Abstract
Background and Purpose
Heme oxygenase-1 (HO-1) is an inducible phase-2 enzyme that degrades toxic heme; its role in cerebral ischemia is not fully understood. We hypothesize that chemically induced HO-1 upregulation with the novel triterpenoid CDDO-Im (2-cyano-3,12 dioxooleana-1,9 dien-28-oyl imidazolide), a robust inducer of phase-2 genes, protects neurons against ischemic injury.
Methods
Using three different models of ischemia, including oxygen-glucose deprivation (OGD) in neuronal cultures, global ischemia in rats and focal ischemia in mice, we determined 1) whether CDDO-Im induces HO-1 expression and protect against ischemic injury, and 2) whether HO-1 inhibition disrupts the neuroprotective effect of CDDO-Im.
Results
CDDO-Im treatment (50–300 nmol/L) resulted in 8-fold HO-1 upregulation in cultured neurons and protected against OGD. The protection was abolished when the cultures were transfected with Nrf2-shRNA or co-incubated with tin protoporphyrin IX (Sn-PPIX), a specific HO-1 inhibitor. In the rat model of global ischemia, intracerebroventricular (ICV) infusion of CDDO-Im (0.5–1.5 μg) augmented HO-1 expression in hippocampal neurons and resulted in significant increases in CA1 neuronal survival after global ischemia. To further strengthen the clinical relevance of the CDDO-Im treatment, we tested its effects in the mouse model of temporary focal ischemia (60 min). Post-ischemic intraperitoneal injection of CDDO-Im (10–100 μg) enhanced HO-1 expression and significantly reduced neurological dysfunction and infarct volume. ICV infusion of Sn-PPIX reduced the neuroprotective effect of CDDO-Im against global and focal ischemia.
Conclusions
CDDO-Im confers neuroprotection against ischemic injury by upregulating HO-1, suggesting that enhance of HO-1 expression may be a legitimate strategy for therapeutic intervention of stroke.
Keywords: stroke, cytoprotective, CDDO, Nrf2
Introduction
Apart from its role in hemoglobin and myoglobin, heme is also a key component of several cytoplasmic and mitochondrial enzyme complexes, such as NADPH oxidase, cyclooxygenases and cytochrome c oxidase. Heme contains an iron and plays an important role in the electron transfer mediated by these enzymes. When released under pathological conditions such as cellular stresses and ischemia, free heme may still be functional and act as a source of free radicals 1. Cells have therefore evolved a system to degrade heme, a system composed of inducible heme oxygenases 1 (HO-1) and constitutive HO-2. The end-products of the degradation include cytoprotective biliverdin and carbon monoxide; as a result, heme oxygenases are potentially neuroprotective against ischemic brain injury 2, 3. HO-1 is especially attractive because of its characteristic inducibility.
HO-1 expression is controlled by a transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Nrf2 is silent under physiological conditions, as it is bound by kelch-like ECH-associated protein 1 (Keap1). This association to Keap1 facilitates its degradation via the ubiquitin proteasome pathway. However, when Nrf2 is dissociated from Keap1, it translocates to the nucleus and initiates transcription of phase-2 enzyme target genes, with subsequent antioxidative and cytoprotective effects. Several compounds exert neuroprotective effects against strokes through activation of Nrf2 and HO-1, such as sulforaphane 4–6, Ginkgo biloba 7, 8 and polyphenols 9–11. However, the efficacy of these compounds is low and high doses are required in order to achieve neuroprotection. Thus, there remains a critical need to find potent compounds that can activate Nrf2 and HO-1 at lower doses in order to avoid potential side effects.
To address this critical need, a group of triterpenoids that demonstrate extremely potent effects in activating Nrf2 have been recently designed 12. Among them, 2-cyano-3,12 dioxooleana-1,9 dien-28-oyl imidazolide (CDDO-Im) is the most potent as it exerts effects in the picomolar to nanomolar range 13–15, is 100 times more effective than sulforaphane and 5000 times more effective than oltipraz 16. Additionally, CDDO-Im appears to be able to across the blood-brain barrier (BBB), as oral administration of CDDO-Im increases Nrf2 activity by 1.5-fold in intact mouse brain 15. CDDO-Im therefore has the potential to provide us with a potent method of inducing Nrf2 and HO-1 in injured neurons and may thereby protect them against ischemic events. The purpose of this study was to test the hypothesis that CDDO-Im protects the brain from ischemic injury via the activation of Nrf2 and upregulation of HO-1 at a low nanomoler dose. If this potent compound is found to be neuroprotective in multiple rodent animal models, this would open the door for experiments in larger species and show promise for future clinical studies.
