Abstract
Background and purpose
The fibronectin isoform containing the alternatively-spliced extra domain A (EDA+-FN) is normally absent from the circulation, but plasma levels of EDA+-FN can become markedly elevated in several human pathological conditions associated with inflammation including ischemic stroke. It remains unknown whether EDA+-FN contributes to stroke pathogenesis or is simply an associative marker. Several in vitro studies suggest that EDA+-FN can activate toll-like receptor 4 (TLR4), an innate immune receptor that triggers pro-inflammatory responses. We undertook a genetic approach in mice to investigate the ability of EDA+-FN to mediate inflammatory brain damage in a focal cerebral ischemia/reperfusion injury model.
Methods
We used genetically modified EDA+/+ mice, which constitutively express EDA+-FN. Extent of injury, neurological outcome and inflammatory mechanisms were assessed following one hour cerebral ischemia/23 hour reperfusion injury and compared to wild-type (WT) mice.
Results
We found that EDA+/+ mice developed significantly larger infarcts and severe neurological deficits that was associated with significant increased neutrophil and macrophage infiltration as quantitated by immunohistochemistry. Additionally, we found upregulation of NF-κB, COX-2, and inflammatory cytokines TNFα, IL-1β and IL-6 in the EDA+/+ mice compared to WT mice. Interestingly, increased brain injury and neurological deficits were largely abrogated in EDA+/+ mice by treatment with a specific TLR4 inhibitor.
Conclusions
These findings provide the first evidence that EDA+-FN promotes inflammatory brain injury following ischemic stroke and suggest that the elevated levels of plasma EDA+-FN observed in chronic inflammatory conditions could worsen injury and outcome in patients following acute stroke.
Keywords: Fibronectin, Inflammation, Cerebral ischemia, mice
Introduction
Ischemic stroke is the third leading cause of morbidity and mortality worldwide, and remains a major challenge to public health. Elucidation of novel cellular and molecular pathways that influence the pathogenesis of ischemic stroke could lead to development of new therapeutic approaches and greater insight into disease pathophysiology.
Recently, the dimeric glycoprotein fibronectin (FN) has emerged as a key factor contributing to the pathogenesis of several diseases associated with thrombosis and inflammation (Extensively reviewed).1 FN is present in plasma and in tissue extracellular matrix.2 FN is a ligand for many members of the integrin family, and the various domains of FN interact with thrombosis-related proteins including collagen, fibrin, and heparin (Supplementary Figure 1). FN protein diversity is generated by alternative splicing of the primary transcript at three sites: extra domain A (EDA), extra domain B (EDB), and the type III homologies connecting segment (IIICS) (Supplementary Figure 1). Two major isoforms of FN exist in humans and mice: 1) plasma FN, which is synthesized by hepatocyte and does not contain the alternatively-spliced EDA or EDB domains; and 2) cellular FN, which is synthesized by cells such as fibroblasts, endothelial cells, and smooth muscle cells. Cellular FN can contain either of the EDA or EDB domains independently, or both domains in different proportions.1 The alternative splicing of EDA and EDB is independent and conserved across species, including mice, rats, chickens and humans. Alternative splicing of IIICS generates additional FN isoforms that are species-specific.3
Fibronectin-containing the alternatively-spliced extra domain A (EDA+-FN) is normally absent from the plasma of humans and mice4, 5 but high plasma levels of EDA+-FN have been found in patients with chronic inflammation and ischemic stroke.6-9 However, it remains unclear in humans whether these elevated levels of EDA+-FN are actively contributing to disease pathogenesis, or rather simply serving as an associated marker. Several recent findings led us to hypothesize that EDA+-FN may have the ability to exacerbate tissue damage and neurological outcome in acute ischemic stroke, a pathophysiological process that is mediated by both thrombotic and inflammatory components. First, recently we have demonstrated that EDA+-FN accelerates thrombosis in ferric-chloride injured mesenteric arterioles, suggesting that EDA+-FN is pro-thrombotic.10 Second, the EDA domain of FN contains binding sites for integrins α4β1 and α9β1 present on leukocytes and endothelial cells,11 suggesting the EDA+-FN may influence inflammatory processes. Third, several in vitro studies demonstrate that the EDA domain of FN can activate toll-like receptor 4 (TLR4), an innate immune receptor that triggers pro-inflammatory responses. 12-14
In this study, we compared EDA+/+ and wild-type (WT) mice to test the hypothesis that EDA+-FN aggravates ischemia/reperfusion (I/R) brain injury and examined the mechanistic role of TLR4 in this process. The EDA+/+ strain constitutively expresses EDA+-FN in the plasma and tissues.5 WT mice contain the wild-type FN allele. Under normal conditions, plasma FN in WT mice lacks the EDA domain; however, EDA+-FN can appear in the plasma during conditions of chronic inflammation such as atherosclerosis.4 In present study, we show that EDA+-FN promotes ischemia/reperfusion brain injury through a TLR4-dependent mechanism.
