Abstract
Background
The physiological control of feeding behavior involves modulation of the intake inhibitory effects of gastrointestinal satiation signaling via endogenous hindbrain leptin receptor (LepR) and glucagon-like-peptide-1 receptor (GLP-1R) activation.
Design and Results
Using a variety of dose-combinations of hindbrain delivered (4th icv) leptin and the GLP-1R agonist exendin-4, experiments demonstrate that hindbrain LepR and GLP-1R signaling interact to control food intake and body weight in an additive fashion. In addition, the maximum intake suppressive response that could be achieved by 4th icv leptin alone in non-obese rats (~33%) was shown to be further suppressed when exendin-4 was co-administered. Importantly, it was determined that the interaction between hindbrain LepR signaling and GLP-1R signaling is relevant to endogenous food intake control, as hindbrain GLP-1R blockade by the selective antagonist exendin-(9–39) attenuated the intake inhibitory effects of hindbrain leptin delivery.
Conclusions
Collectively, the findings reported here show that hindbrain LepR and GLP-1R activation interact in at least an additive fashion to control food intake and body weight. As evidence is accumulating that combination pharmacotherapies offer greater sustained food intake and body weight suppression in obese individuals when compared to mono-drug therapies or lifestyle modifications alone, these findings highlight the need for further examination of combined CNS GLP-1R and LepR signaling as a potential drug target for obesity treatment.
Keywords: leptin, GLP-1, food intake, NTS, exendin-4, exendin-9, synergy, additivity, obesity
Introduction
Greater than 2/3 of US citizens are classified as obese or overweight 1–2. Clinical trials show that weight loss of ≥10% will substantially reduce the risk of overweight / obese individuals from developing other diseases that are obesity co-morbidities 3. Unfortunately, it is clear that for the vast majority of the population, this degree of sustained weight loss cannot be achieved by diet and exercise alone or by the limited range of available single drug therapies 3–6. Therefore, recent focus is given to combination-based pharmacotherapies to enhance obesity treatment efficacy 3, 7–9; however, the specific pharmacological treatments and mechanisms of putative interaction between drugs that make one combination approach more effective than another remain unclear. Recent attention has focused on the incretin hormone glucagon-like-peptide-1 (GLP-1) and its receptor (GLP-1R) as a promising target for both type 2 diabetes mellitus (T2DM) and obesity treatment. Indeed, GLP-1R agonists reduce food intake in both humans and animal models 10–15; an effect dependent in part on direct GLP-1R signaling within the CNS following peripheral ligand administration 16–17. This paper pursues the idea that more progress could be made in the treatment of obesity, if research identified other energy balance systems that interact with and enhance CNS GLP-1R-mediated intake inhibitory effects.
GLP-1R populations within the hindbrain stand out among other GLP-1R-expressing CNS nuclei as critical to food intake regulation. Utilizing the chronic decerebrate rat model 18, research has shown that processing within the caudal brainstem is sufficient to mediate the intake inhibitory effects produced by either peripheral- or hindbrain-delivery of a GLP-1R agonist 13. More recently, we have shown that GLP-1R expressed specifically within the medial subnucleus of the NTS (mNTS), at the level of the area postrema, are critical to food intake regulation 19–20. It is interesting to note that neurons in this region of the mNTS also process: 1] vagally mediated satiation signals that arise from the gastrointestinal (GI) tract following ingestion of food 21–22, and 2] signals conveying long-term energy storage, such as the adipose-tissue derived hormone leptin 23–24. Therefore, it may be that GLP-1R signaling specific to the mNTS participates in the control of food intake by interacting with and enhancing the intake inhibitory effects of these other anorectic signals that require mNTS processing.