Methods
All experimental procedures were approved by the Institutional Animal Use and Care Committee of the University of Pittsburgh, and all animals were randomly allocated into control and treatment groups. CDDO-Im was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM as the stock solution. OGD (60 min) was induced in rat primary cortical neuronal cultures, transient global cerebral ischemia (12 min) was induced in rats and transient focal cerebral ischemia (60 min) was induced in mice. Detailed methods are available online (http://stroke.ahajournals.org).
Results
1. CDDO-Im upregulates HO-1 expression in primary neurons
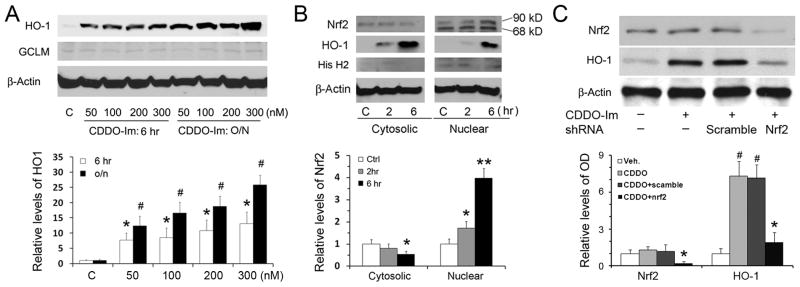
A previous report showed that CDDO-Im was a strong inducer of HO-1 in several lines of non-neural cells 14. To determine if CDDO-Im has a similar effect on neurons, we treated primary neurons with CDDO-Im and detected HO-1 levels using Western blot. HO-1 levels were barely detectable in the control group and at one hour after the CDDO-Im treatment (data not shown). However, HO-1 was increased more than 8-fold after 6-hr treatments with 50, 100, 200 and 300 nmol/L CDDO-Im (Figure 1A), supporting previous report that CDDO derivatives can activate Nrf2 pathway 17. HO-1 levels were further increased following overnight incubation. We noticed that not all phase-2 enzymes were upregulated after CDDO-Im treatment; one example was the modifier subunit of glutamate cysteine ligase (Figure 1A), suggesting HO-1 upregulation is somewhat selective under these conditions. We decided upon the 100 nmol/L concentration of CDDO for follow-up experiments, because it is sufficient to induce HO1, and higher CCDO-Im concentrations such as 300 nM may kill neurons.
Figure 1. CDDO-Im upregulates HO-1 in primary neurons.
(A) Western blots show that CDDO-Im induced HO-1 expression in both a time- and a concentration-dependent manner. n=3, *p<0.01 vs. control, #p<0.01 vs. control and p<0.05 vs. 6-hr groups of the same time-points. (B) CDDO-Im induces nuclear translocation of Nrf2 in neurons. Cultures were treated with 100 nmol/L CDDO-Im at indicated time-points, and cytosolic and nuclear fractions were extracted and subjected to Western blot analysis. n=3, *p<0.05 and **p<0.01 vs. controls. (C) Nrf2 knockdown with shRNA blocked CDDO-induced HO-1 upregulation. Neurons were first transfected with scramble or Nrf2 shRNA lentiviral particles, and then treated with for 6 hr. Whole cell proteins were extracted and subjected to Western blots. n=3, * p<0.05 vs. vehicle, CDDO-Im only and CDDO-Im with scramble shRNA; #p<0.01 vs. vehicle and Nrf2 shRNA groups.
To detect if CDDO-Im activates Nrf2 signaling, we incubated neurons with CDDO-Im for 2 or 6 hr and then extracted nuclear fractions. As shown in Figure 1B, CDDO-Im treatment increased Nrf2 levels in nuclei at both 2 hr and 6 hr, which was accompanied by increased expression of HO-1. To determine if Nrf2 is necessary for HO-1 upregulation after CDDO-Im treatment, we transfected neurons with lentiviral particles containing either rat Nrf2 shRNA or scrambled shRNAs, and then treated them with CDDO-Im 3 days later. Figure 1C shows that Nrf2 levels were decreased after Nrf2 shRNA silencing and CDDO-induced HO-1 upregulation was blocked, while the scrambled shRNAs failed to suppress either Nrf2 or HO-1. Collectively, our findings reveal that CDDO-Im activates Nrf2 signaling and upregulates HO-1 in primary neurons in both a concentration-dependent and a time-dependent manner.
