Abstract
Background/purpose
Local-regional control (LRC) rates for NSCLC after chemoradiotherapy were studied (using two different definitions of LRC) for the association between LRC and survival.
Materials/Methods
Seven legacy RTOG trials of chemoradiotherapy for locally advanced NSCLC were analyzed. Two different definitions of LRC were studied: 1. Freedom from local progression (FFLP--LRC), the traditional RTOG methodology, in which a failure is intrathoracic tumor progression by WHO criteria; and 2. Response-mandatory (strict) Local-regional control (strict-LRC), in which any patient not achieving at least partial response was considered to have failure at Day 0. Testing for associations between LRC and survival was performed using a Cox multivariate model that included other potential predictive factors.
Results
A total of 1,390 patients were analyzed. The LRC rate at 3 years was 38% based on the FFLP-LRC definition and 14% based on the strict-LRC definition. Performance status, concurrent chemotherapy and radiotherapy dose intensity (BED) were associated with better LRC (using either definition). With the strict-LRC definition (but not FFLP-LRC), age was also important. There was a powerful association between LRC and overall survival (p<0.0001) on univariate and multivariate analyses. Age, performance status, chemotherapy sequencing and BED were also significantly associated with survival. Histology and gender were also significant if the strict-LRC model was used.
Conclusions
LRC is associated with survival. The definition of LRC affects the results of these analyses. A consensus definition of LRC, incorporating functional imaging and/or central review, is needed, with the possibility of using LRC as a surrogate endpoint in future trials.
Keywords: Non-small cell lung cancer, Local control, chemoradiotherapy
Introduction
It is axiomatic that cure of cancer can not be achieved without control of the primary tumor site (local control). There have been many studies investigating the relationship between local control and survival in a variety of malignancies, including non-small cell lung cancer (NSCLC)1 Most of these studies show that cancer patients who have local control live longer than those who do not have local control.
A challenge in studying local control in stage III unresectable NSCLC is that it is difficult to assess local tumor status in this disease. With rare exceptions, these cancers are not evaluable on clinical office examination. Interpretation of chest radiography and computed tomography is hindered by extensive radiation-induced inflammation and fibrosis, which can mimic persistently active or recurrent/progressive tumor.
Data show that tumor control and survival has improved with the use of chemoradiotherapy when compared with radiotherapy alone2 However, the reported rate of local-regional control in scientific studies has varied widely, despite relatively similar radiotherapy techniques and chemotherapy regimens. This likely depends upon the means with which local-regional control is assessed and analyzed. For example, an early RTOG study of radiotherapy alone for NSCLC suggested that with an XRT dose of 60 Gy continuous course, 2-year local-regional control was above 60%3 In contrast, a randomized trial by LeChevalier – in which post-radiotherapy bronchoscopy/biopsy was routinely performed – suggested that true local-regional control was only achieved in about 20% of patients4. No other major, large randomized trial in unresectable stage III lung cancer required an attempt at post-radiotherapy pathologic assessment of local control.
Since radiotherapy is a local-regional anti-cancer treatment, it is important to assess local-regional control in studies that involve radiotherapy even if pathologic assessment is not feasible.
We performed several analyses of the RTOG database to examine the probability of local-regional control after chemoradiotherapy. Our study specifically evaluates two different definitions of local-regional control: 1. The “traditional” RTOG measure of local-regional control, also referred to as Freedom from Local Progression (FFLP-LRC); and 2. A more rigorous definition of local-regional control which requires objective local-regional tumor response in addition to freedom from local progression, similar to the definition of LRC often used in studies of head and neck cancer (strict-LRC). We hypothesized that there would be significant differences in the analyses depending upon how local-regional control is defined.
Materials and Methods
This is a retrospective analysis of prospective data collected on patients treated with chemoradiotherapy in prospective RTOG protocols from 1988 through 2002. All patients eligible for analysis were included.
The studies analyzed were:
RTOG 88–08 (Phase III trial : chemo-RT arm only)5: This consisted of induction cisplatin/vinblastine chemotherapy followed by definitive radiotherapy (60 Gy).
RTOG 90–156: Phase I/II trial of concurrent cisplatin/vinblastine with definitive bid radiotherapy (69.6 Gy)
RTOG 91–067 Phase I/II trial of concurrent cisplatin/etoposide with definitive bid radiotherapy (69.6 Gy)
RTOG 92–048: Phase IIR trial; one arm was the same treatment as in RTOG 91–06, while the second arm was induction cisplatin/vinblastine followed by concurrent cisplatin/radiotherapy (63 Gy).