Previous research shows that leptin and GLP-1R signaling interact in food intake and body weight regulation 25–27, however the CNS nuclei mediating this interaction and the nature of the interaction (i.e. additive, synergistic) within the CNS remains poorly understood. Here, using a range of dosing combinations we examine the intake inhibitory and body weight suppressive effects resulting from combined hindbrain administration of leptin and the GLP-1R agonist exendin-4. With most pharmacological treatments aimed at reducing food intake, as the dose of the drug increases, eventually a maximum intake suppressive response is achieved, such that increasing doses will not produce a further enhancement of the intake suppression produced by that drug; an effect commonly illustrated in logarithmic dose-response curves. If hindbrain leptin and GLP-1 receptor signaling reduce food intake in an additive manner, then a reasonable outcome following combined administration is that the maximum response achieved by a single drug alone (i.e. leptin) can be further augmented when combined with the second drug (i.e. exendin-4). To assess this possibility, we determined a dose of 4th icv leptin that produced the maximum intake suppressive response and subsequently examined whether food intake could be further suppressed beyond what is achievable by leptin alone when exendin-4 was co-administered. Finally, to determine whether the interaction between hindbrain LepR signaling and GLP-1R signaling is physiologically relevant to food intake control, we examined whether hindbrain GLP-1R blockade by the selective antagonist exendin-(9–39) attenuates the intake inhibitory effects of 4th icv leptin. Collectively, the findings reported here show that hindbrain LepR and GLP-1R activation interact in at least an additive fashion to control for food intake and body weight regulation.
Materials and Methods
Subjects
Adult male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) housed individually in hanging wire-bottom metal cages maintained on a 12h light cycle (22:00-10:00) / 12h dark cycle (10:00–22:00), had ad libitum access to rodent chow (500I Rodent diet, Lab Diets, St Louis, MO) and water. All rats were 9–12 weeks of age at the start of testing. All protocols and procedures conformed to the institutional standards of the University of Pennsylvania Animal Care and Use Committee.
4th icv Cannulation
Rats were under ketamine (90mg/kg, Butler Animal Health Supply, Dublin, OH), xylazine (2.7mg/kg, Anased, Shenandoah, Iowa), and acepromazine (0.64mg/kg, Bulter Animal Health Supply, Dublin, OH) anesthesia and analgesia (Metacam 2mg/kg, Boehringer Ingelheim Vetmedica, Inc. St Joseph, MO) for surgeries. Guide cannulae (26 gauge, Plastics One, Inc., Roanoke, VA) were implanted with the tip stereotaxically positioned 2.0mm above the fourth ventricle at the following coordinates: on midline, 2.5mm anterior to occipital suture, 5.2mm ventral from skull surface.
Intended anatomical positions of 4th icv injection sites were evaluated 1 week post-surgery by measurement of the sympathoadrenal-mediated glycemic response to an injection of 5-thio-D-glucose (210 μg 4th icv or 24 μg unilateral mNTS injection) 28. A post-injection elevation in baseline plasma glucose level of at least 100% was required for subject inclusion using this verification criterion.
Food Intake Assessment
Effects of combined hindbrain leptin and GLP-1 receptor activation on food intake
Two groups of rats with 4th icv cannula were used to measure the effects of GLP-1R and LepR activation in a series of dosage combinations. All the rats had their food removed at 0830 h. Each rat received counterbalanced 1μl 4th icv injection of the GLP-1R agonist exendin-4 (Ex-4; 0.05, 0.1μg; American Peptide, Sunnyvale, CA) or vehicle (aCSF) beginning at 0930 h. Fifteen minutes later, each rat received the second 1μl counterbalanced 4th icv injection of either leptin (0.4, 3.0μg; Harbor-UCLA Research and Education Institute, Torrance, CA) or vehicle (0.01M NaHCO3). Pre-weighed food was returned immediately before dark onset at 1000 h. Subsequent food intake was recorded at 1, 3, 6, and 24hr (spillage accounted for) and body weight was recorded at 0 and 24 hr. All the conditions were separated by 48–72 hr. The low doses of exendin-4 and leptin were chosen from 13, 19, 24, 29 and pilot data and were designed to be subthreshold for effect on food intake alone, while the higher doses for each were chosen from 29–31 and were designed to provide significant suppression of intake alone, yet retain the ability to see enhanced suppression of intake at all time points.
Ability of hindbrain GLP-1 receptor activation to enhance the maximum intake suppressive effect of hindbrain leptin
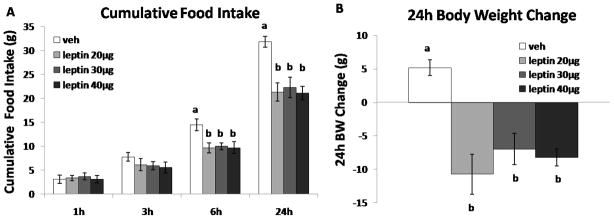
To determine the maximum suppression of intake by hindbrain delivered leptin, a separate group of animals received counterbalanced 4th icv injections of extremely high doses of leptin (20, 30, 40μg/2μl) or vehicle immediately before the dark onset. Food intake and body weight measurements occurred as described above.