2. CDDO-Im protects primary neurons against OGD via Nrf2 and HO-1
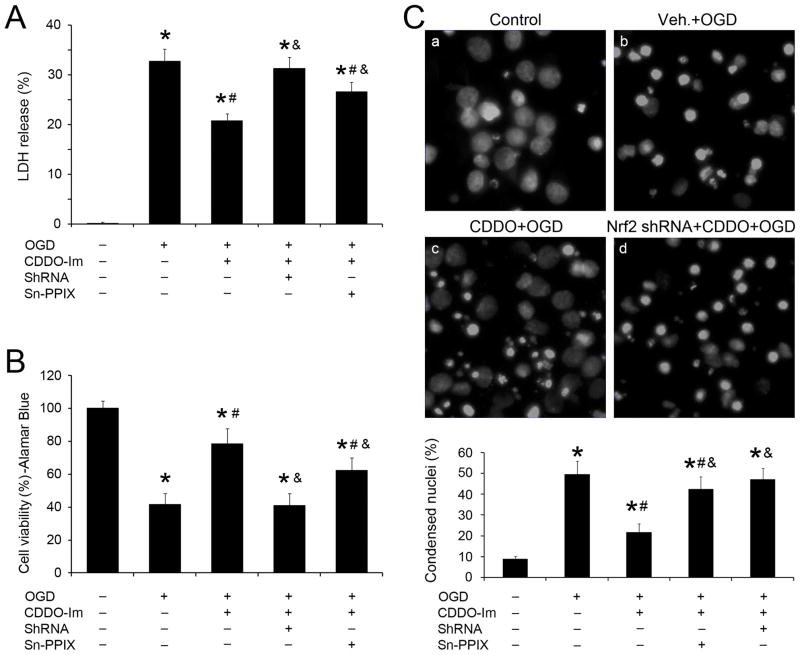
We next determined whether CDDO-Im pretreatment protected neurons from ischemic injury induced by OGD and if HO-1 and Nrf2 played a critical role in the protection. Cultures were treated with CDDO-Im overnight, followed by 60 min OGD, and then subjected to LDH release and Alamar blue assays 24 hr later. Compared to vehicle, CDDO-Im treatment significantly reduced LDH release (Figure 2A) and maintained Alamar blue fluorescence (Figure 2B), indicating that CDDO-Im attenuated neuronal injury. This was further confirmed by Hoechst staining and cell counting (Figure 2C). The protective effects of CDDO-Im were partially blocked when cultures were transfected with Nrf2 shRNA lenti-particles or co-treated with Sn-PPIX, a competitive inhibitor of HO-1 activity (Figure 2), indicating a critical role for both HO-1 and Nrf2 in CDDO-Im-mediated neuroprotection against injury.
Figure 2. CDDO-Im protects primary neurons against OGD.
Primary neurons were treated with 100 nM CDDO-Im in combination with HO-1 inhibitor Sn-PPIX (10 μmol/L) or Nrf2 shRNA (2.0×105 IFU) or scramble shRNA. Neurons were subjected to 60 min OGD, followed at 24 hr by the (A) LDH release and (B) Alamar blue assays. n=3; *p<0.05 vs. control, #p<0.05 vs. OGD, &p<0.05 vs. CDDO-treated OGD group. Another set of cultures underwent similar treatments but were stained with Hoechst and are shown in (C). Dead neurons were counted and shown in the lower panel. *p<0.05 vs. control, #p<0.05 vs. OGD, and p<0.05 vs. CDDO-treated OGD group.
3. CDDO-Im upregulates HO-1 expression in hippocampal CA1
We next investigated whether CDDO-Im can upregulate HO-1 expression in rat brain and render similar neuroprotection in vivo. To bypass the BBB 15 and avoid systemic effects of CDDO-Im 18, 19, we injected 0.5 μg CDDO-Im into ICV, harvested cortex, striatum and hippocampus at the indicated times. As in culture, HO-1 was barely detectable in vehicle-infused brains, and little HO-1 upregulation was observed at 1 hour after the injection of CDDO-Im (data not shown). At 4 hr after the injection, HO-1 was only upregulated in hippocampus; at 24 hr, HO-1 levels were significantly increased in all regions, with the highest level in hippocampus (Figure 3A). The smallest effect was seen in the cortex, which may simply reflect the longer distance of cortex from the injection center than striatum and hippocampus. We next assessed the dose responsiveness of HO-1 to CDDO-Im in the hippocampus. As shown in Figure 3B, higher doses (1.0 and 1.5 μg) of CDDO-Im led to increased levels of HO-1 24 hr after the injection, indicating that CDDO-Im induced HO-1 expression in hippocampus in both a time-dependent and a dose-dependent manner. In hippocampal CA1, one of the vulnerable structures to global ischemia, HO-1 level peaked at 48 hr after the injection and remained elevated for at least 3 days (Figure 3C). Finally, we studied the cellular distribution of HO-1 in the CA1 region. With occasional exceptions, HO-1 staining surrounded the nuclear NeuN signal within CA1 neurons (Figure 3D), indicating a predominantly neuronal distribution of HO-1 following CDDO-Im treatment.
