Abstract
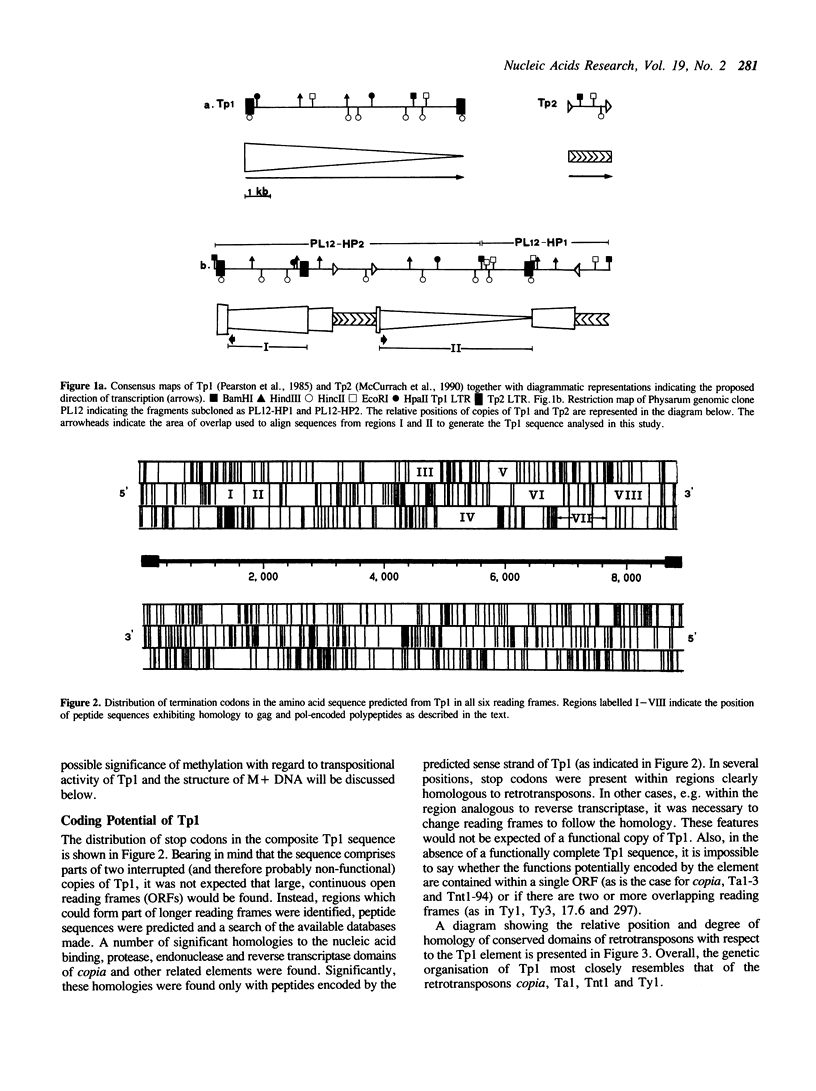
The repetitive fraction of the genome of the eukaryotic slime mould Physarum polycephalum is dominated by the Tp1 family of highly repetitive retrotransposon-like sequences. Tp1 elements consist of two terminal direct repeats of 277bp which flank an internal domain of 8.3kb. They are the major sequence component in the hypermethylated (M+) fraction of the genome where they have been found exclusively in scrambled clusters of up to 50kb long. Scrambling is thought to have arisen by insertion of Tp1 into further copies of the same sequence. In the present study, sequence analysis of cloned Tp1 elements has revealed striking homologies of the predicted amino acid sequence to several highly conserved domains characteristic of retrotransposons. The relative order of the predicted coding regions indicates that Tp1 elements are more closely related to copia and Ty than to retroviruses. Self-integration and methylation of Tp1 elements may function to limit transposition frequency. Such mechanisms provide a possible explanation for the origin and organisation of M + DNA in the Physarum genome.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. DNA methylation. The effect of minor bases on DNA-protein interactions. Biochem J. 1990 Jan 15;265(2):309–320. doi: 10.1042/bj2650309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeby P., Spicher A., de Chastonay Y., Müller F., Tobler H. Structure and genomic organization of proretrovirus-like elements partially eliminated from the somatic genome of Ascaris lumbricoides. EMBO J. 1986 Dec 1;5(12):3353–3360. doi: 10.1002/j.1460-2075.1986.tb04650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Retroviruses and retrotransposons: the role of reverse transcription in shaping the eukaryotic genome. Cell. 1985 Mar;40(3):481–482. doi: 10.1016/0092-8674(85)90190-4. [DOI] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Cappello J., Cohen S. M., Lodish H. F. Dictyostelium transposable element DIRS-1 preferentially inserts into DIRS-1 sequences. Mol Cell Biol. 1984 Oct;4(10):2207–2213. doi: 10.1128/mcb.4.10.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H., Razin A. DNA methylation and development. Biochim Biophys Acta. 1990 May 24;1049(1):1–8. doi: 10.1016/0167-4781(90)90076-e. [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Walbot V. DNA modification of a maize transposable element correlates with loss of activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1767–1771. doi: 10.1073/pnas.83.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Clare J., Farabaugh P. Nucleotide sequence of a yeast Ty element: evidence for an unusual mechanism of gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2829–2833. doi: 10.1073/pnas.82.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J., Bilanchone V. W., Haywood L. J., Dildine S. L., Sandmeyer S. B. A yeast sigma composite element, TY3, has properties of a retrotransposon. J Biol Chem. 1988 Jan 25;263(3):1413–1423. [PubMed] [Google Scholar]
- Collier D. A., Griffin J. A., Wells R. D. Non-B right-handed DNA conformations of homopurine.homopyrimidine sequences in the murine immunoglobulin C alpha switch region. J Biol Chem. 1988 May 25;263(15):7397–7405. [PubMed] [Google Scholar]
- Cooke D. J., Dee J. Methods for the isolation and analysis of plasmodial mutants in Physarum polycephalum. Genet Res. 1974 Oct;24(2):175–187. doi: 10.1017/s0016672300015202. [DOI] [PubMed] [Google Scholar]
- Covey S. N. Amino acid sequence homology in gag region of reverse transcribing elements and the coat protein gene of cauliflower mosaic virus. Nucleic Acids Res. 1986 Jan 24;14(2):623–633. doi: 10.1093/nar/14.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Eden F. C., Musti A. M., Sobieski D. A. Clusters of repeated sequences of chicken DNA are extensively methylated but contain specific undermethylated regions. J Mol Biol. 1981 May 15;148(2):129–151. doi: 10.1016/0022-2836(81)90509-x. [DOI] [PubMed] [Google Scholar]