Having established that the maximum intake suppression achieved by hindbrain delivered leptin is approximately 33% in non-obese rats and is achieved by a 20μg dose of leptin (see Figure 3), we next determined whether this maximum intake and body weight suppression could be further enhanced when leptin is combined with 4thicv Ex-4. Counterbalanced 4th icv injections of Ex-4 (0.3μg/1μl) or vehicle, followed by leptin (20μg/2μl) or vehicle, as well as food intake and body weight measures were carried out as described above.
Figure 3.
Three very high doses (20μg, 30μg and 40μg) of leptin were administered 4th icv to determine the maximum intake suppression that could be achieved by hindbrain leptin delivery. Each dose significantly suppressed 6h and 24h food intake and 24h body weight gain (A, B). However, no significant difference was achieved between each dose. Bars with different letters are considered significantly different (P< 0.05).
Examine the effects of blocking endogenous hindbrain GLP-1 receptor signaling on food intake suppression by hindbrain leptin
A separate group of rats received counterbalanced 4th icv injections of the GLP-1R antagonist exendin-(9–39) (Ex-9; 10μg/1μl American Peptide, Sunnyvale, CA) or vehicle followed 15min later by a second 4th icv injection of leptin (8μg/1μl) or vehicle. Food intake and body weight measurements occurred as described above. This 4th icv dose of Ex-9 has been previously shown to be without effect on food intake alone but sufficient to block the intake suppressive effects of other anorectic stimuli (e.g. lipopolysaccharide and gastric distension) 20,32.
Data and Statistical Analysis
All data are expressed as mean ± SEM, and analyzed by two-way repeated measures ANOVA, followed by Newman Keuls post-hoc tests when main effects or interactions were significant. All statistical analyses were performed using Statistica Software (Statsoft, Tulsa, OK). P<0.05 was interpreted as a significant difference.
Results
Hindbrain leptin and GLP-1 receptor activation additively suppress food intake
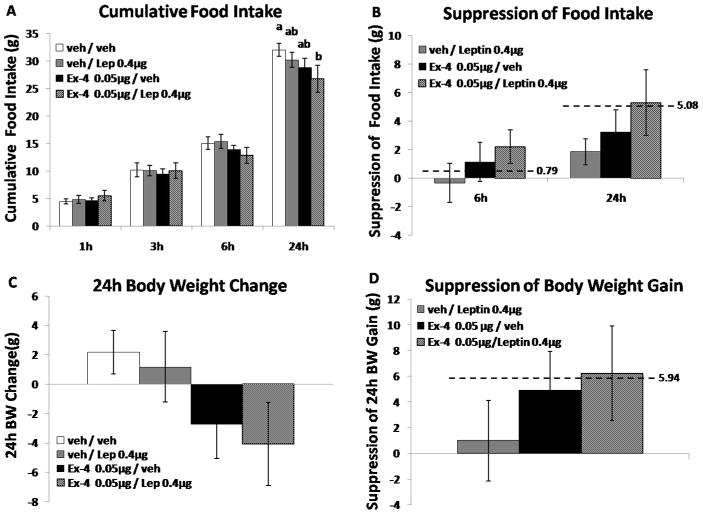
Exendin-4 0.05μg + Leptin 0.4μg
Administered alone, neither 4th icv leptin (0.4μg) nor Ex-4 (0.05μg) significantly affected food intake (n=11, BW=518.0g ± 12.9g at testing); however, when combined food intake was suppressed significantly at 24h compared to vehicle/vehicle treatments (Fig.1A, Ps<0.05). Two-way repeated measures ANOVA revealed a significant main effect for Ex-4 at 24h [F(1,8) = 6.56; P < 0.05]. This low-dose drug combination produced a degree of intake suppression that was roughly equivalent to the predicted additive suppression, defined as the algebraic sum of intake suppression induced by leptin alone and Ex-4 alone relative to vehicle (the dashed horizontal line in Fig. 1B). Co-administration of Ex-4 (0.05μg) and leptin (0.4μg) did not significantly suppress 24h body weight gain compared to vehicle/vehicle injections (Fig. 1C). However, while not significantly different from the body weight gain following vehicle/vehicle injections, the suppression in body weight by the combination of Ex-4 and leptin was equivalent to the predicted additive suppression (Fig. 1D).