Figure 3. CDDO-Im induces HO-1 expression in rat brains.
(A) Western blots show that ICV injection of CDDO-Im (0.5 μg) induced strong HO-1 expression in hippocampus and striatum but modest expression in cortex. n=3, *p<0.05, **p<0.01 vs. vehicle groups. (B) Western blots show that CDDO-Im upregulated CA1 HO-1 in a dose-dependent manner. n=3, *p<0.05, **p<0.01 vs. vehicle groups. (C) Time course of HO-1 expression in CA1 after a single injection of CDDO-Im. (D) Immunohistochemical staining for HO-1 expression in CA1 at 24 hr after CDDO-Im (1.0 μg) ICV injection. HO-1 was expressed predominantly in neurons. Bar=50 μm.
4. Time course and cellular distribution of HO-1 in CA1 after global ischemia in rats
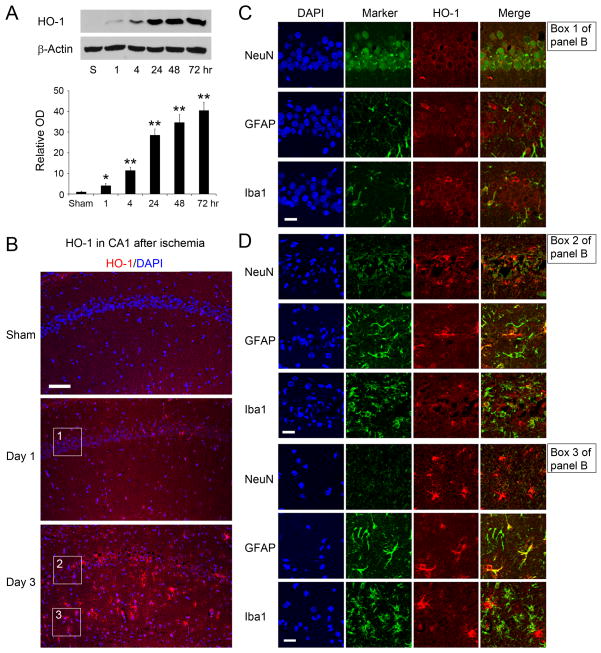
HO-1 was barely detectable in sham-operated rats (Figure 4A). Following ischemia, HO-1 was slightly upregulated at 1 hour, further increased at 4 hr and thereafter, and peaked at 72 hr after ischemia. Immunostaining validated the Western data that global ischemia stimulated HO-1 expression in hippocampal CA1 (Figure 4B). Double-labeling studies revealed that HO-1 was primarily co-localized with NeuN-positive cells in CA1 24 hr after ischemia, a time-point when CA1 neurons were still alive, indicating a neuronal expression of HO-1 (upper panel, Figure 4C). HO-1 occasionally co-localized with Iba-1 positive cells (lower panel, Figure 4C) but seldom with GFAP-positive cells at 24 hr (middle panel, Figure 4C). HO-1 distribution was dramatically altered 3 days after ischemia, however. While HO-1 was still detectable in dead or injured neurons in the pyramidal layer, it was now also detected in astrocytes and microglia in the pyramidal layer (Figure 4D, upper). The strongest HO-1 signal appeared in the radiant layer of hippocampal CA1 (Figure 4D, lower), the white matter that contains septal and commissural fibers. This HO-1 signal localized in GFAP- and Iba1-positive cells, indicating a shift of HO-1 expression from a neuronal to a glial distribution 3 days after ischemia, when CA1 neurons had died.
Figure 4. HO-1 expression in CA1 after global cerebral ischemia in rats.
(A) Representative Western blots showing the temporal profiles of HO-1 expression in hippocampal CA1 after global ischemia. n=3, *p<0.05, **p<0.01 vs. veh.-groups. (B) Representative low-magnification photomicrograph of HO-1 immunostaining in CA1, showing increased levels of HO-1 after ischemia. Bar=100 μm. Boxes 1, 2, and 3 indicate the areas where the high-magnification photos shown in Fig. 4C&D (upper and lower panels) were taken. (C) Cellular distribution of HO-1 in CA1 region 24 hr after ischemia, showing predominantly neuronal expression of HO-1. Bar=25 μm. (D) Cellular distribution of HO-1 in CA1 region 72 hr after ischemia, indicating a primarily glial localization of HO-1 in the radiant layer (stratum radiatum). Bar=25 μm.