RTOG 93–099: Phase III study of immediate concurrent chemoradiotherapy (cisplatin/etoposide/RT (61 Gy)) with or without surgical resection (potentially operable IIIA only) – for this analysis only the patients randomized to no surgery were included.
RTOG 94–1010 : Phase III trial comparing chemo-RT as given in RTOG 88–08 versus immediate concurrent chemo-RT (cisplatin/vinblastine/RT (63 Gy)) versus the RTOG 91–06 regimen.
RTOG 98–0111: Phase III trial of induction chemotherapy (carboplatin/paclitaxel) followed by concurrent chemoradiotherapy (carboplatin/paclitaxel/bid RT (69.6 Gy)), with or without amifostine.
Radiotherapy techniques and doses were similar for all of these studies. Specifically, all of these studies included elective nodal irradiation to the entire mediastinum and in some cases the supraclavicular and/or contralateral hilar nodes to 45 Gy. These comprehensive radiotherapy treatment fields were then followed by a boost to gross disease to at least 60 Gy (maximum 69.6 Gy in 1.2 Gy bid fractionation). “High technology” forms of modern radiotherapy such as intensity modulated radiation therapy (IMRT), image guided radiation therapy (IGRT), adaptive radiotherapy, respiratory gated radiotherapy, or air/tissue inhomogeneity corrected radiotherapy dosimetry were not used. CT-based simulation/planning and 3D conformal planning and delivery of radiotherapy was allowed, but not routinely used and certainly not required in any of these studies. .
Unfortunately, however, RTOG did not collect detailed information about the type of simulation and treatment planning that was used in the patients in these studies (as opposed to 3D conformal specific RTOG studies 93–11 and 01–17, which are not included in this analysis). The studies included in this analysis required that the prescription dose (60 to 69.6 Gy, depending on the exact study) be specified to isocenter, rather than renormalization of dose to a peripheral isodose.
Instructions for the assessment for tumor control was consistent among these studies. Specifically, all patients were required to undergo a post-radiotherapy CT scan of the chest (including liver/adrenals) approximately 6 months after completing radiotherapy, then every 6 months for two years, and then annually. Additional CT scans were allowable at other intervals as clinically indicated, for example if there was clinical suspicion for recurrence/progression. It was recommended that these CT scans be performed both with and without contrast, and that CT slices be 5 mm or smaller. Bone scan and/or head CT/MRI scanning in followup was only performed if metastatic disease was suggested by clinical evaluation. PET scans were not used for staging or post-treatment assessment in this study (patients in this analysis were treated between 1988 and 2002).
Local-regional failure was determined by the individual site, radiation oncology physician-investigators, who were charged with determining whether an “event” (progression of lung cancer) has occurred, and if so if it was in-field, at the edge of the field, or out of field. Any one of the following events constituted a local-regional failure:
Enlargement by > 25% in the bidimensional product of two dimensions of a measurable index (pre-treatment) lesion.
For a non-measurable lesion, estimated enlargement by >25% of tumor bulk, after taking into account post-radiation pneumonitis/fibrosis.
The development of severe tumor-related local-regional complications such as post-obstructive pneumonia and/or hemoptysis were also considered as criteria for local-regional failure if these clinical events could not be attributed to radiation toxicity and/or intercurrent disease.
The appearance of a new malignant lesion within the radiation field or at the edge of the radiation field
Positive biopsy and/or surgical specimen after radiotherapy showing viable non-small cell lung carcinoma following radiotherapy.
It was not necessary to have more than one post-treatment CT scan to document local-regional failure. Biopsy confirmation of local-regional failure was also not required and was in fact rarely performed in these studies. All of the above assessments (other than biopsy results) were based on an assessment performed by the local (site) investigator radiation oncologist. These assessments were done at the time of post-treatmentfollowup visits/scans and reported to RTOG headquarters on data collection forms. These assessments thus represent “prospective” assessments of LRC by the local radiation oncologists/investigators, performed in most cases prior to patient death or last followup. Retrospective re-analysis by RTOG headquarters was not performed, and actual CT images were not collected by RTOG.