Figure 1.
A–B: Neither 4th icv administration of leptin 0.4μg nor Ex-4 0.05μg influenced food intake, whereas the combination of two drugs produced significant suppression of 24h food intake. The degree of intake suppression of drug combination was equivalent to the predicted additive suppression (showed as dotted line). C–D: Co-administration of leptin 0.4μg and Ex-4 0.05μg induced a moderate but non-significant 24h body weight loss. However, the suppression of body weight by the drug combination was equivalent to the predicted additive suppression (showed as dotted line). Bars with different letters are considered significantly different (P< 0.05).
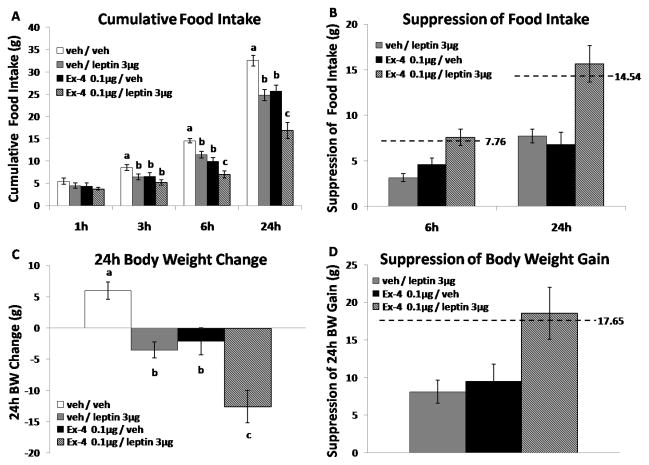
Exendin-4 0.1μg + Leptin 3μg
In a separate group of rats (n=8, BW=423.7g ± 9.0g at testing), there were significant main effects at 3, 6, 24h for leptin alone [Fs(1,7) > 18.90; Ps < 0.01] and for Ex-4 alone [Fs(1,7) > 5.28; Ps ≤ 0.05]. Post-hoc analysis revealed that administration of a higher dose of leptin (3μg) or Ex-4 (0.1μg) alone significantly reduced food intake at 3h, 6h, and 24h (Fig. 2A, Ps < 0.05) and produced a suppression in 24h body weight gain (Fig. 2C, Ps < 0.05). When combined the drug combination produced a significantly greater suppression of food intake at 6h and 24h and a greater degree of body weight loss than that observed by either drug alone (Figs. 2A, 2C, Ps < 0.05). The magnitude of the 6h and 24h food intake suppression and 24h body weight suppression was roughly equivalent to the predicted additive suppression (Figs. 2B, 2D).
Figure 2.
4th icv leptin 3μg or Ex-4 0.1μg alone significantly reduced 3h, 6h and 24h food intake and decreased 24h body weight (A, C). Co-administration of leptin 3μg and Ex-4 0.1μg produced a significantly greater degree of intake suppression at 6h and 24h (A), as well as greater degree of 24h body weight loss (C) relative to each drug administered alone. The magnitude of 6h and 24h intake suppression and 24h body weight loss produced by the drug combination was equivalent to the predicted additive suppression (B, D, showed as dotted line). Bars with different letters are considered significantly different (P< 0.05).
Hindbrain GLP-1 receptor activation augments the maximum intake suppressive effect of hindbrain leptin
To determine the maximum intake suppressive response that can be achieved by 4th icv leptin, food intake and body weight were measured following three very high doses of leptin (20μg, 30μg and 40μg). There was a significant main effect of leptin at 6h [F(1,7) = 80.10; P < 0.05] and 24h [F(1,7) = 361.95; P < 0.01]. Post-hoc analysis revealed that each dose significantly reduced 6h and 24h food intake (n=8, BW=455.0g ± 8.6g at testing), as well as 24h body weight relative to vehicle (Figs. 3A, 3B, Ps < 0.05). However, there was no significant difference in the magnitude of response between the doses. Therefore, we chose the 20μg dose of leptin to be co-administered with Ex-4 (0.3μg) in the next experiment.
