Abstract
Tamoxifen decreases breast cancer recurrence, mortality, and breast cancer risk in high-risk women. Despite these proven benefits, tamoxifen use is often limited due to side effects. We identified predictors of tamoxifen-induced side effects based on clinical variables and serum tamoxifen metabolite biomarkers in a cross-sectional study of patients taking tamoxifen. We enrolled 241 women and collected data on demographics, tamoxifen use and side effects, as well as potential clinical and serum predictors. We used logistic regression models and adjusted for age, body mass index, ethnicity, education, prior post-menopausal hormone therapy, tamoxifen duration, and endoxifen levels to identify factors associated with side effects. Common tamoxifen attributed side effects were hot flashes (64%), vaginal dryness (35%), sleep problems (36%), weight gain (6%), and depression, irritability or mood swings (6%). In multi-variate models, tamoxifen duration, age, prior post-menopausal hormone therapy, and endoxifen levels all predicted side effects. Women who had been on tamoxifen for >12 months were less likely to report side effects (OR 0.15, 95% CI, 0.04–0.58) or severe side effects (OR 0.05, 95% CI, 0.005–0.58) compared to women on tamoxifen for <12 months. Compared to women younger than 50, women who were age 60–70 and older than 70 were less likely to report side effects (OR 0.22, 95% CI, 0.03–1.35; OR 0.13, 95% CI, 0.01–0.99; respectively). Women who previously took post-menopausal hormone therapy were more likely to report severe side effects. Women with higher endoxifen levels were more likely to report side effects (OR 1.67, 95% CI, 1.01–2.77 per standard deviation increase in endoxifen). Clinicians should consider closely monitoring adherence in women taking tamoxifen, especially in younger women, and women who previously took hormone therapy. The association between endoxifen levels and side effects is consistent with the data that suggest that endoxifen is the most highly active metabolite of tamoxifen.
Keywords: Tamoxifen, side effects, predictors, biomarkers, endoxifen, breast cancer treatment
Introduction
Tamoxifen, a selective estrogen receptor modulator (SERM), acts as an estrogen receptor antagonist in breast tissue, and decreases breast cancer recurrence and mortality in women with estrogen receptor-positive breast cancer [1]. Tamoxifen is also effective in primary prevention of breast cancer in high-risk women [2]. However, the use of tamoxifen for prevention is limited due to its side effect profile [3].
Tamoxifen commonly causes a range of side effects such as hot flashes and occasionally causes more serious adverse events such endometrial hyperplasia or endometrial cancer [4–6] and venous thromboembolic disease [7]. Other side effects attributed to tamoxifen are night sweats, gynecologic symptoms (vaginal dryness, vaginal discharge), depression, forgetfulness, sleep alterations, weight gain, and diminished sexual functioning [8–12]. Women with side effects are also more likely to take medications that inhibit cytochrome P450 2D6 enzyme (CYP2D6) [13–16].
Several studies have demonstrated that women with more side effects from tamoxifen are less likely to have a recurrence of breast cancer compared to women who have no side effects [17,18]. Therefore, determining the key predictors of side effects from tamoxifen may help to understand the factors that make tamoxifen more effective.
Tamoxifen is a prodrug and undergoes extensive first-pass oxidative metabolism into more active metabolites by the cytochrome P450 (CYP450) pathway, predominantly the CYP2D6, to 4-hydroxy-tamoxifen (4-OH-tam) and 4-hydroxy-N-desmethyl-tamoxifen, also known as endoxifen [19–21]. Both metabolites have a 30- to 100-fold higher affinity respectively for the estrogen receptor (ER) compared to tamoxifen, but only endoxifen has significant bioavailability [20–26]. CYP2D6 is the rate-limiting enzyme that converts N-desmethyl-tamoxifen (ND-tam), a less active antiestrogen, into endoxifen and modulates its plasma concentrations [21,24,25]. Some studies have suggested an association between CYP2D6 genotypes and side effects of tamoxifen, while other studies have failed to demonstrate such an association [27–30].
We sought to identify clinical predictors and serum biomarkers that may affect the risk of side effects among women taking tamoxifen. In particular, we examined a series of demographic and clinical factors to determine if any of these can be used to predict side effects. In addition, we examined the association between side effects and the levels of tamoxifen metabolites, and cytochrome P450 2D6 (CYP2D6) genetic variants.