5. CDDO-Im pretreatment attenuates CA1 neuronal death after global ischemia in rats
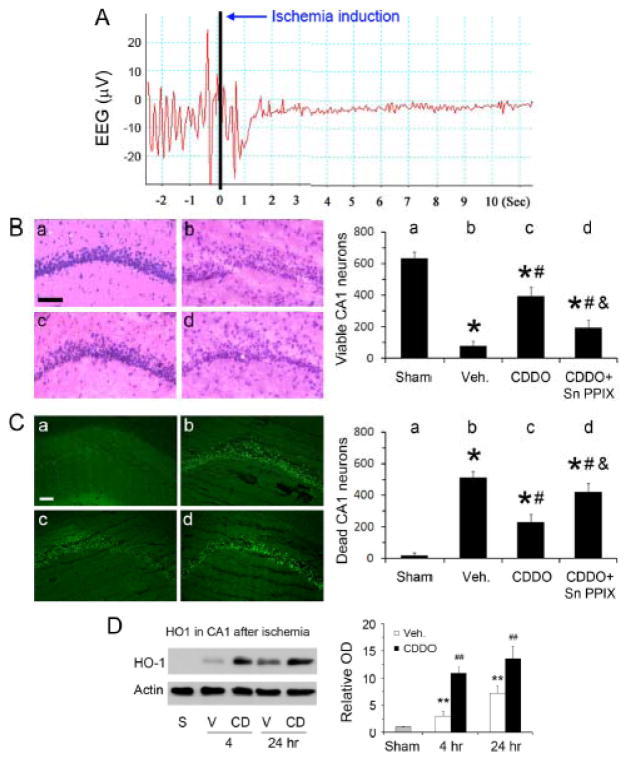
We then investigated if CDDO-Im could protect CA1 neurons from ischemic injury induced by global ischemia. EEG isoelectricity immediately after the occlusion of common carotid arteries verified the success of ischemia (Figure 5A). In vehicle-treated rats, ischemia killed about 90% of CA1 neurons; CDDO-Im treatment clearly attenuated neuronal injuries, indicated by an increased number of viable neurons (Figure 5B) and a decreased number of apoptotic neurons (Figure 5C). To determine the role of HO-1 in the protection, we compared the time courses of HO-1 expression in CA1 between vehicle and CDDO-Im groups early after ischemia, and found differences between these two groups, with a high level of pre-existing HO-1 in the CDDO-Im group (Figure 5D). Administration of Sn-PPIX (30 μg) partially but significantly blocked the protective effects of CDDO-Im against ischemic neuronal death (Figure 5B&C).
Figure 5. CDDO-Im induces HO-1 expression in hippocampal CA1 neurons in rats.
(A) A representative EEG recording in a rat undergoing global cerebral ischemia, showing rapid isoelectricity after the onset of ischemia. (B) H&E stain of CA1 regions and quantitative analysis of viable neurons after global ischemia in rats. a, sham; b, vehicle-treated ischemia; c, CDDO-Im-treated ischemia; and d, CDDO-Im- and Sn-PPIX-treated ischemia. Bar=50 μm; n=8, *p<0.05 vs. sham, #p<0.05 vs. vehicle-treated ischemia, and &p<0.05 vs. CDDO-treated ischemia. (C) PANT stain of CA1 regions and quantitative analysis of dead neurons. a, sham; b, vehicle-treated ischemia; c, CDDO-Im-treated ischemia; and d, CDDO-Im- and Sn-PPIX-treated ischemia. Bar=100 μm; n=8, *p<0.05 vs. sham, #p<0.05 vs. vehicle-treated ischemia, and &p<0.05 vs. CDDO-treated ischemia. (D) Western blots of HO-1 in CA1, demonstrating pre-existence of HO-1 in CA1 after CDDO-Im treatments. n=3, **p<0.01 vs. sham, ##p<0.01 vs. sham and vehicle-treated ischemia. S, sham; V, vehicle treated; CD, CDDO-Im treated.
