789k. [DOI] [PubMed] [Google Scholar]
- 26.Karrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindstrom G. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health Perspect. 2007;115(2):226–230. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Ehrenstein OS, Fenton SE, Kato K, Kuklenyik Z, Calafat AM, Hines EP. Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod. Toxicol. 2009;27(3–4):239–245. doi: 10.1016/j.reprotox.2009.03.001. (Elmsford, N.Y.) [DOI] [PubMed] [Google Scholar]
- 28.Keller JM, Calafat AM, Kato K, Ellefson ME, Reagen WK, Strynar M, O’Connell S, Butt CM, Mabury SA, Small J, Muir DC, Leigh SD, Schantz MM. Determination of perfluorinated alkyl acid concentrations in human serum and milk standard reference materials. Anal. Bioanal. Chem. 2010;397(2):439–451. doi: 10.1007/s00216-009-3222-x. [DOI] [PubMed] [Google Scholar]
- 29.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand. J. Work Environ. Health. 2010;36(5):413–421. doi: 10.5271/sjweh.2908. [DOI] [PubMed] [Google Scholar]
- 30.Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczeny O, Koletzko B, Volkel W. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ. Sci. Technol. 2010;44(18):7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- 31.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(6 Suppl):S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 32.Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Health Perspect. 2007;115(11):1670–1676. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int. Arch. Occup. Environ. Health. 2007;80(7):643–648. doi: 10.1007/s00420-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 34.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ. Health Perspect. 2007;115(11):1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fei C, McLaughlin JK, Tarone RE, Olsen J. Fetal growth indicators and perfluorinated chemicals: a study in the Danish National Birth Cohort. Am. J. Epidemiol. 2008;168(1):66–72. doi: 10.1093/aje/kwn095. [DOI] [PubMed] [Google Scholar]
- 36.Hamm MP, Cherry NM, Chan E, Martin JW, Burstyn I. Maternal exposure to perfluorinated acids and fetal growth. J. Expos. Sci. Environ. Epidemiol. 2010;20(7):589–597. doi: 10.1038/jes.2009.57. [DOI] [PubMed] [Google Scholar]
- 37.Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ. Health Perspect. 2009;117(4):660–667. doi: 10.1289/ehp.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolan LA, Nolan JM, Shofer FS, Rodway NV, Emmett EA. The relationship between birth weight, gestational age and perfluorooctanoic acid (PFOA)-contaminated public drinking water. Reprod. Toxicol. 2009;27(3–4):231–238. doi: 10.1016/j.reprotox.2008.11.001. (Elmsford, N.Y.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolan LA, Nolan JM, Shofer FS, Rodway NV, Emmett EA. Congenital anomalies, labor/delivery complications, maternal risk factors and their relationship with perfluorooctanoic acid (PFOA)-contaminated public drinking water. Reprod. Toxicol. 2010;29(2):147–155. doi: 10.1016/j.reprotox.2009.10.012. (Elmsford, N.Y.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein CR, Savitz DA, Dougan M. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am. J. Epidemiol. 2009;170(7):837–846. doi: 10.1093/aje/kwp212. [DOI] [PubMed] [Google Scholar]
- 41.Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ. Health Perspect. 2008;116(10):1391–1395. doi: 10.1289/ehp.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hines EP, Rayner JL, Barbee R, Moreland RA, Valcour A, Schmid JE, Fenton SE. Assays for endogenous components of human milk: comparison of fresh and frozen samples and corresponding analytes in serum. J. Hum. Lact. 2007;23(2):144–156. doi: 10.1177/0890334407300334. [DOI] [PubMed] [Google Scholar]
- 43.Butenhoff JL, Kennedy GL, Jr., Frame SR, O’Connor JC, York RG. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 2004;196(1–2):95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 44.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007;96(1):133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 45.Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, Nakayama S, Lindstrom AB, Strynar MJ, Lau C. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol. Sci. 2007;98(2):571–581. doi: 10.1093/toxsci/kfm110. [DOI] [PubMed] [Google Scholar]
- 46.Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, Flanders WD, Heron J, McGeehin MA, Marcus M. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ. Int. 2011;37(1):129–135. doi: 10.1016/j.envint.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.C8 Science Panel, Science Panel files Status Report with the Court on C8 and patterns of age of puberty. 2010 [Google Scholar]
- 48.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 49.Elford J, Whincup P, Shaper AG. Early life experience and adult cardiovascular disease: longitudinal and case–control studies. Int. J. Epidemiol. 1991;20(4):833–844. doi: 10.1093/ije/20.4.833. [DOI] [PubMed] [Google Scholar]
- 50.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 51.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303(6809):1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newbold RR. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones (Athens, Greece) 2010;9(3):206–217. doi: 10.14310/horm.2002.1271. [DOI] [PubMed] [Google Scholar]