Materials and methods
Study design and population
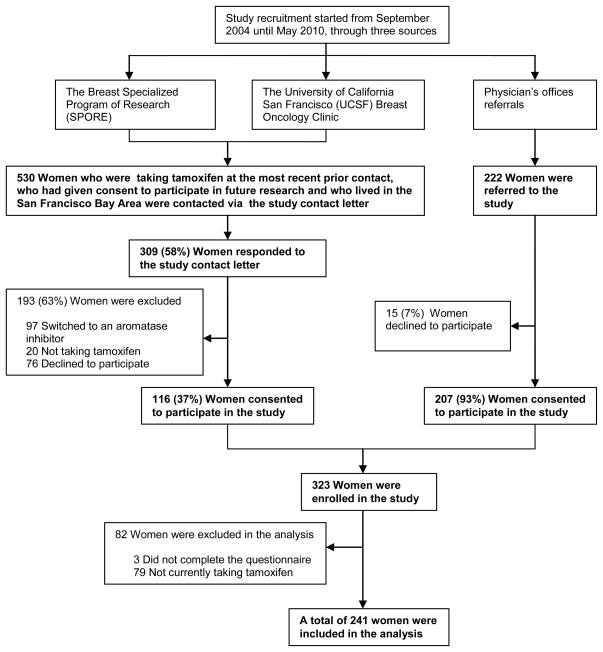
Potential participants included women who were currently taking tamoxifen. Participants were excluded if they were currently taking post-menopausal hormone therapy (HT), had a bleeding disorder or were taking anticoagulation medication (e.g. coumadin or heparin) or could not give informed consent. From September 2004 until May 2010, participants were recruited through three sources: 1) The Breast Specialized Program of Research (SPORE), a well-established, large cohort of San Francisco Bay Area women with breast cancer; 2) the University of California San Francisco (UCSF) Breast Oncology Clinic; and 3) physician referral. The Breast SPORE includes women from UCSF, California Pacific Medical Center (CPMC) and San Francisco General Hospital (SFGH, affiliated with UCSF). From the Breast SPORE database and the UCSF Breast Oncology Clinic and via the study contact letter, we identified 530 women who had been taking tamoxifen at the most recent prior contact, had previously given consent to be contacted for participation in future research, and who lived in the San Francisco Bay area. We received responses from 309 (58%) of the women we contacted. Among the respondents, 116 women (37%) were enrolled in the study, 117 (38%) reported that they had switched to an aromatase inhibitor (AI, N = 97) or were not on tamoxifen (N = 20), and 76 (25%) declined to participate. Another 222 were referred to the study from physician’s offices. Of these, 15 (7%) did not agree to participate, leaving 207 (93%) women referred from physician’s offices who consented to the study. From all recruitment sources, 323 women were enrolled in this study. Of these enrollees, 79 were not currently taking tamoxifen and 3 were excluded from the data analysis because they did not complete the questionnaire. Thus, a total of 241 women were included in our analyses (Fig. 1). The institutional review boards at UCSF (including its affiliated SFGH) and CPMC approved the study and all women provided written informed consent at study entry.
Fig. 1.
Overall recruitment of the study population
Demographic, breast cancer risk factors and side effects from tamoxifen data collection
We conducted in person interviews through a questionnaire that was administered by a trained research associate. The questionnaires collected the following information: demographics, past medical history, breast cancer history, tamoxifen adherence, duration of use, and side effects. Participants also provided blood and serum samples. All participants in the study were taking tamoxifen at the time of enrollment. All demographic, lifestyle, reproductive factors and symptoms were self-reported. Body mass index (BMI) was measured as weight in kilograms (kg) divided by the square of height in meters (m2) and categorized according to the World Health Organization categories. Women were classified as pre-menopausal, if they indicated having a menstrual period in the prior 3 months and no change in menstrual regularity in the prior year; and were considered post-menopausal, if they had no vaginal bleeding (amenorrhea) for at least 6 months without other obvious pathological or physiological cause. Patients were asked if they were experiencing hot flashes, vaginal dryness, sleep problems and any other side effects from tamoxifen. Dichotomous reported of side effects variables with yes/no responses were considered for analysis. The number, intensity, duration, and severity of side effects were also reported in the questionnaire. Severity of each side effect was rated on a Likert-type scale with responses ranging from 1 (mild) to 5 (extremely severe).
Laboratory procedures
After the interview, two 10cc tubes of blood were drawn from each participant. One tube of blood collected into EDTA (ethylene diamine tetraacetic acid) as a preservative was used for genomic DNA extraction that was performed at UCSF DNA Bank and at the UCSF Clinical Pharmacogenomics Laboratory. DNA was extracted from whole blood using the Qiagen QIAamp Blood DNA Kit (Frederick, MD, USA). After extraction, DNA was quantified and stored at −20°C. A second blood sample was collected in a serum separator tube and stored at −70°C to measure tamoxifen metabolites, specially, endoxifen levels. Tamoxifen and metabolite concentrations were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS; Model 3200, Applied Biosystems/Sciex, Foster City, CA, USA) as previously described [31]. Tamoxifen metabolite measurements were not reported to patients or clinicians since there was no clinical data on their use at the time of the study was conceived and designed.
CYP2D6 genotype
The analysis of CYP2D6 polymorphisms was performed at the UCSF Clinical Pharmacogenomics Laboratory, a Clinical Laboratory Improvement Amendments Act (CLIA)-certified clinical laboratory, using the AmpliChip CYP450 Test (Roche Molecular Systems, Inc., Branchburg, NJ, USA), a test that is cleared for clinical used by the U.S. Food and Drug Administration (FDA). This test uses the Affymetrix microarray platform and screens for 27 different alleles of the CYP2D6 gene (including gene duplications and deletions) and 3 alleles of the CYP2C19 gene. The AmpliChip CYP450 Data Analysis Software was used to infer the genotype, and to predict the individual’s CYP2D6 enzymatic activity. We classified subjects into 4 classes: Ultra-rapid metabolizers (UMs), extensive metabolizers (EMs), intermediate metabolizers (IMs), and poor metabolizers (PMs). The test and assay conditions for this study followed the manufacturer’s instructions [32]. In approximately 1 to 2% of samples, the test results in a “no genotype” call, presumably because of a rare variant not detected by the chip that interferes with the usual hybridization patterns. In every case of a “no genotype” result from the AmpliChip, we repeated the assay at least once to confirm that the result could not be obtained. Additionally, we categorized CYP2D6 genotypes using the activity score rating as previously described by Gaedigk et al [33].