- Emori Y., Shiba T., Kanaya S., Inouye S., Yuki S., Saigo K. The nucleotide sequences of copia and copia-related RNA in Drosophila virus-like particles. 1985 Jun 27-Jul 3Nature. 315(6022):773–776. doi: 10.1038/315773a0. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J. Transposable elements in eukaryotes. Int Rev Cytol. 1985;93:281–326. doi: 10.1016/s0074-7696(08)61376-5. [DOI] [PubMed] [Google Scholar]
- Fourcade-Peronnet F., d'Auriol L., Becker J., Galibert F., Best-Belpomme M. Primary structure and functional organization of Drosophila 1731 retrotransposon. Nucleic Acids Res. 1988 Jul 11;16(13):6113–6125. doi: 10.1093/nar/16.13.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick R. J., Henderson L. E., Hanser J. P., Rein A. Point mutants of Moloney murine leukemia virus that fail to package viral RNA: evidence for specific RNA recognition by a "zinc finger-like" protein sequence. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8420–8424. doi: 10.1073/pnas.85.22.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Grandbastien M. A., Spielmann A., Caboche M. Tnt1, a mobile retroviral-like transposable element of tobacco isolated by plant cell genetics. Nature. 1989 Jan 26;337(6205):376–380. doi: 10.1038/337376a0. [DOI] [PubMed] [Google Scholar]
- Hansen L. J., Chalker D. L., Sandmeyer S. B. Ty3, a yeast retrotransposon associated with tRNA genes, has homology to animal retroviruses. Mol Cell Biol. 1988 Dec;8(12):5245–5256. doi: 10.1128/mcb.8.12.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman N., Jack P. L., Brown A. J., McLachlan A. Distribution of inverted repeat sequences in nuclear DNA from Physarum polycephalum. Eur J Biochem. 1979 Feb 15;94(1):179–187. doi: 10.1111/j.1432-1033.1979.tb12884.x. [DOI] [PubMed] [Google Scholar]
- Hardman N., Jack P. L., Fergie R. C., Gerrie L. M. Sequence organisation in nuclear DNA from Physarum polycephalum. Interspersion of repetitive and single-copy sequences. Eur J Biochem. 1980 Jan;103(2):247–257. doi: 10.1111/j.1432-1033.1980.tb04309.x. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Inouye S., Yuki S., Saigo K. Complete nucleotide sequence and genome organization of a Drosophila transposable genetic element, 297. Eur J Biochem. 1986 Jan 15;154(2):417–425. doi: 10.1111/j.1432-1033.1986.tb09414.x. [DOI] [PubMed] [Google Scholar]
- Inouye S., Yuki S., Saigo K. Sequence-specific insertion of the Drosophila transposable genetic element 17.6. 1984 Jul 26-Aug 1Nature. 310(5975):332–333. doi: 10.1038/310332a0. [DOI] [PubMed] [Google Scholar]
- Jin Y. K., Bennetzen J. L. Structure and coding properties of Bs1, a maize retrovirus-like transposon. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6235–6239. doi: 10.1073/pnas.86.16.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. S., McClure M. A., Feng D. F., Gray J., Doolittle R. F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Ando Y., Shiba T. Unusual priming mechanism of RNA-directed DNA synthesis in copia retrovirus-like particles of Drosophila. 1986 Oct 30-Nov 5Nature. 323(6091):824–826. doi: 10.1038/323824a0. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Purifoy D. J., Powell K. L., Darby G. Site-specific mutagenesis of AIDS virus reverse transcriptase. 1987 Jun 25-Jul 1Nature. 327(6124):716–717. doi: 10.1038/327716a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Liu Q. R., Chan P. K. Identification of a long stretch of homopurine.homopyrimidine sequence in a cluster of retroposons in the human genome. J Mol Biol. 1990 Apr 5;212(3):453–459. doi: 10.1016/0022-2836(90)90324-F. [DOI] [PubMed] [Google Scholar]
- Magill J. M., Magill C. W. DNA methylation in fungi. Dev Genet. 1989;10(2):63–69. doi: 10.1002/dvg.1020100202. [DOI] [PubMed] [Google Scholar]