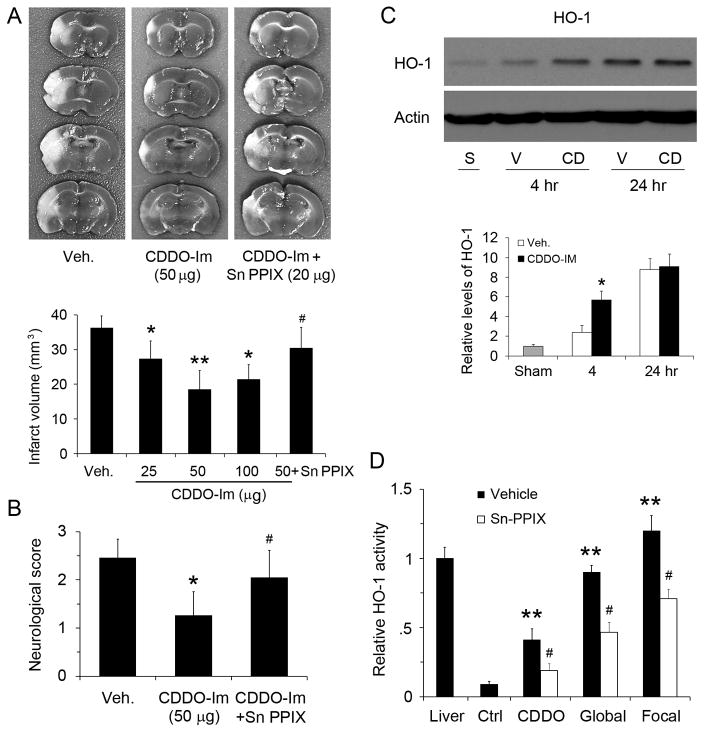
6. Post-ischemic CDDO-Im treatment decreases infarct after focal ischemia in mice
To further strengthen the clinical relevance of the CDDO-Im treatment, we tested its effects in a mouse model of focal ischemia, with a post-ischemia treatment. CDDO-Im was IP injected immediately after ischemia when the permeability of the blood-brain barrier was increased, and ischemic outcomes were evaluated 48 hr later. Infarct volume was 35.8 mm3 in the vehicle-treated group (Figure 6A). CDDO-Im at 10 μg per mouse did not protect the brain (data not shown), while 25, 50 and 100 μg CDDO-Im reduced infarcts significantly, with the best result in the 50 μg group, indicating that CDDO-Im protected brain from focal ischemia in a dose-dependent manner even when it was injected after ischemia. In supportive, Fluoro-Jade B staining of brain sections at 48 hr after MCAO demonstrated that CDDO-Im attenuated neuronal death compared with vehicle group (Figure S1). The protection of CDDO-Im was also demonstrated at the behavioral level by improved neurological function (Figure 6B). To investigate the role of HO-1 in this mouse model, we injected 20 μg Sn-PPIX into ICV after ischemia, and found that the protective effect of CDDO-Im was again partially blocked, indicated by the relapse of infarct volume (Figure 6A). Western blots showed CDDO-Im treatments resulted in increased HO-1 expression early after middle cerebral artery occlusion (MCAO, Figure 6C). To confirm that HO-1 activity is changed after CDDO-Im treatment and ischemia, and that Sn-PPIX truly inhibits HO-1 activity, we performed HO-1 activity assay in brain tissues, and found that HO-1 activities were increased after protein levels were upregulated, and that Sn-PPIX inhibited HO-1 activity by half in CA1 and cortical tissues (Figure 6D). Ischemia by itself was a strong inducer of HO-1, suggestive of endogenous neuroprotection in the brain after a stroke and consistent with previous studies suggesting that HO-1 knockout increases infarcts 8. Taken together, our results indicate that even a low dose of CDDO-Im is neuroprotective against ischemic injury and that HO-1 mediated the protective effects of CDDO-Im in three models and two species.
Figure 6. CDDO-Im post-treatment protected mice from focal cerebral ischemia.
(A) TTC stains 48 hr after MCAO in mice, showing that CDDO-Im reduced infarct size in a dose-dependent manner. n=6–8, *p<0.05 and **p<0.01 vs vehicle group, #p<0.05 vs 50 μg CDDO-Im. (B) CDDO-Im attenuated neurological dysfunction after ischemia. n=8, *p<0.05 vs vehicle group, #p<0.05 vs CDDO-Im post-treatment. (C) CDDO-Im increased HO-1 level early after MCAO. S, sham; V, vehicle; CD, CDDO-Im. n=3, *p<0.01 vs control. (D) HO-1 activity assay was performed with rat CA1 tissues (CDDO-Im and global ischemia) or mouse cortical tissues (focal ischemia) at 24 hours after CDDO-Im injection or ischemia and verified that Sn-PPIX was effective in inhibiting HO-1 activity. n=3, *p<0.01 vs control, and #p<0.05 vs vehicle.