Statistical analysis
Baseline study population characteristics were described using frequencies for categorical variables and mean or median (when needed) for continuous variables. The Wald chi-squared test (χ2 test) of proportions (or Fisher’s exact test, when needed) was used for categorical comparisons of baseline characteristics to determine whether the distribution of these variables differed. Measures of association between each potential predictor and each side effect outcome were calculated using χ2 tests. Logistic regression was then used to determine which factors were predictive of the outcome and to estimate the occurrence of tamoxifen side effects. We first estimated unadjusted associations expressed in terms of odds ratios (ORs) of each independent variable. A final model was developed that included only those factors with statistically significant (P ≤ 0.05 for the Wald χ2 test) predictive ability. We used logistic regression adjusting for age, BMI, ethnicity, education level, prior post-menopausal hormone therapy (HT), length of tamoxifen treatment and endoxifen levels to identify factors that are independently associated with common side effects. The co-variates included account for clinical predictors such as current age, age at menarche (as continuous variable), menopausal status (pre-menopausal versus post-menopausal), BMI (as continuous variable), and previous use of HT (ever/never). Other common risk factors, including age at menopause, were unavailable or did not apply for some of the participants and were not, therefore, included in the analysis. The χ2 test, Fisher’s exact test and logistic regression analyses were carried out using STATA (version 10) statistical software package (StataCorp LP, College Station, TX, USA).
Results
The mean age among participants was 50.8 years old (range from 23 to 83 years old) and most (66%) were pre-menopausal at the time of breast cancer diagnosis (Table 1). Among the 79 women who were post-menopausal at the time of the study, 66% (52) had previously used hormonal therapy. Of these prior hormone users, 38% used estrogen alone, 29% used a combination of estrogen and progesterone, 8% used progesterone alone and 25% did not recall the type of HT used. The majority of women were Caucasian (69%) or Asian (22%). Most of the participants (75%) had graduated from college and completed a post-graduate degree. Only 3 women in the study were taking tamoxifen for prevention of breast cancer. The median duration of tamoxifen treatment in the overall population was 16 months. Most of the women in the study (69%) were recruited from a University hospital, 24% were recruited from community hospitals and 6% from a public hospital.
Table 1.
Baseline demographic, reproductive, prior post-menopausal hormone therapy use, breast cancer and tamoxifen treatment duration characteristics of study participants
| Baseline characteristics | Number of participants taking tamoxifen (N = 241)
|
|
|---|---|---|
| N/Mean/Median | Percent/SD/IQR | |
| Mean Age (years)a | 50.8 | ± 11.39 |
| Mean BMI (kg/m2)a | 24.19 | ± 3.91 |
| Self-reported ethnicity | ||
| Caucasian | 166 | 69 |
| Asian/East Asian | 54 | 22 |
| African American/Black | 3 | 1.5 |
| Latina/Hispanic | 13 | 5 |
| Pacific Islander | 1 | 0.5 |
| Other/Mixed | 1 | 0.5 |
| Declined/refused/do not know | 3 | 1.5 |
| Number married (yes) | 172 | 71 |
| Number full time working | 97 | 40 |
| Education levels | ||
| High school graduated or less | 9 | 4 |
| Some college | 45 | 19 |
| College graduated | 86 | 36 |
| Completed post-graduate degree | 95 | 39 |
| Socio economic status | ||
| Income < $50,000 | 34 | 14 |
| Income ≥ $50,000 to < $100,000 | 57 | 24 |
| Income ≥ $100,000 | 115 | 48 |
| Breast cancer (yes) | 238 | 99 |
| Had at least one other chronic health problem | 96 | 40 |
| Mean age at menarche (years)a | 12.70 | ± 1.31 |
| Pre-menopausal status at the time of diagnosis | 157 | 66 |
| Mean age at menopause at the time of diagnosis (years)ab | 47.94 | ± 6.20 |
| Had natural menopauseb | 50 | 63 |
| Had hysterectomy and oopherectomyb | 12 | 15 |
| Prior post-menopausal hormone therapy useb | 52 | 66 |
| Median length of post-menopausal hormone therapy use (years)bc | 4 | 2 – 9 |
| Median duration of tamoxifen treatment (months)b | 16 | 2 – 36 |
N number of participants; SD standard deviation; BMI body mass index; IQR interquartile range (Q1 = 25 percentile - Q3 = 75 percentile)
Data presented as Mean ± SD
Data reported for post-menopausal women at the time of diagnosis (N = 79, 34%)
Data presented as Median (IQR, Q1 and Q3)
Seventy three percent of the participants experienced some side effects that they attributed to tamoxifen (Table 2). However, only 51 (21%) reported a severe side effect, defined as a side effect that was rated a 4 or 5 in severity on a Likert-type scale. The most common side effect reported was hot flashes (64%) with 20% reporting severe hot flashes. Other common side effects included vaginal dryness (35%), sleep problems (36%), depression or irritability (6%) and weight gain (6%). Side effects tended to be reported most commonly among women who had been on tamoxifen for fewer than 12 months (Table 2). However, weight gain is the only side effect that seems to increase report with duration of tamoxifen use.
Table 2.