Supraclavicular nodal metastases were not common, but when they occurred, they were considered local-regional failure in those cases where the supraclavicular fossa was part of the original AP-PA field(s). Malignant effusions (pleural and/or pericardial) were considered distant metastases rather than local-regional failure.
For analysis of strict-LRC, the initial response assessment was based on the CT scan performed approximately 6 months after radiotherapy, except in cases when there was clear progression prior to six months.
Local-regional control was assessed using two different methodologies, described below:
Freedom from Local-regional Progression (FFLP – for this paper the abbreviation FFLP-LRC will be used): With this definition, which is the traditional RTOG definition of local-regional control for lung cancer, all patients are presumed to have local-regional control at Day 0 (date of randomization). Subsequently, the development of progressive lung cancer within or adjacent to the radiotherapy field (including regional nodes) was considered to be local-regional failure. Patients were censored at the date of death if they died from distant metastases or died without documented progressive cancer; they were censored at the date of last follow-up if they were still alive with no evidence of local-regional failure.
Response-mandatory (strict) Local-regional Control (strict-LRC): With this more stringent definition, patients are not considered to have local-regional control unless they achieve at least a partial response of their primary tumor by imaging (≥ 50% reduction in the product of two dimensions of the dominant tumor lesion). Patients who do not achieve objective response are considered to have suffered local-regional failure at Day 0 (date of randomization). Patients who do achieve objective response are then evaluated similarly to FFLP-LRC (as above). The development of progressive lung cancer within or adjacent to the radiotherapy field after a response was considered to be local-regional failure at the date of progression. The distinction of whether progressive lung cancer was considered to be within or adjacent to the radiotherapy field was determined by the individual site investigator. Central review of post-treatment imaging was not performed. Patients were censored at the date of death if they died from distant metastases or died without documented progressive cancer; they were censored at the date of last follow-up if they were still alive with no evidence of local-regional failure..
Local-regional control rates were analyzed using the Kaplan-Meier method. Factors potentially predictive for local-regional control were assessed, including patient related factors (age (≤ 70 vs. > 70, gender (female vs. male), and KPS (70–80 vs. 90–100), tumor related factors (stage (IIA/IIIA vs. IIIB), and histology (non-squamous vs. squamous)), and treatment related factors (chemotherapy sequencing and biologically equivalent dose (BED)). Local-regional control was modeled multivariately using the Cox proportional hazards method to find associations with these potentially predictive factors.
Finally, for each methodology, an assessment of the relationship between local-regional control and survival was performed. For these analyses, local-regional control was analyzed in a binary fashion (no progression per the given methodology versus progression per the given methodology). Overall survival rates by local-regional control were generated using the Kaplan-Meier method. Local-regional control was then considered as a variable in multivariate Cox proportional hazards models for association with survival.
Results
A total of 1,390 patients are included in this analysis. Patient characteristics are shown in Table 1. As shown in this table, most patients within these RTOG trials were relatively young (<70 years old), had good performance status, and had minimal or no weight loss. Approximately two thirds of the patients were male and about two thirds had non-squamous histology. Approximately half of the population had stage II/IIIA disease and half had IIIB disease.
Table 1.
Pretreatment Characteristics
| n=1390 | |
|---|---|
| Age | |
| Mean | 60.4 |
| Median | 61 |
| Range | 31 – 84 |
| ≤ 70 | 1066 (77%) |
| > 70 | 324 (23%) |
| Gender | |
| Male | 907 (65%) |
| Female | 483 (35%) |
| Race | |
| White | 1125 (81%) |
| Hispanic | 15 (1%) |
| Black | 123 (9%) |
| Other/Unknown | 30 (2%) |
| Not collected | 97 (7%) |
| KPS | |
| 90–100 | 1066 (77%) |
| 70–80 | 324 (23%) |
| Weight Loss | |
| ≤ 5% | 1287 (93%) |
| > 5% | 70 (5%) |
| Unknown | 33 (2%) |
| Histology | |
| Squamous | 527 (38%) |
| Non-squamous | 863 (62%) |
| Stage | |
| II/IIIA | 717 (52%) |
| IIIB | 673 (48%) |
| T-stage | |
| T0–T2 | 687 (49%) |
| T3–T4 | 691 (50%) |
| Unknown | 12 (1%) |
| BED | n=1257 |
| Mean | 73.5 |
| Median | 74.7 |
| Range | 1.3 – 86.4 |
Analysis of Local-regional control using FFLP-LRC Methodology
As shown in Figure 1, FFLP-LRC quickly drops from 100% at time 0 to 63% at 1-year, 44% at 2 years; 38% at 3 years and 32% at 5 years. The median duration of FFLP-LRC was 18.8 months. In the multivariate model, gender (p=0.0002), performance status (p=0.04), chemotherapy sequencing (0.01) and BED (<0.0001) were associated with FFLP-LRC.