Discussion
In this study, we demonstrated that CDDO-Im, a synthetic triterpenoid and the strongest inducer of the Nrf2 signaling pathway known so far, induced robust HO-1 expression in neuronal cultures and brains. Using three ischemia models, one in vitro and two in vivo, we found that CDDO-Im treatment attenuated ischemic neuronal injury at extremely low doses. The protective effect of CDDO-Im was blocked when HO-1 activity was inhibited by Sn-PPIX or when Nrf2 was knocked down with specific shRNA, indicating that HO-1 upregulation and Nrf2 activation played important roles in the neuroprotective effects of CDDO-Im.
Triterpenoids belong to a group of five-ring compounds that are produced by many plants, including ginsengs. Natural triterpenoids have been used as alternative medicines for centuries for their mild anti-oxidative, anti-inflammatory and anti-carcinogenic effects. In an effort to enhance their potency, oleanolic acid, a natural triterpenoid, has been further modified by extensive synthetic steps, and a new set of synthetic triterpenoids has been generated 12. Among them, CDDO-Im displays the strongest bioactivities, including anti-cancer, anti-inflammatory and anti-oxidative effects 13–15, 19.
The anti-oxidative role of CDDO-Im is dependent on its ability to activate Nrf2, a master transcription factor that governs phase-2 enzyme expression 14, 15. Under normal conditions, Nrf2 is not active because it binds Keap1, which facilities their proteosomal degradation, resulting in a short half-life and a low basal level of Nrf2 20. Keap1 is rich in cysteine, and Cys-151 and Cys-275 are important for Nrf2 degradation 21. A recent study shows that dihydro-CDDO-trifluoroethyl amide, another derivative of CDDO, dissociates Keap1 from Nrf2 by interacting with Cys-151 of Keap1 via Michael addition 22, leading to upregulation of phase-2 enzymes. CDDO-Im may function in a similar manner to upregulate phase-2 enzymes, though direct evidence for this is currently not available.
As a phase-2 enzyme, HO-1 is neuroprotective against stroke, as HO-1 knockout worsens infarcts 8 and HO-1 overexpression reduces infarcts in mice 2. Our data support this notion. In addition to an increase in levels, HO-1 location is also likely to contribute to its neuroprotective capacity. HO-1 was primarily expressed in neurons after CDDO-Im treatment, as shown in Figures 1&3 and in a previous report 17. This is likely to be important for its neuroprotective role in our hands. HO-1 was upregulated in CA1 neurons during the early stage (<24 hr) after ischemia, further supporting the notion that HO-1 may contribute to protection against ischemic neuronal injury. In the late stage, HO-1 was also strongly expressed in astrocytes and microglia. The role of glial HO-1 remains unclear, though previous reports showed that astrocytic Nrf2 and HO-1 also offered neuronal protection against oxidative stress 4, 23. A prior report also showed that HO-1 could be expressed in cultured astrocytes after CDDO treatment 17; however, we did not notice strong astrocytic HO-1 in vivo, probably because astrocytes were not activated after CDDO-Im injection.
The potential benefits of CDDO-Im in the nervous system are not limited to stroke. For example, CDDO-Im reduces retinal injury from photooxidation 24. Other CDDO derivatives protect against Alzheimer’s disease 25, Huntington’s and Parkinson’s disease 26, and amyotrophic lateral sclerosis 27. In future studies, the neuroprotective resume for CDDO-Im could be extended to other forms of acute brain injuries such as hemorrhagic stroke and traumatic brain injury. Compared to several Nrf2/HO-1 inducers such as sulforaphane 4, 5 and Gingko biloba 7 that have previously shown neuroprotective effects, a clear advantage of using CDDO-Im is its low doses required to achieve neuroprotection. Thus, the strong potency of CDDO-Im with no apparent toxicity makes this drug a potential candidate for clinical neuroprotection. A caveat for the clinical application of CDDO-Im though is its relatively mild capability to across BBB. However, since BBB is likely compromised after stroke, it is possible to systemically administrate CDDO-Im shortly after stroke and achieve neuroprotection, especially in conjunction with tPA treatment that would re-establish blood flow in ischemic regions. Alternatively, a CDDO-Im derivative with increased BBB penetrating properties may be designed and tested in future studies.
In summary, our data reveal for the first time that a low dose of a potent compound CDDO-Im upregulates the inducible phase-2 enzyme HO-1 primarily in neurons and protects neurons and brain from ischemic injury. The protection was apparent in three different models and was blocked with a competitive inhibitor of HO-1. Therefore, CDDO-Im is a promising candidate for protecting brain against human stroke in an Nrf2/HO-1 dependent manner. Nonetheless, several issues still need to be addressed in the future. Examples include whether other phase-2 enzymes or other signaling pathways are involved in the neuroprotective effects of CDDO-Im, as the nuclear factor kappa B, phosphatase and tensin homolog, and mammalian target of rapamycin appear to also mediate the effects of CDDO-Im in non-neuronal cells 12, 28, 29. Clarification of these issues may help develop new strategies for stroke treatment.