- Martin G., Wiernasz D., Schedl P. Evolution of Drosophila repetitive-dispersed DNA. J Mol Evol. 1983;19(3-4):203–213. doi: 10.1007/BF02099967. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Johnson M. S., Feng D. F., Doolittle R. F. Sequence comparisons of retroviral proteins: relative rates of change and general phylogeny. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2469–2473. doi: 10.1073/pnas.85.8.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurrach K. J., Rothnie H. M., Hardman N., Glover L. A. Identification of a second retrotransposon-related element in the genome of Physarum polycephalum. Curr Genet. 1990 May;17(5):403–408. doi: 10.1007/BF00334518. [DOI] [PubMed] [Google Scholar]
- McLachlan A., Hardman N. Analysis of foldback sequences and repetitive sequences in cloned segments of nuclear DNA from Physarum polycephalum. Biochim Biophys Acta. 1982 Apr 26;697(1):89–100. doi: 10.1016/0167-4781(82)90049-5. [DOI] [PubMed] [Google Scholar]
- Mellor J., Kingsman A. J., Kingsman S. M. Ty, an endogenous retrovirus of yeast? Yeast. 1986 Sep;2(3):145–152. doi: 10.1002/yea.320020302. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Rubin G. M. Complete nucleotide sequence of the Drosophila transposable element copia: homology between copia and retroviral proteins. Mol Cell Biol. 1985 Jul;5(7):1630–1638. doi: 10.1128/mcb.5.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel L. E., Crick F. H. Selfish DNA: the ultimate parasite. Nature. 1980 Apr 17;284(5757):604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Developmental expression of Drosophila melanogaster retrovirus-like transposable elements. EMBO J. 1987 Feb;6(2):419–424. doi: 10.1002/j.1460-2075.1987.tb04771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson K. E., Deka N., Schmid C. W., Misra R., Schindler C. W., Rush M. G., Kadyk L., Leinwand L. A transposon-like element in human DNA. Nature. 1985 Jul 25;316(6026):359–361. doi: 10.1038/316359a0. [DOI] [PubMed] [Google Scholar]
- Pearl L. H., Taylor W. R. A structural model for the retroviral proteases. Nature. 1987 Sep 24;329(6137):351–354. doi: 10.1038/329351a0. [DOI] [PubMed] [Google Scholar]
- Pearston D. H., Gordon M., Hardman N. Transposon-like properties of the major, long repetitive sequence family in the genome of Physarum polycephalum. EMBO J. 1985 Dec 16;4(13A):3557–3562. doi: 10.1002/j.1460-2075.1985.tb04117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples O. P., Hardman N. An abundant family of methylated repetitive sequences dominates the genome of Physarum polycephalum. Nucleic Acids Res. 1983 Nov 25;11(22):7777–7788. doi: 10.1093/nar/11.22.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples O. P., Robinson A. C., Whittaker P. A., Hardman N. Sequence organisation in nuclear DNA from Physarum polycephalum. Genomic organisation of DNA segments containing foldback sequences. Biochim Biophys Acta. 1983 Nov 17;741(2):204–213. doi: 10.1016/0167-4781(83)90060-x. [DOI] [PubMed] [Google Scholar]
- Peoples O. P., Whittaker P. A., Pearston D., Hardman N. Structural organization of a hypermethylated nuclear DNA component in Physarum polycephalum. J Gen Microbiol. 1985 May;131(5):1157–1165. doi: 10.1099/00221287-131-5-1157. [DOI] [PubMed] [Google Scholar]