Distribution of tamoxifen-induced side effects by length of tamoxifen treatment
| Side effects from tamoxifen a | Number of participants taking tamoxifen (N = 241)
|
|||||
|---|---|---|---|---|---|---|
| Length of tamoxifen treatment (number of months)
| ||||||
| ≤ 12 months N = 110 (46%) |
13 – 24 months N = 39 (16%) |
25 – 36 months N = 32 (13%) |
> 36 months N = 57 (24%) |
Unknown length N = 3 (1%) |
Total N |
|
| No side effects | 19 (29) | 12 (18) | 16 (24) | 19 (29) | 0 | 66 |
| Hot flashes (yes) | 78 (50) | 24 (16) | 14 (9) | 36 (23) | 3 (2) | 155 |
| Severe hot flashes b | 14 (46) | 4 (13) | 2 (6) | 9 (29) | 2 (6) | 31 |
| Vaginal dryness (yes) | 37 (44) | 21 (25) | 9 (11) | 16 (19) | 1 (1) | 84 |
| Severe vaginal dryness b | 5 (27) | 5 (27) | 1 (6) | 6 (34) | 1 (6) | 18 |
| Sleep problems (yes) | 49 (56) | 18 (21) | 2 (2) | 16 (19) | 2 (2) | 87 |
| Severe sleep problems b | 11 (52) | 5 (24) | 0 | 5 (24) | 0 | 21 |
| Other side effects: | ||||||
| Weight gain | 1 (7) | 2 (14) | 4 (29) | 7 (50) | 0 | 14 |
| Irritability and mood swings | 2 (22) | 2 (22) | 1 (11) | 3 (34) | 1 (11) | 9 |
| Depression | 0 | 3 (60) | 0 | 2 (40) | 0 | 5 |
| Any side effect | 91 (52) | 27 (15) | 16 (9) | 38 (22) | 3 (2) | 175 |
| At least one severe side effect | 25 (49) | 7 (14) | 2 (4) | 15 (29) | 2 (4) | 51 |
N number of participants; % percent
Self-reported tamoxifen side effects
Severe hot flashes, vaginal dryness and sleep problems expressed as scale 4–5 on a Likert-type scale compare to women with no hot flashes, vaginal dryness and sleep problems
Among all of the metabolite levels, only endoxifen levels were significantly associated with side effects, with women who reported no side effects having approximately 2 ng/mL lower levels of endoxifen (Table 3). There was also a trend towards association between lower levels of 4-OH-tam and no side effects. CYP2D6 genotypes were not associated with side effects when analyzed using the activity score rating (Table 3) or when using the designation of poor, intermediate, extensive and ultra-rapid metabolizer status (P = 0.66). However, CYP2D6 genotypes were highly correlated with endoxifen levels (Wu et al, in preparation).
Table 3.
Association of tamoxifen metabolites concentrations (ng/mL) and CYP2D6 genotype by activity score rating and report of tamoxifen-induced side effects and severe side effects
| Tamoxifen metabolites and CYP2D6 genotype by activity score rating | Number of participants taking tamoxifen (N = 241)
|
||||
|---|---|---|---|---|---|
| No side effects | Any side effect | Severe side effects a | |||
| Mean (ng/mL) | Mean (ng/mL) | P value b | Mean (ng/mL) | P value b | |
| Endoxifen | 7.29 | 9.36 | 0.03 | 9.67 | 0.04 |
| Tamoxifen | 87.30 | 94.04 | 0.36 | 95.41 | 0.38 |
| 4-hydroxy-tamoxifen (4-OH-tam) | 3.43 | 7.51 | 0.07 | 9.42 | 0.06 |
| N-desmethyl-tamoxifen (ND-tam) | 178.15 | 187.49 | 0.49 | 193.98 | 0.40 |
| CYP2D6 genotype by activity score rating | N (%) | N (%) | P valuec | N (%) | P valuec |
| 0 | 2 (20) | 8 (80) | 0.20 | 2 (50) | 0.21 |
| 0.5 | 3 (33) | 6 (67) | 0 | ||
| 1 | 7 (14) | 42 (86) | 13 (65) | ||
| 1.5 | 10 (22) | 36 (78) | 14 (58) | ||
| 2 | 23 (30) | 54 (70) | 14 (38) | ||
| 2.5 | 1 (50) | 1 (50) | 0 | ||
| 3 | 0 | 5 (100) | 1 (100) | ||
N number of participants; % percent; P value ≤ 0.05
Severe hot flashes, vaginal dryness and sleep problems expressed as scale 4–5 on a Likert-type scale compare to women with no hot flashes, vaginal dryness and sleep problems
P value for a Student’s t-tests
P value for a Kruskal-Wallis one-way analysis of variance by rank
In multi-variate models, significant predictors of side effects included shorter duration on tamoxifen, younger age, previous use of post-menopausal hormone therapy and higher endoxifen levels (Table 4). The strongest predictor of side effects was duration of therapy with fewer side effects among women who have been on tamoxifen for >12 months. Older women were also less likely to report side effects. Compared to women who were younger than 50, women who were older than 60 were less likely to report side effects (OR 0.22, 95% CI, 0.03–1.35, P = 0.10) as were women who were older than 70 (OR 0.13, 95% CI, 0.01–0.99, P = 0.05) which constituted a significant trend between older age and decreased side effects (P for trend = 0.03). Women who had previously used HT were much more likely to report side effects (OR 3.6, 95% CI, 0.97–13.52, P = 0.05). The mean of endoxifen levels in the overall population was 8.77 ng/mL with a standard deviation of 6.31. For each standard deviation increase in endoxifen levels, we detected an odds ratio of 1.67 (95% CI, 1.01–2.77) in the risk of reporting any side effects.
Table 4.