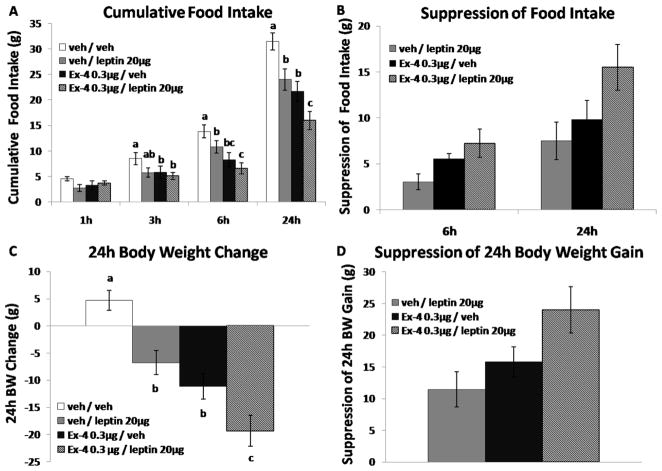
Exendin-4 0.3μg + Leptin 20μg
There was a significant main effect at 6h and 24h for both leptin alone [Fs(1,7) > 8.68; Ps < 0.05] and Ex-4 alone [Fs(1,7) > 23.10; Ps < 0.01]. Post-hoc analysis confirmed that when administered alone, leptin (20μg) or Ex-4 (0.3μg) significantly reduced 6h and 24h food intake, as well as 24h body weight. When combined leptin and Ex-4 yielded greater suppression of food intake at 6h and 24h, as well as 24h body weight loss compared with each drug alone (Figs. 4A–4D, Ps < 0.05). Thus, the maximum intake and body weight suppression achieved by hindbrain leptin alone was augmented when combined with hindbrain GLP-1R activation.
Figure 4.
Leptin 20μg and Ex-4 (4th icv) alone significantly reduced 6h and 24h food intake, as well as 24h body weight. Co-administration of leptin and Ex-4 produced a greater degree of intake suppression at 6h and 24h, as well as 24h body weight loss relative to each drug alone (A, C). The intake and body weight gain suppressions are also illustrated by the combination of Ex-4 and leptin (B, D). Bars with different letters are considered significantly different (P< 0.05).
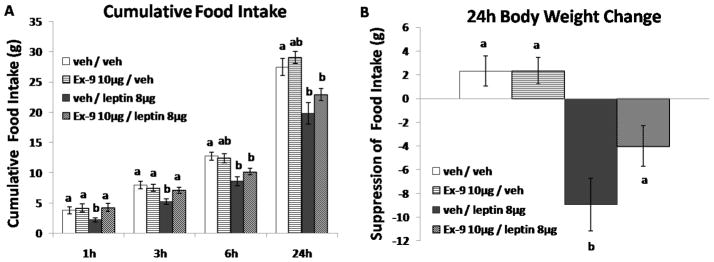
Blockade of endogenous hindbrain GLP-1 receptor signaling attenuates the intake inhibitory effects of hindbrain leptin
In separate rats (n=15, BW=368.8g ± 8.1g at testing), two-way repeated measures ANOVA revealed significant main effects for leptin at 3h, 6h, and 24h [Fs(1,14) > 6.25; Ps < 0.05], Ex-9 at 1h [F(1,14) = 7.68; P < 0.05], and a significant interaction for leptin and Ex-9 at 3h [F(1,14) = 7.15; P < 0.05]. Post-hoc analysis revealed that 4th icv administration of leptin (8μg) significantly inhibited 1h, 3h, 6h and 24h food intake and decreased 24h body weight (Figs. 5A, 5B, Ps < 0.05). Blockade of hindbrain GLP-1R via the antagonist Ex-9 (10μg; 4th icv) produced no effect on food intake. However, when administered in combination, Ex-9 attenuated the leptin-induced suppression of food intake at 1h and 3h (Fig. 5A, Ps < 0.05), as well as the 24h body weight loss (Fig. 5B, Ps < 0.05) produced by leptin.
Figure 5.
A–B: Leptin (8μg; 4th icv) significantly reduced 1h, 3h, 6h and 24h food intake and 24h body weight. GLP-1 receptor antagonist Ex-9 (10μg; 4th icv) had no effect on food intake when administered alone. However, Ex-9 attenuated leptin induced intake suppression at 1h and 3h, as well as 24h body weight loss. Bars with different letters are considered significantly different (P< 0.05).