Figure 1.
Freedom from Local Progression (FFLP-LRC) for all patients (n=1,390)
Analysis of Local-regional control using strict-LRC Methodology
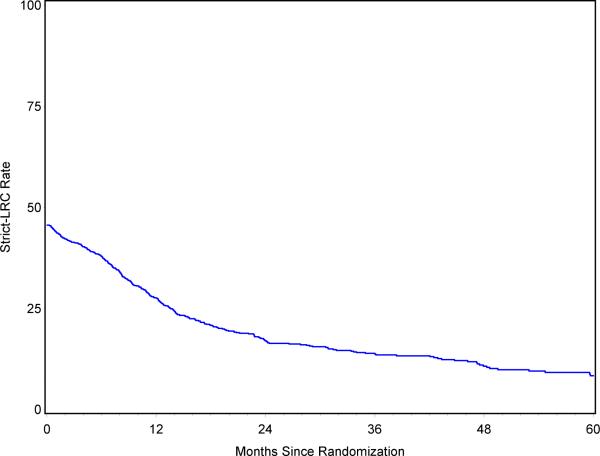
As shown in Figure 2, only 46% of all patients achieved at least a partial response to chemoradiotherapy (thus strict-LRC was 46% at day 1). strict-LRC dropped to 28% at 1 year; 17% at 2 years; 14% at 3 years and 8% at 5 years. Age (p=0.04), performance status (p=0.04), chemotherapy sequencing (p=0.008), and BED (p=0.001) were associated with strict-LRC in the multivariate analysis.
Figure 2.
Response-mandatory Local-regional Control (strict-LRC) for all patients (n=1,390)
Association between Local-regional control and Survival (FFLP-LRC methodology)
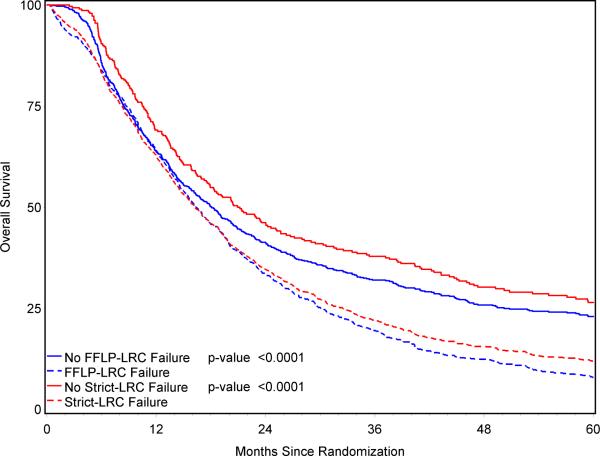
FFLP-LRC was significantly associated with overall survival. The median survival for patients who achieved FFLP-LRC was 18.1 months (2-year survival 41%; 3-year survival 32%; 5-year survival 26%), compared with 16.4 month median survival for patients who suffered local-regional failure by the FFLP-LRC definition (2-year survival 33%; 3-year survival 19%; 5-year survival 8%) (p<0.0001). This is displayed graphically in Figure 3. The overall hazard ratio for death based on FFLP-LRC status was 1.43(95% CI: 1.28, 1.61).
Figure 3.
Overall survival in patients with (solid lines) or without (dashed lines) local control as defined by the FFLP-LRC or strict-LRC definitions.
On multivariate Cox model analysis, FFLP-LRC remained significantly associated with overall survival (p<0.0001) (Table 2). Other factors that were significantly associated with overall survival in this model were age (≤70 versus > 70; p=0.004); Karnofsky performance status (90–100 versus 70–80; p=0.005); chemotherapy sequencing (concurrent versus sequential; p-0.04); and radiotherapy dose intensity delivered (BED as a continuous variable; p<0.0001). Gender and stage were not associated with overall survival.
Table 2.