- 53.Leonard RC, Kreckmann KH, Sakr CJ, Symons JM. Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann. Epidemiol. 2008;18(1):15–22. doi: 10.1016/j.annepidem.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Sakr CJ, Symons JM, Kreckmann KH, Leonard RC. Ischaemic heart disease mortality study among workers with occupational exposure to ammonium perfluorooctanoate. Occup. Environ. Med. 2009;66(10):699–703. doi: 10.1136/oem.2008.041582. [DOI] [PubMed] [Google Scholar]
- 55.Lundin JI, Alexander BH, Olsen GW, Church TR. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology. 2009;20(6):921–928. doi: 10.1097/EDE.0b013e3181b5f395. (Cambridge, Mass). [DOI] [PubMed] [Google Scholar]
- 56.Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ. Health Perspect. 2010;118(5):686–692. doi: 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2009;32(4):702–707. doi: 10.2337/dc08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol. Cell. Endocrinol. 2009;304(1–2):97–105. doi: 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 59.Gueorguiev M, Goth ML, Korbonits M. Leptin and puberty: a review. Pituitary. 2001;4(1–2):79–86. doi: 10.1023/a:1012943029127. [DOI] [PubMed] [Google Scholar]
- 60.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl.):S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 61.Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA) Environ. Health Perspect. 2010 doi: 10.1289/ehp.0901827. (available at http://dx.doi.org/). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general US population. Environ. Health Perspect. 2010;118(2):197–202. doi: 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, Ducatman AM. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project, Arch. Pediatr. Adolesc. Med. 2010;164(9):860–869. doi: 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White SS, Kato K, Jia LT, Basden BJ, Calafat AM, Hines EP, Stanko JP, Wolf CJ, Abbott BD, Fenton SE. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod. Toxicol. 2009;27(3–4):289–298. doi: 10.1016/j.reprotox.2008.11.054. (Elmsford, N.Y.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Emmett EA, Zhang H, Shofer FS, Freeman D, Rodway NV, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum levels and certain health parameters. J. Occup. Environ. Med. 2006;48(8):771–779. doi: 10.1097/01.jom.0000233380.13087.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang C, Tan YS, Harkema JR, Haslam SZ. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod. Toxicol. 2009;27(3–4):299–306. doi: 10.1016/j.reprotox.2008.10.003. (Elmsford, N.Y.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Y, Tan YS, Haslam SZ, Yang C. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol. Sci. 2011;115(1):214–224. doi: 10.1093/toxsci/kfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jorgensen N. Do perfluoroalkyl compounds impair human semen quality? Environ. Health Perspect. 2009;117(6):923–927. doi: 10.1289/ehp.0800517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 2009;24(5):1200–1205. doi: 10.1093/humrep/den490. [DOI] [PubMed] [Google Scholar]
- 70.Ishibashi H, Ishida H, Matsuoka M, Tominaga N, Arizono K. Estrogenic effects of fluorotelomer alcohols for human estrogen receptor isoforms alpha and beta in vitro. Biol. Pharm. Bull. 2007;30(7):1358–1359. doi: 10.1248/bpb.30.1358. [DOI] [PubMed] [Google Scholar]
- 71.Pirali B, Negri S, Chytiris S, Perissi A, Villani L, La Manna L, Cottica D, Ferrari M, Imbriani M, Rotondi M, Chiovato L. Perfluorooctane sulfonate and perfluorooctanoic acid in surgical thyroid specimens of patients with thyroid diseases. Thyroid. 2009;19(12):1407–1412. doi: 10.1089/thy.2009.0174. [DOI] [PubMed] [Google Scholar]
- 72.Bloom MS, Kannan K, Spliethoff HM, Tao L, Aldous KM, Vena JE. Exploratory assessment of perfluorinated compounds and human thyroid function. Physiol. Behav. 2010;99(2):240–245. doi: 10.1016/j.physbeh.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Olsen GW, Burris JM, Burlew MM, Mandel JH. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J. Occup. Environ. Med. 2003;45(3):260–270. doi: 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- 74.Butenhoff J, Costa G, Elcombe C, Farrar D, Hansen K, Iwai H, Jung R, Kennedy G, Jr., Lieder P, Olsen G, Thomford P. Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months. Toxicol. Sci. 2002;69(1):244–257. doi: 10.1093/toxsci/69.1.244. [DOI] [PubMed] [Google Scholar]
- 75.Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB, Butenhoff JL. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS) Toxicology. 2008;243(3):330–339. doi: 10.1016/j.tox.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Poppe K, Velkeniers B, Glinoer D. The role of thyroid autoimmunity in fertility and pregnancy. Nat. Clin. Pract. Endocrinol. Metab. 2008;4(7):394–405. doi: 10.1038/ncpendmet0846. [DOI] [PubMed] [Google Scholar]