Discussion
Combination pharmacotherapies may well offer a better obesity treatment option for sustained food intake and body weight suppression than mono-drug therapies or lifestyle modifications alone 3, 7–9. Long-acting GLP-1R agonists are being pursued as a potential treatment option for obesity, due in large part to the potent intake inhibitory effects produced by their administration 12, 33–34. Previous research shows that GLP-1R agonists, such as exendin-4, reduce food intake in part through direct activation of CNS GLP-1R 16–17. Within the CNS, the GLP-1Rs expressed in the NTS are critical for food intake regulation 19–20. Likewise, LepR signaling in the medial subnucleus of the NTS (mNTS at the level of the AP) is also involved in the normal control of energy balance 23. The current experiments examined whether hindbrain LepR and GLP-1R signaling interact in the control of food intake and body weight. Results demonstrate that endogenous hindbrain GLP-1R signaling mediates, at least in part, the intake inhibitory effects of hindbrain LepR activation, as blockade of GLP-1R by the antagonist Ex-9 attenuated the short-term intake inhibitory effects produced by 4th icv leptin. Using a range of dosing combinations, results also show that when co-administered in the hindbrain, leptin and the GLP-1R agonist exendin-4 interact in at least an additive fashion to suppress food intake and body weight. Most notably, we showed that the maximum intake inhibitory response produced by hindbrain leptin alone can be further enhanced when exendin-4 is co-administered. Collectively, results extend previous finding showing that leptin and GLP-1R activation interact in food intake control 25–27, 35 by demonstrating that: 1] this interaction is additive in nature and occurs in the hindbrain, presumably the mNTS; 2] the maximum intake inhibitory response produced by one of the systems can be further enhanced when the other system is also activated; 3] that endogenous hindbrain GLP-1R signaling mediates the intake inhibitory response of hindbrain leptin.
Support for the notion of synergy in the effects of two drugs has been operationalized in several ways 35–39. In most cases synergy is defined as an interaction that requires a response production beyond that achieved by: 1] either agent alone and 2] beyond the predicted additive response produced by the algebraic sum of the individual responses of each agent when administered alone. However, the simple arithmetical sum of responses to determine predicted additivity has been argued by many [e.g. 35–39] to be insufficient when the attempt is to categorize an interaction between agents as synergistic. Thus, it is extremely difficult to demonstrate convincingly that two agents are, in fact, interacting in a synergistic fashion. Here, however, we set out to examine a more conservative goal – whether: 1] two drugs/hormonal systems interact, and 2] the nature of the interaction is additive (and not synergistic) as indicated by an algebraic sum analysis. We showed that hindbrain leptin and exendin-4 are interacting in at least an additive fashion to control for food intake and body weight. Previous reports have shown that antagonizing the GLP-1R in the CNS will attenuate an intake inhibitory response to leptin 40; however, the site of action for this effect remained unidentified. Here, we established that these two systems interact endogenously to control food intake within the hindbrain, given that antagonism of one system in the hindbrain (GLP-1R blockade) reduced the magnitude of the food intake suppressive response produced by the second system/drug (hindbrain leptin administration). Thus, using conservative mathematical approaches, together with behavior pharmacological strategies, we conclude that leptin and GLP-1 receptor signaling in the hindbrain is interacting in energy balance regulation in at least an additive fashion.
While the use of 4th icv drug administration does confine our results to the hindbrain, it limits our ability to anatomically define the exact nucleus-site-of-action of the ligands delivered. Our previous data 19–20, 23–24, together with the confined localization of LepR in the mNTS 23–24, highlight the relevance of the mNTS as the most logical site for the interaction for leptin and GLP-1R ligands to interact in the control for food intake in body weight regulation following 4th icv delivery. However, we cannot conclude from these data whether the interaction is indeed occurring within mNTS neurons that expresses both the LepR and GLP-1R, nor can we conclude whether the additive interaction reported here for hindbrain leptin and exendin-4 involves multisynaptic communication. While all rats used in these experiments were maintained on chow and fall within the normal body weight range for male Sprague Dawley rats, it is worth noting that the body weights of the rats used in these studies were not perfectly matched between experiments. Thus, we cannot conclude whether these differences in body weight influenced the magnitude of food intake and body weight suppression by Ex-4 and leptin; nor can we conclude what effects the combination of these drugs would have in an obese rat model. Additionally, further analyses would be needed to determine if this additive interaction between leptin and GLP-1R signaling is occurring at other energy balance relevant nuclei within the CNS.