Methods
An expanded version of the method section is available in the Online Data Supplementary section.
Mice
EDA+/+, EDA+/wt mice have been described and characterized previously.5 Both strains have been backcrossed >15 generations to C57BL/6J background. Control mice were heterozygous littermates or age matched WT C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). All mice used were males between 8-10 weeks of age. All experiments were approved by the University of Iowa Animal Care and Use Committee.
Cerebral ischemia and reperfusion injury
Transient focal cerebral ischemia was induced by 60 minutes of occlusion of the right middle cerebral artery with a 7.0 siliconized filament followed by either 5 hours or 23 hours of reperfusion. Mice were anesthetized with 1-1.5% isoflurane mixed with medical air. Body temperature was maintained at 37°C ± 1.0 using a heating pad. Laser Doppler flowmetry (Perimed instruments, Sweden) was used to confirm induction of ischemia and reperfusion (Supplementary Table 1). Physiological parameters, including pH, pO2, and pCO2 (Supplementary Table 2) were analyzed using a blood analyzer (Radiometer, USA). Mean arterial blood pressure was determined by non-invasive blood pressure system (CODA Monitor, Kent scientific, USA). Body temperature was measured by infrared thermometer (Kent scientific). Prior to sacrifice mice were evaluated for motor-deficits by an observer blinded to the identity of the groups. The motor-deficit was scored on a four-point neurological scale as described:15 0, no observable neurological deficit (normal); 1, failure to extend left forepaw on lifting the whole body by tail (mild); 2, circling to the contralateral side but normal posture at rest (moderate); 3, leaning to the contralateral side at rest (severe); 4, no spontaneous motor activity. For morphometric measurement eight coronal serial sections were cut at 1-mm intervals from the frontal pole using a mouse Brain Matrix (Roboz surgical instrument). Coronal sections were stained with 2% triphenyl-2, 3, 4-tetrazolium-chloride (TTC) for 15 min at 37o C. Sections were scanned, digitalized and infarct areas were measured blindly using the Nikon NIS element software. To correct for brain swelling due to edema after ischemia the corrected total infarct volume (%) was calculated as follows: Corrected infarct volume (%) = {[volume of contralateral hemisphere- (volume of ipsilateral hemisphere-volume of infarct)]}/ volume of contralateral hemisphere X 100.
Statistics
Results are reported as the mean ± SEM. Statistical significance of the difference between means was assessed by unpaired Student’s t test (for comparison of 2 groups) or by ANOVA followed by Boneferroni’s multiple comparison test. ANOVA on ranks was applied to test for significant differences in the neuroscore. Treatment and genotype effects were analyzed by 2-way ANOVA followed by Holm-Sidak multiple comparison tests. Values of P < 0.05 were considered significant.