Multivariate Cox Proportional Hazards Models of Overall Survival
| Model | Covariate | Comparison | Hazard Ratio† | (95% CI) | p-value‡ |
|---|---|---|---|---|---|
| LRC endpoint = Freedom from Local Progression (FFLP-LRC) | FFLP-LRC | No failure vs. failure | 1.42 | (1.26, 1.60) | <0.0001 |
| Age | ≤ 70 vs. > 70 | 1.29 | (1.09, 1.53) | 0.004 | |
| KPS | 90, 100 vs. 70, 80 | 1.22 | (1.06, 1.40) | 0.005 | |
| Chemotherapy Order | Concurrent vs. Sequential | 1.15 | (1.01, 1.32) | 0.04 | |
| BED | Continuous | 0.97 | (0.96, 0.98) | <0.0001 | |
|
| |||||
| LRC endpoint = Response-mandatory | strict-LRC | No failure vs. failure | 1.49 | (1.28, 1.73) | <0.0001 |
| Age | ≤ 70 vs. > 70 | 1.24 | (1.05, 1.47) | 0.01 | |
| Local-Regional Control (strict-LRC) | Gender | Female vs. Male | 1.14 | (1.00, 1.29) | 0.047 |
| KPS | 90, 100 vs. 70, 80 | 1.17 | (1.02, 1.35) | 0.02 | |
| Histology | Non-squamous vs. Squamous | 1.15 | (1.02, 1.30) | 0.02 | |
| Chemotherapy Order | Concurrent vs. Sequential | 1.17 | (1.02, 1.33) | 0.02 | |
| BED | Continuous | 0.97 | (0.96, 0.98) | <0.0001 | |
A stepwise model was used with the following covariates : LRC endpoint (no failure vs. failure), age (≤ 70 vs. > 70), gender (female vs. male), KPS (90, 100 vs. 70, 80), histology (non-squamous vs. squamous), AJCC stage grouping (II/IIIA vs. IIIB), chemotherapy order (concurrent vs. sequential) and BED (continuous or cut at the median (74.67353)). The entry criterion was p ≤ 0.05, and the exit criterion was p-value > 0.05. Only covariates in the final model are listed.
A hazard ratio greater than 1 indicates an increased risk of death for the second level listed. The hazard ratio for continuous BED indicates how much risk is decreased for each increase by 1 in BED
Log-rank chi-square test
Association between Local-regional control and Survival (strict-LRC methodology)
strict-LRC was significantly associated with overall survival (Table 2). The median survival for patients who achieved strict-LRC was 20.9 months (2-year survival 46%; 3-year survival 38%; 5-year survival 26%), compared with 16.3 month median survival for patients who suffered local-regional failure by the strict-LRC definition (2-year survival 35%; 3-year survival 22%; 5-year survival 12%) (p<0.0001). This is displayed graphically in Figure 3. The overall hazard ratio for death based on strict-LRC status was 1.57 (95% CI: 1.36, 1.82).
On multivariate Cox model analysis, strict-LRC remained significantly associated with overall survival (p<0.0001). Other factors that were significantly associated with overall survival in this model were age (≤70 versus > 70; p=0.01); gender (female versus male; p=0.05), Karnofsky performance status (90–100 versus 70–80; p=0.02); histology (nonsquamous vs. squamous; p=0.02); chemotherapy sequencing (concurrent versus sequential; p=0.02) and radiotherapy dose intensity delivered (BED as a continuous variable; p<0.0001).
Discussion
Our study shows that local-regional control after chemoradiotherapy for stage III NSCLC is suboptimal. Using the traditional, FFLP-LRC definition, the 5-year local-regional control probability was 32% and using the more rigorous strict-LRC definition local control, the 5-year local-regional control probability was 8%. Unlike studies conducted by the RTOG and others in the 1970's-`80s, which suggested local control rates of about 50%, these more modern trials used rigorous CT scan based assessment of response and local control. The results of our study are similar to those reported by LeChevalier, in which patients were required to undergo postradiotherapy biopsy to assess local disease status.
However, none of these trials used the modern state-of-the-art technique for assessment of NSCLC status, FDG-PET scan, which has shown value for the staging and followup of patients with stage III NSCLC.12, as reviewed by Mac Manus.