Association of clinical and serum predictors, and report of any tamoxifen-induced side effects
| Clinical and serum predictors | Number of participants taking tamoxifen (N = 241)
|
|
|---|---|---|
| Report of any side effects (yes/no)
| ||
| Unadjusted | Adjusted a | |
| OR (95% CI) P value | OR (95% CI) P value | |
| Age | ||
| Age ≤ 40 | - | - |
| Age 41 – 50 | 1.44 (0.63, 3.28) 0.38 | 0.89 (0.23, 3.34) 0.86 |
| Age 51 – 60 | 2.45 (0.90, 6.61) 0.07 | 1.79 (0.36, 8.78) 0.47 |
| Age 61 – 70 | 0.88 (0.32, 2.35) 0.79 | 0.22 (0.03, 1.35) 0.10 |
| Age > 70 | 0.42 (0.12, 1.43) 0.16 | 0.13 (0.01, 0.99) 0.05 |
| Body mass index (BMI) | 1.0 (0.92, 1.08) 0.97 | 0.89 (0.79, 1.01) 0.09 |
| Ethnicity | ||
| Caucasian | - | - |
| Latina/Hispanic | 1.09 (0.28, 4.16) 0.89 | 5.75 (0.44, 73.69) 0.17 |
| Black/AA | 0.65 (0.05, 7.42) 0.73 | 4.67 (0.15, 140.84) 0.37 |
| Asian/East Asian | 0.55 (0.28, 1.07) 0.08 | 0.50 (0.17, 1.43) 0.20 |
| Education | 1.13 (0.58, 2.21) 0.71 | 1.48 (0.57, 3.82) 0.41 |
| Previously used post-menopausal hormone therapy (HT) | 1.18 (0.58, 2.41) 0.64 | 3.63 (0.97, 13.52) 0.05 |
| Months on tamoxifen | 0.71 (0.56, 0.89) 0.004 | 0.65 (0.46, 0.92) 0.01 |
| ≤ 12 months | - | - |
| 13 – 24 months | 0.46 (0.20, 1.08) 0.07 | 0.15 (0.04, 0.58) 0.005 |
| 25 – 36 months | 0.20 (0.08, 0.48) <0.0001 | 0.10 (0.02, 0.36) <0.0001 |
| > 36 months | 0.41 (0.19, 0.87) 0.02 | 0.23 (0.07, 0.78) 0.01 |
| Endoxifen levelsb | 1.47 (1.02, 2.12) 0.03 | 1.67 (1.01, 2.77) 0.04 |
N number of participants; OR odds ratios; CI confidence interval; P value ≤ 0.05, AA African American
Odd ratios adjusted for age, BMI, ethnicity, level of education, prior post-menopausal hormone therapy use, tamoxifen length (in months) and endoxifen levels
Odd ratio for endoxifen levels rescaled to the standard deviation
We also performed analyses of severe side effects (Table 5) since we reasoned that these were more likely to lead to decrease adherence. The risk factors for severe side effects were very similar to the risk factors for any side effects report. Younger age was also a significant risk factor for severe side effects (Table 5). Women on tamoxifen for >12 months were much less likely to report severe side effects. Women who had previously taken post-menopausal hormone therapy were also more likely to report severe side effects (OR 16.16, 95% CI, 1.42–183.28, P = 0.02). There was a trend towards an association between higher endoxifen levels and severe side effects.
Table 5.
Association of clinical and serum predictors, and report of severe side effects from tamoxifen
| Clinical and serum predictors | Number of participants taking tamoxifen (N = 241)
|
|
|---|---|---|
| Report of severe side effects
a | ||
| Unadjusted | Adjusted a | |
| OR (95% CI) P value | OR (95% CI) P value | |
| Age | ||
| Age ≤ 40 | - | - |
| Age 41 – 50 | 1.14 (0.38, 3.34) 0.81 | 0.59 (0.05, 5.95) 0.65 |
| Age 51 – 60 | 2.16 (0.63, 7.44) 0.21 | 1.34 (0.11, 15.93) 0.81 |
| Age 61 – 70 | 1.0 (0.28, 3.54) 1.0 | 0.04 (0.001, 1.13) 0.06 |
| Age > 70 | 0.56 (0.11, 2.78) 0.48 | 0.08 (0.003, 1.89) 0.11 |
| Body mass index (BMI) | 1.06 (0.96, 1.17) 0.23 | 0.99 (0.82, 1.18) 0.92 |
| Ethnicity | ||
| Caucasian | - | - |
| Latina/Hispanic | 2.0 (0.44, 9.02) 0.36 | 23.98 (0.50, 1146.26) 0.11 |
| Black/AA | 2.41 (0.20, 27.75) 0.48 | 40.80 (0.51, 3213.88) 0.09 |
| Asian/East Asian | 0.54 (0.21, 1.34) 0.18 | 0.58 (0.13, 2.48) 0.46 |
| Education | 0.61 (0.27, 1.38) 0.24 | 1.62 (0.42, 6.19) 0.48 |
| Previously used post-menopausal hormone therapy (HT) | 1.69 (0.71, 4.03) 0.23 | 16.16 (1.42, 183.28) 0.02 |
| Months on tamoxifen | 0.78 (0.57, 1.05) 0.10 | 0.79 (0.48, 1.29) 0.35 |
| ≤ 12 months | - | - |
| 13 – 24 months | 0.44 (0.14, 1.34) 0.15 | 0.05 (0.005, 0.58) 0.01 |
| 25 – 36 months | 0.09 (0.01, 0.46) 0.004 | 0.03 (0.004, 0.38) 0.006 |
| > 36 months | 0.60 (0.24, 1.47) 0.26 | 0.35 (0.07, 1.75) 0.20 |
| Endoxifen levelsb | 1.54 (0.99, 2.40) 0.05 | 1.79 (0.90, 3.56) 0.09 |
N number of participants; OR odds ratios; CI confidence interval; P value ≤ 0.05; AA African American
Odd ratios adjusted for age, BMI, ethnicity, level of education, prior post-menopausal hormone therapy use, tamoxifen length (in months) and endoxifen levels
Odd ratio for endoxifen levels rescaled to the standard deviation
We had data on co-medication use from 166 of 241 participants. Of these women, 49 reported taking co-medications described as inhibitors of CYP2D6 enzyme activity based on the Cytochrome P450 Drug Interaction Table from the Indiana University School of Medicine website [34]. In univariate analyses these women were more likely to report side effects (OR 2.73, 95% CI, 1.01–7.34, P = 0.04) and to report severe side effects (and OR 4.17, 95% CI, 1.37–12.34, P = 0.01,). The association between co-medications and report of side effects was not significant after adjusting for age, BMI, race, education, HT, tamoxifen length and endoxifen levels.. Adding co-medication use to the multivariate models did not appreciably change the other factors associated with side effects.