Results
Exacerbated brain injury and worsened neurological outcome following ischemic stroke in mice expressing EDA+-FN
To test the hypothesis that EDA+-FN exacerbates brain injury after ischemic stroke, we compared infarct volume and neurological outcome in the EDA+/+, EDA+/wt and WT mice following 60 min ischemia/23 h reperfusion injury. EDA+/+ and EDA+/wt mice had significantly increased in infarct volume (EDA+/+ mice, mean ± SEM: 37.3 % ± 4.1 %; EDA+/wt mice, mean ± SEM: 36.1 % ± 3.9 %, P<0.01, ANOVA) in the ischemic brain hemisphere compared with WT mice (mean ± SEM: 22.3 % ± 3.4 %, Figure 1A & B). The increased infarct volume in the EDA+/+ and EDA+/wt mice was associated with severe neurological deficits compared with WT mice (P<0.01, Figure 1C). Exacerbated infarct volume in the EDA+/+ and EDA+/wt mice was not associated with increased mortality when compared to WT mice (not shown). Laser Doppler measurements (Supplementary Table 1) and physiological parameters (Supplementary Table 2) were similar among groups during and after ischemia. Together these findings demonstrate that EDA+-FN exacerbates brain damage and worsens neurological outcome following ischemic stroke.
Figure 1. EDA+-FN promotes brain injury and worsens neurological outcome during cerebral ischemia.
(A) Representative TTC stained serial coronal brain sections from one mouse of each genotype following 60 min of ischemia/ 23 h of reperfusion injury. (B) Corrected mean infarct volumes (%) of each genotype (N=11-13/group). (C) Neurological score from each genotype prior to sacrifice (N=11-13/group). Data are medium ± SD. ANOVA on ranks was used to test for significant differences.
Enhanced brain tissue inflammation in mice expressing EDA+-FN
To determine whether EDA+-FN-exacerbated brain injury is associated with increased inflammation, we measured myeloperoxidase (MPO) activity in the supernatant fractions of brain homogenates prepared from the infarcted and surrounding areas in the EDA+/+ and WT mice following 60 min ischemia/23 h reperfusion injury. EDA+/+ mice demonstrated significantly increased MPO activity in the ischemic hemisphere compared with WT mice (Figure 2A), suggesting enhanced inflammation in the damaged tissue. No differences in MPO activity were observed in the uninjured contralateral hemisphere of WT and EDA+/+ mice (Figure 2A), suggesting that EDA+-FN promotes inflammation only in the setting of injury. In concordance with the increased tissue MPO activity, neutrophils and macrophages were significantly elevated in EDA+/+ mice compared to WT (P<0.01, Figure 2B & C) but not in the contralateral hemisphere (not shown). These findings demonstrate that EDA+-FN exacerbates tissue inflammation following ischemic stroke.
Figure 2. EDA+-FN enhances inflammatory response during cerebral ischemia.
(A) MPO activity from each genotype quantified by ELISA from the ipsilateral (infarct and surrounding region) or contralateral hemispheres (N=7/group). (B) The left panel shows representative coronal brain sections stained for neutrophil marker (NIMP positive cells are stained as brown), and counter stained with hematoxylin (blue) from one mouse of each genotype. The right panel shows quantification of NIMP-stained cells in the infarct region. The scale bar = 20 μm. (C) The left panel shows representative coronal brain sections stained for macrophages (Mac-3 positive cells are stained as brown), and counter stained with hematoxylin (blue) from one mouse of each genotype. The right panel shows quantification of Mac-3 stained cells in the infarct region. The scale bar = 20 μm. Quantification was done as described in method section. Nine coronal sections per mouse (separated by 100μm from the frontal pole) were analyzed from 4 mice of each genotype (N = 36 sections/genotype).