Supplementary Material
Acknowledgments
We thank Pat Strickler for secretarial support.
Sources of Funding
This work was supported by funds from the National Institutes of Health (NS36736, NS43802 and NS45048 to J.C.], the VA Merit Review Grant (to J.C.) and the American Heart Association (10SDG2560122 to F.Z.).
Footnotes
Disclosures
None.
References
- 1.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 3.Dore S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, et al. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- 4.Kraft AD, Johnson DA, Johnson JA. Nuclear factor e2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. The Journal of Neuroscience. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neuroscience Letters. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 6.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, et al. Transcription factor nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 7.Saleem S, Zhuang H, Biswal S, Christen Y, Dore S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39:3389–3396. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah ZA, Nada SE, Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in gingko biloba (egb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih AY, Li P, Murphy TH. A small-molecule-inducible nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. The Journal of Neuroscience. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh T, Okamoto S-i, Cui J, Watanabe Y, Furuta K, Suzuki M, et al. Activation of the keap1/nrf2 pathway for neuroprotection by electrophillic phase ii inducers. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah ZA, Li R-c, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, et al. The flavanol (−)-epicatechin prevents stroke damage through the nrf2/ho1 pathway. J Cereb Blood Flow Metab. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. New synthetic triterpenoids: Potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2010;74:537–545. doi: 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda T, Honda Y, Favaloro FG, Jr, Gribble GW, Suh N, Place AE, et al. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg Med Chem Lett. 2002;12:1027–1030. doi: 10.1016/s0960-894x(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 14.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, et al. The synthetic triterpenoids, cddo and cddo-imidazolide, are potent inducers of heme oxygenase-1 and nrf2/are signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 15.Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 16.Kensler TW, Wakabayashi N. Nrf2: Friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graber DJ, Park PJ, Hickey WF, Harris BT. Synthetic triterpenoid cddo derivatives modulate cytoprotective or immunological properties in astrocytes, neurons, and microglia. J Neuroimmune Pharmacol. 2010;6:107–120. doi: 10.1007/s11481-010-9240-9. [DOI] [PubMed] [Google Scholar]
- 18.Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG, Jr, Suh N, et al. Synthetic oleanane and ursane triterpenoids with modified rings a and c: A series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 2000;43:4233–4246. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- 19.Place AE, Suh N, Williams CR, Risingsong R, Honda T, Honda Y, et al. The novel synthetic triterpenoid, cddo-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin Cancer Res. 2003;9:2798–2806. [PubMed] [Google Scholar]
- 20.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by nrf2 through binding to the amino-terminal neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, et al. The antioxidant defense system keap1-nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. Dihydro-cddo-trifluoroethyl amide (dh404), a novel nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One. 2009;4:e8391. doi: 10.1371/journal.pone.0008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vargas MR, Johnson JA. The nrf2-are cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitha-Rowe I, Liby K, Royce D, Sporn M. Synthetic triterpenoids attenuate cytotoxic retinal injury: Cross-talk between nrf2 and pi3k/akt signaling through inhibition of the lipid phosphatase pten. Invest Ophthalmol Vis Sci. 2009;50:5339–5347. doi: 10.1167/iovs.09-3648. [DOI] [PubMed] [Google Scholar]
- 25.Dumont M, Wille E, Calingasan NY, Tampellini D, Williams C, Gouras GK, et al. Triterpenoid cddo-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of alzheimer’s disease. J Neurochem. 2009;109:502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, et al. Neuroprotective effects of the triterpenoid, cddo methyl amide, a potent inducer of nrf2-mediated transcription. PLoS One. 2009;4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M, et al. Neuroprotective effect of nrf2/are activators, cddo ethylamide and cddo trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic Biol Med. 2011;51:88–96. doi: 10.1016/j.freeradbiomed.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yore MM, Kettenbach AN, Sporn MB, Gerber SA, Liby KT. Proteomic analysis shows synthetic oleanane triterpenoid binds to mtor. PLoS One. 2011;6:e22862. doi: 10.1371/journal.pone.0022862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid cddo-me blocks the nf-kappab pathway by direct inhibition of ikkbeta on cys-179. J Biol Chem. 2006;281:35764–35769. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.