Discussion
We performed a cross-sectional analysis of women taking tamoxifen to identify predictors of commonly reported side effects. We found that the risk of side effects was lower among women who were on tamoxifen for longer durations. In addition, we found that younger age, previous use of post-menopausal hormone therapy and higher serum endoxifen levels were the most significant predictors in a multi-variate model. We did not detect an association between CYP2D6 genotype and side effects.
The rate of side effects in our study is consistent with previous studies; about half of the women experienced hot flashes, vaginal dryness and sleep problems were also among the most prevalent side effects. Loprinzi et al., conducted 2 studies of tamoxifen side effects and found that approximately 50% of women in both studies had hot flashes [35,36]. The incidence of hot flashes was slightly lower in their studies compared to in our population; however, since all of their patients were post-menopausal, while most of ours were pre-menopausal, and since younger age was predictive of more side effects in our study, the higher incidence in our population may be expected. Loprinzi et al., also found that a history of moderate to severe hot flashes and a history of prior estrogen therapy use were associated [35]. Our finding of an association between severity of tamoxifen side effects and prior use of HT is consistent with previous reports in which women who use HT are more likely to have side effects with menopause. Although these findings do not point to an etiology, they may help clinicians predict which women may have the most severe side effects.
Demissie et al., identified predictors of adjuvant tamoxifen use, side effects, and discontinuation in older women. They found that women who were ≥ 75 years of age were significantly less likely to report side effects. Although our population is younger, the association of fewer side effects among older women is consistent with our data. Demissie et al., also found that women with better emotional health had significantly lower odds of reporting side effects. A similar finding was seeing when the researchers restricted the analysis to hot flashes. In addition, they also observed that educational attainment, especially women who completed at least the 12th grade were more than 5 times likely to report hot flashes than those who did not complete high school [37]. We did not find any association between level of education and increase reported of hot flashes; however, women in our study were highly educated on average so that the variation may not have been the same as in the Demissie et al.
Our study is the first, to our knowledge, to report the association between any side effects from tamoxifen and endoxifen levels. We found that only endoxifen, the most active metabolite of tamoxifen significantly associated with side effects. We did not see any association between tamoxifen levels and side effects, suggesting that the association between serum endoxifen levels and side effects is not a marker of adherence since adherence would presumably have a more direct effect on tamoxifen levels. We observed a trend towards association between side effects and 4-hydroxy-tamoxifen. While this association was not significant, the trend may be consistent with other data that 4-hydroxy-tamoxifen is a highly active metabolite. However, since endoxifen has much higher bioavailability compared to 4-hydroxy-tamoxifen, its effect may be clinically more important.
We did not observe an association between CYP2D6 genotype and side effects. Recently, the WHEL study [31] reported that women with the lowest level of endoxifen have higher incidence of recurrence. They found no association between recurrence and CYP2D6 genotype. Women who experienced hot flashes from tamoxifen in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial were significantly more protected from recurrence [18]. However, the ATAC study also recently reported no association between CYP2D6 genotype and recurrence [38]. Taken together, these findings may indicate that endoxifen levels may predict both side effects from tamoxifen and risk of recurrence. In contrast, CYP2D6 genotype appears to be a less consistent predictor of recurrence, possibly because the CYP2D6 genotype only partially explains the variability in endoxifen levels. Endoxifen concentration varies not only according to the number of functional CYP2D6 alleles [39] but also in the presence of medications that inhibit CYP2D6 enzyme. Agents such as the selective serotonin reuptake inhibitors (SSRIs) paroxetine or fluoxetine, and the antiarrhythmic quinidine are among the most potent inhibitors [20,40]. When these medications are co-administered with tamoxifen to women with an EM phenotype, endoxifen concentrations are similar to those observed in PM, and have the potential, therefore, to reduce tamoxifen efficacy. Other commonly used medications such as buproprion, duloxetine, clomipramine and pimozide exhibit inhibition close to that of paroxetine, fluoxetine and quinidine [40]. Beside CYP2D6 genotypes, CYP2C19, CYP2B6, CYP2C9 and CYP3A5 are responsible for converting tamoxifen to its active metabolites. Murdter et al., found that CYP2C9 carriers of reduced-function (*2 and *3 alleles) had lower plasma concentrations of active metabolites, pointing to the role of additional pathways [41]. The inter-individual variations of the activity of these enzymes due to genetic polymorphisms could therefore be predictors of outcome during tamoxifen treatment. These findings suggest that future studies of tamoxifen efficacy and side effects should focus on direct assessment of endoxifen levels.
Our study found an association between increased risk of side effects and use of medications which inhibit CYP2D6. Since serotonin selective receptor inhibitors (SSRI’s) are among the most common CYP2D6 inhibitors used, it is likely that these medications were prescribed to treat side effects among some of the women. Therefore, the association between them and side effects may be due to the confounding effect of physicians prescribing these medications for patients with side effects.
Another important aspect of predicting side effects is ensuring adherence to treatment. Hot flashes are common among tamoxifen users and are associated with drug discontinuation [37,42]. In both ATAC and the Breast International Group (BIG) 1–98 trials, hot flashes were reported significantly less frequently with the aromatase inhibitors [43] compared to tamoxifen [44,45]. Thus, accurately predicting hot flashes may help clinicians target certain patients with pharmacologic and non-pharmacologic interventions (or treatments) to prevent or reduce the severity of hot flashes in patients who are candidates for tamoxifen treatment. In addition, in situations in which equally effective alternative treatments may be available (such as raloxifene for breast cancer prevention) accurately predicting side effects may help clinicians select therapies.
Few studies have identified specific factors that predict which women will experience side effects from tamoxifen. Predictors of menopausal hot flashes, a mechanistically related phenotype, include smoking, maternal history of hot flashes, early age of menopause, surgical menopause, higher body mass index, lower physical activity, higher follicle-stimulating hormone (FSH) levels, anxiety, alcohol use, higher parity, and lower socioeconomic status [46–51]. However, it is unknown whether any of these factors predict which women may experience hot flashes associated with tamoxifen therapy.