Up regulation of NF-κB, COX-2 and pro-inflammatory cytokines expression following 60 min ischemia/ 23 h reperfusion injury in mice expressing EDA+-FN
The EDA domain of FN has been shown to trigger gene expression of NF-κB in in vitro.12 We measured NF-κB p65 (a marker of NF-κB activation) in nuclear extracts by Western blotting (Figure 3A). EDA+/+ mice demonstrated significantly higher levels of NF-κB p65 compared with WT mice. We next investigated expression of the inflammatory enzyme COX-2, which is an NF-κB responsive gene, by Western blotting of cytoplasmic fractions (Figure 3B). Similar to NF-κB p65, we found significantly higher protein levels of COX-2 in injured tissue in the EDA+/+ mice compared to WT mice. In addition to COX-2, NF-κB regulates the coordinated expression of many genes, including genes encoding pro-inflammatory cytokines that amplify and perpetuate the inflammatory response.16 We therefore investigated the effect of EDA+-FN on pro-inflammatory cytokine production (Figure 3C). EDA+/+ mice demonstrated significantly elevated levels of IL-6, IL-1β and TNF-α compared to WT in the supernatant fractions of brain homogenates prepared from the infarcted and surrounding areas. No differences in levels of IL-6, IL-1β and TNF-α were observed in the uninjured contralateral hemisphere of WT and EDA+/+ mice or at baseline in sham-operated EDA+/+ animals (not shown).
Figure 3. EDA+-FN up regulates expression levels of NF-κB, COX-2 and pro-inflammatory cytokines after ischemia/reperfusion injury.
(A) Representative Western blots of subunit NF-κB p65 and β-actin. # 1 and # 2 are samples from individual mice from each genotype. (B) Representative Western blots of COX-2 and β-actin. #1 and # 2 are samples from individual mice from each genotype. Corresponding histogram shows mean densitometric analysis of bands after normalizing with β-actin as a loading control of each genotype (N=4/ group). Data are mean ± SEM. AU indicates arbitrary units. (C) Quantification of IL-6, I IL-1β, and TNF-α by ELISA in the supernatant of brain homogenates from the ischemic region (infarcted and surrounding area) from each genotype (N =8 /group). Data are mean ± SEM.
EDA+-FN promote early tissue inflammation following 60 min ischemia/5 h reperfusion injury
Increased markers of inflammation at the 24 hours time point may merely represent a late response to increased lesion size in the EDA+/+ mice. To examine the inflammatory response at an earlier time point, WT and EDA+/+mice were subjected to 60 min ischemia/ 5 h reperfusion injury. EDA+/+ mice demonstrated significantly increased MPO activity and neutrophil influx in the ischemic hemisphere compared with WT mice (Figure 4 A& B). No differences in MPO activity were observed in the uninjured contralateral hemisphere of WT and EDA+/+ mice (Figure 4A). Next, we investigated the effect of EDA+-FN on pro-inflammatory cytokines production in the ischemic hemisphere. EDA+/+ mice demonstrated significantly elevated levels of inflammatory cytokines, including IL-6, IL-1β and TNF-α compared to WT (Figure 4C) in the supernatant fractions of brain homogenates prepared from the infarcted and surrounding areas. Together these results suggest that the increased inflammatory response observed in EDA+/+ mice is not simply a late consequence of increased lesion size at the 24 hours time point.
Figure 4. EDA+-FN enhances early inflammatory response following 60 min ischemia/5 h reperfusion injury.
(A) MPO activity from each genotype quantified by ELISA from the ipsilateral (infarct and surrounding region) or contralateral hemispheres (N=6/group). (B) The left panel shows representative coronal brain sections stained for neutrophils (NIMP positive cells are stained as brown), and counter stained with hematoxylin (blue) from one mouse of each genotype. The right panel shows quantification of NIMP-stained cells in the infarct region. The scale bar = 20 μm. Three coronal sections per mouse (separated by 100μm) were analyzed from 3 mice of each genotype (N = 9 sections/genotype). (C) Quantification of IL-6, IL-1β and TNF-α by ELISA in the supernatant of brain homogenates from the ischemic region (infarcted and surrounding area) from each genotype. Data are mean ± SEM. N = 6 /group.