- Prats A. C., Sarih L., Gabus C., Litvak S., Keith G., Darlix J. L. Small finger protein of avian and murine retroviruses has nucleic acid annealing activity and positions the replication primer tRNA onto genomic RNA. EMBO J. 1988 Jun;7(6):1777–1783. doi: 10.1002/j.1460-2075.1988.tb03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Fink G. R. DNA rearrangements associated with a transposable element in yeast. Cell. 1980 Aug;21(1):239–249. doi: 10.1016/0092-8674(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Rotman G., Itin A., Keshet E. Promoter and enhancer activities of long terminal repeats associated with cellular retrovirus-like (VL30) elements. Nucleic Acids Res. 1986 Jan 24;14(2):645–658. doi: 10.1093/nar/14.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigo K., Kugimiya W., Matsuo Y., Inouye S., Yoshioka K., Yuki S. Identification of the coding sequence for a reverse transcriptase-like enzyme in a transposable genetic element in Drosophila melanogaster. Nature. 1984 Dec 13;312(5995):659–661. doi: 10.1038/312659a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Selker E. U. DNA methylation and chromatin structure: a view from below. Trends Biochem Sci. 1990 Mar;15(3):103–107. doi: 10.1016/0968-0004(90)90193-f. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Smyth D. R., Kalitsis P., Joseph J. L., Sentry J. W. Plant retrotransposon from Lilium henryi is related to Ty3 of yeast and the gypsy group of Drosophila. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5015–5019. doi: 10.1073/pnas.86.13.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Drosophila genome organization: conserved and dynamic aspects. Annu Rev Genet. 1981;15:219–264. doi: 10.1146/annurev.ge.15.120181.001251. [DOI] [PubMed] [Google Scholar]
- Stucka R., Lochmüller H., Feldmann H. Ty4, a novel low-copy number element in Saccharomyces cerevisiae: one copy is located in a cluster of Ty elements and tRNA genes. Nucleic Acids Res. 1989 Jul 11;17(13):4993–5001. doi: 10.1093/nar/17.13.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Kikuno R., Hayashida H., Miyata T., Kugimiya W., Inouye S., Yuki S., Saigo K. Close structural resemblance between putative polymerase of a Drosophila transposable genetic element 17.6 and pol gene product of Moloney murine leukaemia virus. EMBO J. 1985 May;4(5):1267–1272. doi: 10.1002/j.1460-2075.1985.tb03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torruella M., Gordon K., Hohn T. Cauliflower mosaic virus produces an aspartic proteinase to cleave its polyproteins. EMBO J. 1989 Oct;8(10):2819–2825. doi: 10.1002/j.1460-2075.1989.tb08428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Voytas D. F., Ausubel F. M. A copia-like transposable element family in Arabidopsis thaliana. Nature. 1988 Nov 17;336(6196):242–244. doi: 10.1038/336242a0. [DOI] [PubMed] [Google Scholar]
- Weinstock K. G., Mastrangelo M. F., Burkett T. J., Garfinkel D. J., Strathern J. N. Multimeric arrays of the yeast retrotransposon Ty. Mol Cell Biol. 1990 Jun;10(6):2882–2892. doi: 10.1128/mcb.10.6.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Tabata S., Pachl C. The clustered and scrambled arrangement of moderately repetitive elements in Drosophila DNA. Cell. 1979 Dec;18(4):1231–1246. doi: 10.1016/0092-8674(79)90235-6. [DOI] [PubMed] [Google Scholar]
- Whittaker P. A., Hardman N. Methylation of nuclear DNA in Physarum polycephalum. Biochem J. 1980 Dec 1;191(3):859–862. doi: 10.1042/bj1910859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Similarity of reverse transcriptase-like sequences of viruses, transposable elements, and mitochondrial introns. Mol Biol Evol. 1988 Nov;5(6):675–690. doi: 10.1093/oxfordjournals.molbev.a040521. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Honma H., Zushi M., Kondo S., Togashi S., Miyake T., Shiba T. Virus-like particle formation of Drosophila copia through autocatalytic processing. EMBO J. 1990 Feb;9(2):535–541. doi: 10.1002/j.1460-2075.1990.tb08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki S., Ishimaru S., Inouye S., Saigo K. Identification of genes for reverse transcriptase-like enzymes in two Drosophila retrotransposons, 412 and gypsy; a rapid detection method of reverse transcriptase genes using YXDD box probes. Nucleic Acids Res. 1986 Apr 11;14(7):3017–3030. doi: 10.1093/nar/14.7.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]