It should also be noted that since these RTOG studies have been completed, there have been considerable improvements in 3-dimensional radiotherapy planning and delivery13, 14, 15. It is possible that modern results of chemoradiotherapy are better than we found here; future rigorous analyses of patterns of failure are needed as data mature. The difference in the numerical rates of local-regional control depending upon the definition of local-regional control is not surprising. The strict-LRC definition not only requires the absence of progressive tumor within the radiation field, it also requires evidence of an objective response (50% reduction in the product of two dimensions of the dominant tumor mass for measurable disease, as per RECIST criteria). It is quite possible that even more dramatic differences could be observed if central review of response and LRC was performed; unfortunately, the RTOG has not had sufficient resources to perform this type of rigorous central review in its lung cancer studies. The lack of central review of response and LRC is a shortcoming of this current analysis.
Nonetheless, regardless of the methodology used to calculate local-regional control rates, there is a very strong association between local-regional control and survival. This is axiomatic -- it is biologically implausible to believe that a lung cancer patient can survive for very long without achieving control of his/her local tumor, irrespective of the occurrence of distant metastases. It is interesting to note, however, that the magnitude of the difference in median survival between patients locally controlled and those not locally controlled was modest (18.3 mo. vs. 16.9 mo. using FFLP-LRC definition; 23.5 mo. vs. 17.0 mo. using strict-LRC definition).
The association between local-regional control and survival as measured in this study is not perfect. This is related to several factors. First is the high likelihood and lethality of distant metastases in this patient population. A second factor may be the use of `salvage' chemotherapy. A third and potentially more important factor, however, may be the limitations in our ability to determine local-regional control, including differences in how individual radiation oncologists/investigators assess and score local-regional control and progression. Some patients thought to have persistent/recurrent cancer might actually have exuberant inflammatory and/or fibrotic tissue following chemoradiotherapy. Other patients thought to have had complete response of their cancer may actually have microscopic yet highly virulent foci of residual disease. It is possible that the use of more sophisticated means to assess the viability of a post-treatment mass could provide better information about the relationship between local control and survival than current means. Studies suggest that FDG-PET scan following radiotherapy may be more informative than other methods of followup.16, 17
Factors associated with a higher likelihood of local-regional control and survival did depend upon the methodology used. Using the FFLP-LRC model, local-regional control was associated with KPS, chemotherapy sequencing (concurrent was better), and BED. Using the strict-LRC model, KPS, chemotherapy sequencing and BED remained important; age was also found to be significant. The finding that BED was strongly associated with LRC is highly relevant to current and future trials of radiotherapy dose escalation, and has been analyzed in further detail in a separate RTOG manuscript by our group18.
The details of the association between local-regional control and survival also appeared to depend upon the definition used for LRC. This is evident from visual analysis of the curves in Figures 3 and 4. Figure 3 shows the relationship between LRC and survival using the FFLP-LRC definition; although the difference is highly statistically significant, there is not a large difference in the curves until after the first year of followup. This suggests that FFLP-LRC is not particularly useful as an early marker of long-term survival. In contrast, the strong relationship between strict-LRC and survival (Figure 3) becomes clear within the first few months of followup. Thus, strict-LRC rates might be a useful surrogate endpoint for survival in future studies of novel treatment regimens. This might be particularly true if future study populations are enriched by improvements in pre-treatment staging (i.e. excluding patients with distant metastases identified by PET and/or other means). A currently active collaborative trial between the American College of Radiology Imaging Network (ACRIN) and the RTOG is rigorously testing this concept. Specifically, this protocol (ACRIN 6668/RTOG 0235) is collecting pre- and post-treatment FDG-PET scans for analysis of SUV as an imaging based early marker of long-term survival. If this study is positive, future trials of novel agents plus radiotherapy for stage III NSCLC may consider using response/local-regional control as a surrogate endpoint for survival.
Conclusions
In summary, this analysis reveals that local-regional control rates are suboptimal after standard chemoradiotherapy. The exact results depend upon the definition of local-regional control; however, regardless of the definition, there is a strong association between local-regional control and survival in locally advanced NSCLC.