Although our findings identify some factors that may affect side effects from tamoxifen, our study also has several important limitations. First, our study included women who were mostly Caucasian and Asian and were well-educated; thus, our study may not be generalizable to all populations. There are reports that Asian American women report fewer peri-menopausal hot flashes [52–54], although it is unknown whether the same finding is seen with tamoxifen. Our study did include 55 Asian American women and we saw no significant difference in the report of side effects in this group. However, we were likely underpowered to detect a clinically important difference. Second, we used a cross-sectional study design. Therefore, we were unable to determine whether these side effects diminished among individual women with time or whether they led to discontinuation of the treatment.. In addition, the cross-sectional study design may have biased the results in other ways since the women on Tamoxifen for more than 1 year may be a biased subset of the women who started the therapy and thus may have different risk factors for side effects. Third, we studied tamoxifen use during a period of time when the therapeutic guidelines were changing and more new clinical trials results were recommending the use of aromatase inhibitors in clinical practice for post-menopausal women. Finally, our study only had information on co-medication use from 166 of 241 participants.
In summary, we found that women who are younger, taking tamoxifen for less than 12 months, those with higher endoxifen levels, and who had previously used post-menopausal hormone therapy are more likely to report side effects from tamoxifen. The association between side effects and endoxifen levels is intriguing because of the connection of higher endoxifen levels with breast cancer outcomes (no breast events, breast cancer recurrence and new primary breast cancer) in the WHEL trial. Large scale studies of endoxifen levels, side effects and recurrence should be pursued to definitively determine the association between these factors.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (NIGMS) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Award T32 GM007546, University of California San Francisco, Clinical Pharmacology Fellowship Training Program (W. Lorizio); California Breast Cancer Research Program (CBCRP) Grant 14OB-0166 (E. Ziv); National Cancer Institute (NCI) Grant P50 CA58207 funded UCSF Breast SPORE; the Center for Translational and Policy Research in Personalized Medicine (TRANSPERS) National Institutes of Health/National Cancer Institute (NIH/NCI) Grant P01 CA130818-02A1 (M. S. Beattie), and materials and instrumentation for the AmpliChip CYP450 Test were donated by Roche Molecular Systems, Inc. We thank Viktoriya Krepkiy (Ziv Lab) for helping with participants and administrative support.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2 (3):205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 2.Group EBCTC. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365 (9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95 (7):526–532. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 4.Kloos I, Delaloge S, Pautier P, Di Palma M, Goupil A, Duvillard P, Cailleux PE, Lhomme C. Tamoxifen-related uterine carcinosarcomas occur under/after prolonged treatment: report of five cases and review of the literature. Int J Gynecol Cancer. 2002;12 (5):496–500. doi: 10.1046/j.1525-1438.2002.01134.x. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, Boyle P. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361 (9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86 (7):527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 7.Hendrick A, Subramanian VP. Tamoxifen and thromboembolism. Jama. 1980;243 (6):514–515. [PubMed] [Google Scholar]
- 8.Love RR, Cameron L, Connell BL, Leventhal H. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151 (9):1842–1847. [PubMed] [Google Scholar]
- 9.Ganz PA, Rowland JH, Meyerowitz BE, Desmond KA. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 1998;152:396–411. doi: 10.1007/978-3-642-45769-2_38. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov NV, Wolmark N, Wickerham DL, Fisher ER, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320 (8):479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 11.Ganz PA. Impact of tamoxifen adjuvant therapy on symptoms, functioning, and quality of life. J Natl Cancer Inst Monogr. 2001;(30):130–134. doi: 10.1093/oxfordjournals.jncimonographs.a003450. [DOI] [PubMed] [Google Scholar]
- 12.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17 (9):2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 13.Kimmick GG, Lovato J, McQuellon R, Robinson E, Muss HB. Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J. 2006;12 (2):114–122. doi: 10.1111/j.1075-122X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 14.Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, LaVasseur BI, Barton DL, Novotny PJ, Dakhil SR, Rodger K, Rummans TA, Christensen BJ. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356 (9247):2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 15.Loprinzi CL, Sloan JA, Perez EA, Quella SK, Stella PJ, Mailliard JA, Halyard MY, Pruthi S, Novotny PJ, Rummans TA. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20 (6):1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 16.Loprinzi CL, Sloan J, Stearns V, Slack R, Iyengar M, Diekmann B, Kimmick G, Lovato J, Gordon P, Pandya K, Guttuso T, Jr, Barton D, Novotny P. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. J Clin Oncol. 2009;27 (17):2831–2837. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortimer JE, Flatt SW, Parker BA, Gold EB, Wasserman L, Natarajan L, Pierce JP. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108 (3):421–426. doi: 10.1007/s10549-007-9612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuzick J, Sestak I, Cella D, Fallowfield L. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9 (12):1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 19.Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49 (8):2175–2183. [PubMed] [Google Scholar]