Previous studies have attempted to characterize whether a synergistic or additive suppression of food intake and body weight results from combining leptin or GLP-1R agonists with agonists for other hormone systems. These studies show that in addition to GLP-1, leptin interacts with a number of within-meal satiation signals, including but not limited to amylin 35, 41, cholecystokinin (CCK) 23, 42–43, and gastric distension 24, 44. It is interesting to note that the suppression of food intake by all of these satiation signals involves NTS processing. Whether NTS GLP-1R signaling plays a role in the interaction between leptin and these aforementioned anorectic signals is unknown. Likewise, it is also not clear whether the interaction reported here for hindbrain LepR and GLP-1R signaling is occurring within the same NTS neuron. Nonetheless, it is clear that intake suppression produced by CNS LepR or GLP-1R activation involves similar intracellular signaling pathways including the mitogen-activated protein kinase (MAPK) 19, 45 and adenosine monophosphate protein kinase (AMPK) 23, 29, 46–47. Thus, combined activation of common intracellular signaling pathways, putatively within the same NTS neuron, could allow for potentiation of intake suppression observed when GLP-1R and LepR are concomitantly activated. Such an effect may potentially make NTS neurons more sensitive to other satiation signals (e.g. amylin, CCK, and gastric distension) whose intake suppression may also involve these same intracellular signaling pathways [see 12, 48–50 for review].
In summary, using various dosing combinations of leptin and the GLP-1R agonist exendin-4, results demonstrate that hindbrain GLP-1R and LepR signaling interact in control of food intake and body weight regulation in at least an additive fashion. The finding that blockade of GLP-1R attenuated the intake inhibitory effects of hindbrain leptin, together with previous reports 20, 23, suggests that this interaction may be physiologically relevant for the normal control of food intake. From a clinical perspective of developing combination pharmacotherapies aimed at treating obesity, it is noteworthy to find that a maximum response produced by one drug can be further enhanced when the second drug is combined. Current results demonstrated such a response for leptin and exendin-4, with the maximum intake inhibitory response produced by leptin alone being augmented when combined with exendin-4 in the hindbrain. Given that systemically administered long-acting GLP-1R agonists are penetrating into the brain 51 and having direct action on CNS GLP-1R 52 and that CNS LepR are required for energy balance regulation 53, future research aimed at designing drugs that target both CNS GLP-1R and LepR in specific energy balance-relevant nuclei, such as the NTS, may prove advantageous in treating obesity.
Acknowledgments
We thank Andrea Spaeth, Amber Alhadeff, Theresa Leichner, and Samantha Fortin for their technical assistance and Dr. Lori Flanagan-Cato for valuable scientific discussion. This work was supported by the China Scholarship Council (SZ) and NIH grants: DK021397 (HJG), DK089752 (SEK), DK085435 (MRH).
References
- 1.Center for Disease Control and Prevention. Overweight and Obesity: Data and Statistics. 2009. [Google Scholar]
- 2.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol. 2010;6(10):578–88. doi: 10.1038/nrendo.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Wadden TA, Fujioka K, Toubro S, Gantz I, Erondu NE, Chen M, et al. A randomized trialof lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity (Silver Spring) 2010;18(12):2301–10. doi: 10.1038/oby.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 7.Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009;17(1):30–9. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 8.Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5(10):749–57. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 9.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32(7):1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–16. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 11.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8(4):436–47. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 12.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010;100(5):503–10. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149(8):4059–68. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56(1):8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 15.Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R983–7. doi: 10.1152/ajpregu.00323.2007. [DOI] [PubMed] [Google Scholar]
- 16.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and Central GLP-1 Receptor Populations Mediate the Anorectic Effects of Peripherally Administered GLP-1Receptor Agonists, Liraglutide and Exendin-4. Endocrinology. 2011 doi: 10.1210/en.2011-0174. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–81. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201(4352):267–9. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 19.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular Signals Mediating the Food Intake-Suppressive Effects of Hindbrain Glucagon-like Peptide-1 Receptor Activation. Cell Metab. 2011;13(3):320–30. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150(6):2654–9. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 2009;33 (Suppl 1):S11–5. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- 22.Moran TH. Gut peptide signaling in the controls of food intake. Obesity (Silver Spring) 2006;14 (Suppl 5):250S–253S. doi: 10.1038/oby.2006.318. [DOI] [PubMed] [Google Scholar]