Brain injury by EDA+-FN occurs via a TLR4-dependent mechanism
To investigate the molecular mechanism by which EDA+-FN exacerbates brain injury; we targeted TLR4, a proinflammatory receptor that has previously shown to interact with EDA domain of FN in vitro.12-14 Mice were injected intravenously with the specific TLR4 inhibitor CLI-095 (1mg/Kg) or vehicle 30 minutes prior to 60 min ischemia/ 23 h reperfusion injury. TLR4 inhibitor CLI-095 at this dose has been previously demonstrated to specifically suppress TLR4 signaling and downstream cytokine production in several murine models.14, 17, 18 Administration of CLI-095 to WT mice resulted in a modest, but statistically significant reduction of infarct volume (P<0.05, Figure 5A and B), and improvement of neurological outcome (P<0.01, Figure 5C). Interestingly, the same treatment in EDA+/+ mice resulted in an even more robust reduction of infarct volume (P<0.001 [Figure 5A and B]) and improvement of neurological outcome (P<0.01, Figure 5C), protecting nearly to the extent of injury seen in CLI-095-treated WT mice. There was a two-fold reduction of infarct volume in CLI-095-treated EDA+/+ mice compared to CLI-095-treated WT mice (P<0.0001, [Figure 5B]). Two-way ANOVA analysis showed that the interaction of genotype and treatment with the TLR4 inhibitor CLI-095 was significant (P=0.018, F= 6.149). This observation suggests that a majority of the effect introduced by EDA+-FN is occurring via a TLR4-dependent mechanism.
Figure 5. Brain injury in the EDA+/+ mice is TLR4-dependent.
(A) Representative TTC stained serial coronal brain sections from one mouse of each genotype following 60 min of ischemia /23 h of reperfusion injury (B) Corrected mean infarct volumes (%) of each genotype (N=9-10/group). There was a two-fold reduction of infarct volume in CLI-095-treated EDA+/+ mice compared to CLI-095-treated WT mice (P<0.0001). Two-way ANOVA analysis showed that interaction of genotype and treatment with the TLR4 inhibitor CLI-095 was significant (P=0.018, F= 6.149) (C) Neurological score from each genotype prior to sacrifice (N=9-10/group). Data are medium ± SD. ANOVA on ranks was used to test for significant differences.
Discussion
Several human pathological conditions including ischemic stroke are associated with elevated plasma levels of EDA+-FN, an endogenous splice variant of FN, which is normally absent from the circulation.6-9, 19 The significance of the elevated EDA+-FN levels in circulation observed in ischemic stroke and other chronic inflammatory conditions is not known. In this study, we show that EDA+/+ mice constitutively expressing EDA+-FN exhibit exacerbated brain injury, worsened neurological outcome, and enhanced post-ischemic inflammation, demonstrating an active functional role for EDA+-FN in disease pathogenesis.
We demonstrated that EDA+-FN promotes the recruitment of inflammatory cells (neutrophil and macrophage) in the infarcted tissue following reperfusion injury. This observation is consistent with neutrophil influx correlating positively with enhanced ischemic damage, as documented by other studies.20 We next demonstrated enhanced activation of transcription factor NF-κB in the ischemic hemisphere of mice constitutively expressing EDA+-FN. NF-κB activation is known to promote brain injury during focal cerebral ischemia, via a cell death-promoting mechanism.21 Interestingly, the EDA domain of FN has been shown to trigger gene expression of NF-κB in in vitro.12 Activation of NF-κB also induces pro-inflammatory genes encoding enzymes, cytokines, and other adhesion molecules, all of which are known to promote inflammatory tissue injury.22 Consisting with this mechanism, we detected enhanced expression of COX-2, TNF-α, IL-1β, and IL-6 in the infarcted and surrounding areas in mice constitutively expressing EDA+-FN.