- 20.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95 (23):1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69 (5):1722–1727. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85 (2):151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 23.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339 (22):1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 24.Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75 (2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- 25.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310 (3):1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97 (1):30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 27.Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, Desta Z, Perez EA, Ingle JN. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23 (36):9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 28.Henry NL, Rae JM, Li L, Azzouz F, Skaar TC, Desta Z, Sikora MJ, Philips S, Nguyen AT, Storniolo AM, Hayes DF, Flockhart DA, Stearns V. Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat. 2009;117 (3):571–575. doi: 10.1007/s10549-009-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madlensky L, Flatt SW, Natarajan L, Lawrence HJ, Nikoloff DM, Fontecha M, Hao S, Hillman G, Johnson A, Parker BA, JPP Hot flashes are associated with CYP2D6 genotype in breast cancer survivors taking tamoxifen. Cancer Res. 2009;69(suppl):6045 abstract. [Google Scholar]
- 30.Leyland-Jones B, Regan MM, Bouzyk M, Kammler R, Tang W, Pagani O, Maibach R, Dell’Orto P, Thurlimann B, Price KN, Viale G Group. B-CGaIBCS. Outcome according to CYP2D6 genotype among postmenopausal women with endocrine-responsive early invasive breast cancer randomized in the BIG 1–98 trial. Cancer Res. 2010;70(24 Suppl 2):Abstract nr S1–8. [Google Scholar]
- 31.Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, Nikoloff DM, Hillman G, Fontecha MR, Lawrence HJ, Parker BA, Wu AH, Pierce JP. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89 (5):718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebsamen MC, Desmeules J, Daali Y, Chiappe A, Diemand A, Rey C, Chabert J, Dayer P, Hochstrasser D, Rossier MF. The AmpliChip CYP450 test: cytochrome P450 2D6 genotype assessment and phenotype prediction. Pharmacogenomics J. 2009;9 (1):34–41. doi: 10.1038/tpj.2008.7. [DOI] [PubMed] [Google Scholar]
- 33.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83 (2):234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 34.Flockhart DA. Drug Interactions: Cytochrome P450 Drug Interaction Table. Indiana University School of Medicine; 2007. [ http://medicine.iupui.edu/clinpharm/ddis/tableaspx] [Google Scholar]
- 35.Loprinzi CL, Zahasky KM, Sloan JA, Novotny PJ, Quella SK. Tamoxifen-induced hot flashes. Clin Breast Cancer. 2000;1 (1):52–56. doi: 10.3816/cbc.2000.n.004. [DOI] [PubMed] [Google Scholar]
- 36.Perez DG, Zahasky KM, Loprinzi CL, Sloan J, Novotny P, Barton D, Pritchard KI. Tamoxifen-associated hot flashes in women. Support Cancer Ther. 2007;4 (3):152–156. doi: 10.3816/SCT.2007.n.009. [DOI] [PubMed] [Google Scholar]
- 37.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19 (2):322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 38.Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP, Salter J, Pineda S, Cuzick J, Dowsett M. Lack of correlation between gene variants in tamoxifen metabolizing enzymes with primary endpoints in the ATAC trial. Cancer Res. 2010;70(24 Suppl 2):Abstract nr S1–7. [Google Scholar]
- 39.Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, Hillman G, Hayes DF, Stearns V, Flockhart DA. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80 (1):61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Sideras K, Ingle JN, Ames MM, Loprinzi CL, Mrazek DP, Black JL, Weinshilboum RM, Hawse JR, Spelsberg TC, Goetz MP. Coprescription of tamoxifen and medications that inhibit CYP2D6. J Clin Oncol. 2010;28 (16):2768–2776. doi: 10.1200/JCO.2009.23.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, Fasching PA, Fehm T, Eichelbaum M, Schwab M, Brauch H. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89 (5):708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 42.Fallowfield L. Acceptance of adjuvant therapy and quality of life issues. Breast. 2005;14 (6):612–616. doi: 10.1016/j.breast.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Bonanni B, Macis D, Maisonneuve P, Johansson HA, Gucciardo G, Oliviero P, Travaglini R, Muraca MG, Rotmensz N, Veronesi U, Decensi AU. Polymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flashes: data from the Italian Tamoxifen Trial. J Clin Oncol. 2006;24(22):3708–3709. doi: 10.1200/JCO.2006.06.8072. author reply 3709. [DOI] [PubMed] [Google Scholar]
- 44.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365 (9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 45.Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25 (5):486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 46.Staropoli CA, Flaws JA, Bush TL, Moulton AW. Predictors of menopausal hot flashes. J Womens Health. 1998;7 (9):1149–1155. doi: 10.1089/jwh.1998.7.1149. [DOI] [PubMed] [Google Scholar]
- 47.Wilbur J, Miller AM, Montgomery A, Chandler P. Sociodemographic characteristics, biological factors, and symptom reporting in midlife women. Menopause. 1998;5 (1):43–51. [PubMed] [Google Scholar]
- 48.Hunter MS. Predictors of menopausal symptoms: psychosocial aspects. Baillieres Clin Endocrinol Metab. 1993;7 (1):33–45. doi: 10.1016/s0950-351x(05)80269-1. [DOI] [PubMed] [Google Scholar]
- 49.Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, Salamone L, Stellato R. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152 (5):463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 50.Freeman EW, Sammel MD, Grisso JA, Battistini M, Garcia-Espagna B, Hollander L. Hot flashes in the late reproductive years: risk factors for Africa American and Caucasian women. J Womens Health Gend Based Med. 2001;10 (1):67–76. doi: 10.1089/152460901750067133. [DOI] [PubMed] [Google Scholar]
- 51.Schwingl PJ, Hulka BS, Harlow SD. Risk factors for menopausal hot flashes. Obstet Gynecol. 1994;84 (1):29–34. [PubMed] [Google Scholar]
- 52.Brown DE, Sievert LL, Morrison LA, Reza AM, Mills PS. Do Japanese American women really have fewer hot flashes than European Americans? The Hilo Women’s Health Study. Menopause. 2009;16 (5):870–876. doi: 10.1097/gme.0b013e31819d88da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, Kagawa-Singer M. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med. 2001;52 (3):345–356. doi: 10.1016/s0277-9536(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 54.Sievert LL, Morrison L, Brown DE, Reza AM. Vasomotor symptoms among Japanese-American and European-American women living in Hilo, Hawaii. Menopause. 2007;14 (2):261–269. doi: 10.1097/01.gme.0000233496.13088.24. [DOI] [PubMed] [Google Scholar]