- 77.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit hyperactivity disorder in U.S. children aged 12–15 years. Environ. Health Perspect. 2010;118(12):1762–1767. doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29(1):160–169. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 79.Onishchenko N, Fischer C, Wan Ibrahim WN, Negri S, Spulber S, Cottica D, Ceccatelli S. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotox. Res. 2011;19(3):452–461. doi: 10.1007/s12640-010-9200-4. [DOI] [PubMed] [Google Scholar]
- 80.Dewitt JC, Copeland CB, Strynar MJ, Luebke RW. Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ. Health Perspect. 2008;116(5):644–650. doi: 10.1289/ehp.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Q, Abedi-Valugerdi M, Xie Y, Zhao XY, Moller G, Nelson BD, DePierre JW. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int. Immunopharmacol. 2002;2(2–3):389–397. doi: 10.1016/s1567-5769(01)00164-3. [DOI] [PubMed] [Google Scholar]
- 82.Yang Q, Xie Y, Alexson SE, Nelson BD, DePierre JW. Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem. Pharmacol. 2002;63(10):1893–1900. doi: 10.1016/s0006-2952(02)00923-1. [DOI] [PubMed] [Google Scholar]
- 83.Yang Q, Xie Y, Depierre JW. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin. Exp. Immunol. 2000;122(2):219–226. doi: 10.1046/j.1365-2249.2000.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Q, Xie Y, Eriksson AM, Nelson BD, DePierre JW. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem. Pharmacol. 2001;62(8):1133–1140. doi: 10.1016/s0006-2952(01)00752-3. [DOI] [PubMed] [Google Scholar]
- 85.Son HY, Lee S, Tak EN, Cho HS, Shin HI, Kim SH, Yang JH. Perfluorooctanoic acid alters T lymphocyte phenotypes and cytokine expression in mice. Environ. Toxicol. 2009;24(6):580–588. doi: 10.1002/tox.20459. [DOI] [PubMed] [Google Scholar]
- 86.Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J. Occup. Environ. Med. 2009;51(3):364–372. doi: 10.1097/JOM.0b013e3181965d80. [DOI] [PubMed] [Google Scholar]
- 87.Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ. Res. 2010;110(8):773–777. doi: 10.1016/j.envres.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 88.C8 Science Panel, PFOA and Immune Biomarkers in Adults Exposed to PFOA in Drinking Water in the Mid Ohio Valley. 2009 [Google Scholar]
- 89.Bernal-Mizrachi C, Weng S, Feng C, Finck BN, Knutsen RH, Leone TC, Coleman T, Mecham RP, Kelly DP, Semenkovich CF. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat. Med. 2003;9(8):1069–1075. doi: 10.1038/nm898. [DOI] [PubMed] [Google Scholar]
- 90.DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden-Adams MM, Luebke RW, Luster MI. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit. Rev. Toxicol. 2009;39(1):76–94. doi: 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- 91.Wolf DC, Moore T, Abbott BD, Rosen MB, Das KP, Zehr RD, Lindstrom AB, Strynar MJ, Lau C. Comparative hepatic effects of perfluorooctanoic acid and WY 14,643 in PPAR-alpha knockout and wild-type mice. Toxicol. Pathol. 2008;36(4):632–639. doi: 10.1177/0192623308318216. [DOI] [PubMed] [Google Scholar]
- 92.Abbott BD, Wolf CJ, Das KP, Zehr RD, Schmid JE, Lindstrom AB, Strynar MJ, Lau C. Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPAR alpha) in the mouse. Reprod. Toxicol. 2009;27(3–4):258–265. doi: 10.1016/j.reprotox.2008.05.061. (Elmsford, N.Y.). [DOI] [PubMed] [Google Scholar]
- 93.Abbott BD, Wood CR, Watkins AM, Das KP, Lau CS. Peroxisome proliferator-activated receptors alpha, beta, and gamma mRNA and protein expression in human fetal tissues. PPAR Res. 2010 doi: 10.1155/2010/690907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Kishi R, Nakazawa H. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ. Health Perspect. 2004;112(11):1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuklenyik Z, Reich JA, Tully JS, Needham LL, Calafat AM. Automated solid-phase extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ. Sci. Technol. 2004;38(13):3698–3704. doi: 10.1021/es040332u. [DOI] [PubMed] [Google Scholar]
- 96.Jin Y, Liu X, Li T, Qin H, Zhang Y. Status of perfluorochemicals in adult serum and umbilical blood in Shenyang. Wei sheng yan jiu (J. Hyg. Res.) 2004;33(4):481–483. [PubMed] [Google Scholar]
- 97.So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, Lam PK. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ. Sci. Technol. 2006;40(9):2924–2929. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- 98.Volkel W, Genzel-Boroviczeny O, Demmelmair H, Gebauer C, Koletzko B, Twardella D, Raab U, Fromme H. Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: results of a pilot study. Int. J. Hyg. Environ. Health. 2008;211(3–4):440–446. doi: 10.1016/j.ijheh.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 99.Karrman A, Domingo JL, Llebaria X, Nadal M, Bigas E, van Bavel B, Lindstrom G. Biomonitoring perfluorinated compounds in Catalonia, Spain: concentrations and trends in human liver and milk samples. Environ. Sci. Pollut. Res. Int. 2010;17(3):750–758. doi: 10.1007/s11356-009-0178-5. [DOI] [PubMed] [Google Scholar]
- 100.Liu J, Li J, Zhao Y, Wang Y, Zhang L, Wu Y. The occurrence of perfluorinated alkyl compounds in human milk from different regions of China. Environ. Int. 2010;36(5):433–438. doi: 10.1016/j.envint.2010.03.004. [DOI] [PubMed] [Google Scholar]