- 23.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11(1):77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology. 2007;148(5):2189–97. doi: 10.1210/en.2006-1572. [DOI] [PubMed] [Google Scholar]
- 25.Nowak A, Bojanowska E. Effects of peripheral or central GLP-1receptor blockade on leptin-induced suppression of appetite. J Physiol Pharmacol. 2008;59(3):501–10. [PubMed] [Google Scholar]
- 26.Bojanowska E, Nowak A. Interactions between leptin and exendin-4, a glucagon-like peptide-1 agonist, in the regulation of food intake in the rat. J Physiol Pharmacol. 2007;58(2):349–60. [PubMed] [Google Scholar]
- 27.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55(12):3387–93. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 28.Slusser PG, Ritter RC. Increased feeding and hyperglycemia elicited by intracerebroventricular 5-thioglucose. Brain research. 1980;202(2):474–8. doi: 10.1016/0006-8993(80)90158-4. [DOI] [PubMed] [Google Scholar]
- 29.Hayes MR, Skibicka KP, Bence KK, Grill HJ. Dorsal hindbrain 5′-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology. 2009;150(5):2175–82. doi: 10.1210/en.2008-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150(4):1705–11. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143(1):239–46. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 32.Grill HJ, Carmody JS, Amanda Sadacca L, Williams DL, Kaplan JM. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brain stem but not forebrain GLP-1-R. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1190–3. doi: 10.1152/ajpregu.00163.2004. [DOI] [PubMed] [Google Scholar]
- 33.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative Effects of the Long-Acting GLP-1 Receptor Ligands, Liraglutide and Exendin-4, on Food Intake and Body Weight Suppression in Rats. Obesity (Silver Spring) 2011;19(7):1342–9. doi: 10.1038/oby.2011.50. [DOI] [PubMed] [Google Scholar]
- 34.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 35.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A. 2008;105(20):7257–62. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallarida RJ, Raffa RB. Testing for synergism over a range of fixed ratio drug combinations: replacing the isobologram. Life Sci. 1996;58(2):PL 23–8. doi: 10.1016/0024-3205(95)02271-6. [DOI] [PubMed] [Google Scholar]
- 37.Roth JD, Trevaskis JL, Turek VF, Parkes DG. Weighing in” on synergy: preclinical research on neurohormonal anti-obesity combinations. Brain Res. 2010;1350:86–94. doi: 10.1016/j.brainres.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41(2):93–141. [PubMed] [Google Scholar]
- 39.Bello NT, Kemm MH, Ofeldt EM, Moran TH. Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R945–52. doi: 10.1152/ajpregu.00275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstone AP, Mercer JG, Gunn I, Moar KM, Edwards CM, Rossi M, et al. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett. 1997;415(2):134–8. doi: 10.1016/s0014-5793(97)01103-4. [DOI] [PubMed] [Google Scholar]
- 41.Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B, et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149(11):5679–87. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- 42.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276(5 Pt 2):R1545–9. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 43.Matson CA, Reid DF, Cannon TA, Ritter RC. Cholecystokinin and leptin act synergistically to reduce body weight. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R882–90. doi: 10.1152/ajpregu.2000.278.4.R882. [DOI] [PubMed] [Google Scholar]
- 44.Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav. 2001;72(1–2):123–8. doi: 10.1016/s0031-9384(00)00393-0. [DOI] [PubMed] [Google Scholar]
- 45.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276(7):4747–55. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 46.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428(6982):569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 47.Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, et al. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci U S A. 2007;104(44):17358–63. doi: 10.1073/pnas.0708385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol. 2010;31(1):61–78. doi: 10.1016/j.yfrne.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berthoud HR, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav. 2006;89(4):517–24. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Potes CS, Lutz TA. Brainstem mechanisms of amylin-induced anorexia. Physiol Behav. 2010;100(5):511–8. doi: 10.1016/j.physbeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7(11):2294–300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 52.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and Central GLP-1 Receptor Populations Mediate the Anorectic Effects of Peripherally Administered GLP-1 Receptor Agonists, Liraglutide and Exendin-4. Endocrinology. 2011;152(8):3103–12. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113–21. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]