Acknowledgment of Research Support
This publication was supported by grant numbers RTOG U10 CA21661, CCOP U10 CA37422, Stat U10 CA32115 from the National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez CA, Bauer M, Edelstein S, Gillespie BW, Birch R. Impact o ftumor control on survival in carcinoma of the lung treated with irradiation. Int J Radiat Oncol Biol Phys. 1986 Apr;12(4):539–47. doi: 10.1016/0360-3016(86)90061-1. PMID 3009368. [DOI] [PubMed] [Google Scholar]
- 2.Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer. 1995 Aug 15;76(4):593–601. doi: 10.1002/1097-0142(19950815)76:4<593::aid-cncr2820760409>3.0.co;2-n. PMID 8625152. [DOI] [PubMed] [Google Scholar]
- 3.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Cancer. 1987 Jun 1;59(11):1874–81. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. Radiation Therapy Oncology Group. PMID 3032394. [DOI] [PubMed] [Google Scholar]
- 4.Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991 Mar 20;83(6):417–23. doi: 10.1093/jnci/83.6.417. PMID 1847977. [DOI] [PubMed] [Google Scholar]
- 5.Sause W, Kolesar P, Taylor S, IV, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000 Feb;117(2):358–64. doi: 10.1378/chest.117.2.358. PMID 10669675. [DOI] [PubMed] [Google Scholar]
- 6.Byhardt RW, Scott CB, Ettinger DS, et al. Concurrent hyperfractionated irradiation and chemotherapy for unresectable nonsmall cell lung cancer. Results of Radiation Therapy Oncology Group 90-15. Cancer. 1995 May 1;75(9):2337–44. doi: 10.1002/1097-0142(19950501)75:9<2337::aid-cncr2820750924>3.0.co;2-k. PMID 7712445. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Scott C, Komaki R, et al. Concurrent chemoradiation therapy with oral etoposide and cisplatin for locally advanced inoperable non-small-cell lung cancer: radiation therapy oncology group protocol 91–06. J Clin Oncol. 1996 Apr;14(4):1055–64. doi: 10.1200/JCO.1996.14.4.1055. PMID 8648357. [DOI] [PubMed] [Google Scholar]
- 8.Komaki R, Scott C, Ettinger D, et al. Randomized study of chemotherapy/radiation therapy combinations for favorable patients with locally advanced inoperable nonsmall cell lung cancer: Radiation Therapy Oncology Group (RTOG) 92-04. Int J Radiat Oncol Biol Phys. 1997 Apr 1;38(1):149–55. doi: 10.1016/s0360-3016(97)00251-4. PMID 9212017. [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009 Aug 1;374(9687):379–86. doi: 10.1016/S0140-6736(09)60737-6. Epub 2009 Jul 24. PMID 19632716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran WJ, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011 Oct 5;103(19):1452–60. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98–01. J Clin Oncol. 2005 Apr 1;23(10):2145–54. doi: 10.1200/JCO.2005.07.167. PMID 15800308. [DOI] [PubMed] [Google Scholar]
- 12.Mac Manus M, Hicks RJ. The use of positron emission tomography (PET) in the staging/evaluation, treatment, and follow-up of patients with lung cancer: a critical review. Int J Radiat Oncol Biol Phys. 2008 Dec 1;72(5):1298–306. doi: 10.1016/j.ijrobp.2008.08.022. PMID 19028270. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig KE, Fox JL, Yorke E. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005 May 15;103(10):2118–27. doi: 10.1002/cncr.21007. PMID 15830346. [DOI] [PubMed] [Google Scholar]
- 14.Bradley JD, Bae K, Graham MV. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010 May 10;28(14):2475–80. doi: 10.1200/JCO.2009.27.1205. Epub 2010 Apr 5. PMID 20368547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Correa CR, Zhao L. The effect of radiation dose and chemotherapy on overall survival in 237 patients with Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009 Apr 1;73(5):1383–90. doi: 10.1016/j.ijrobp.2008.06.1935. Epub 2008 Oct 17. PMID 18929449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003 Apr 1;21(7):1285–92. doi: 10.1200/JCO.2003.07.054. PMID 12663716. [DOI] [PubMed] [Google Scholar]
- 17.Ryu JS, Choi NC, Fischman AJ, et al. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002 Feb;35(2):179–87. doi: 10.1016/s0169-5002(01)00332-4. PMID 11804691. [DOI] [PubMed] [Google Scholar]
- 18.Machtay M, Bae K, Movsas B, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced NSCLC treated with chemoradiation: ana analysis of the Radiation Therapy Oncology Group. Int J RAdiat Oncol Biol Phys. 2010 Oct 25; doi: 10.1016/j.ijrobp.2010.09.004. epub. PMID 20980108. [DOI] [PMC free article] [PubMed] [Google Scholar]