Next we investigated the molecular mechanism by which EDA+-FN exacerbates inflammation after brain injury. Using a specific TLR4 inhibitor that has been shown to be effective in mice (CLI-095),14, 17, 18 we found that inhibition of TLR4 signaling in the EDA+/+ mice resulted in a robust reduction of infarct volume, and improvement of neurological outcome (Figure 6). We propose that EDA+-FN interacts with TLR4 to upregulate the NF-κB pathway, which mediates brain injury after ischemic stroke (Figure 6). In line with our study, previously it was demonstrated that the EDA domain of FN is able to activate TLR4 signaling in vitro.12 In this study, Okamura et al. transfected HEK 293 cells, which do not express endogenous TLR4, with a TLR4 plasmid and demonstrated that the EDA domain of FN stimulates NF-κB via TLR4 activation.12 However, WT mice treated with same inhibitor also exhibited a modest, but statistically significant reduction of infarct volume, presumably because of the presence of other endogenous ligands for TRL4. For example, heat-shock proteins 23 released from necrotic cells, fibrinogen and fibrin deposited during vascular injury 24 have been reported to activate TLR4 and generate an inflammatory response. Interestingly, CLI-095-treated EDA+/+ mice exhibited a reduction of infarct volume down equivalent to the size seen in CLI-095-treated WT mice. This result strongly suggests that majority of the effect introduced by EDA+-FN on brain injury occurs via a TLR4-dependent mechanism. Multiple cell types that express TLR4 might contribute to the inflammatory response to EDA+-FN in the setting of acute brain injury. In line with our studies, recently it was shown that recombinant isolated fibronectin EDA domain is able to promote neutrophil migration both in vitro and in vivo, through a process dependent on TLR4 signaling in neutrophils.14 Thus our data together with these findings suggest that EDA+-FN may aggravate brain injury following ischemia through a TLR4-dependent mechanism involving either neutrophils or macrophages independently, or both via an NF-κB signaling pathway. Although ours and other studies indicate that EDA+-FN/TLR4 signaling is likely a significant mediator of post-ischemic inflammation, it remains possible that some of the pro-inflammatory effects of EDA+-FN are TLR4-independent, perhaps mediated by binding sites for leukocyte integrins α4β1 and α9β1 in the EDA domain.11 Additional future studies will be required to determine if disruption of EDA+-FN-integrin interactions in vivo prevents inflammatory cells recruitment and subsequent tissue damage.
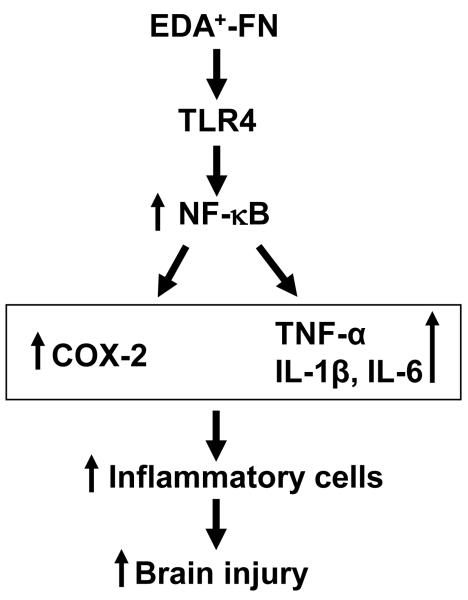
Figure 6. Proposed mechanism of EDA+-FN-mediated brain injury.
Upon ischemia/reperfusion injury plasma EDA+-FN gets incorporated efficiently in the extracellular matrix due to blood brain barrier disruption and enhances activation of TLR-4 signaling pathway. TLR-4 activates NF-κB, which up regulates inflammatory gene encoding enzyme COX-2 and cytokines TNF-α, IL-1β and IL-6 that generate signals for the recruitment of inflammatory cells resulting in aggravated brain tissue injury.
A potential limitation of this study is that EDA+/+ mice have lower plasma levels of total FN compared with WT mice, presumably due to decreased secretion.5, 10 The effect of EDA+-FN on brain injury is unlikely to be due to decreased levels of FN in plasma or brain tissue, because EDA+/wt mice, which have normal levels of FN but contain EDA+-FN,5 also exhibit exacerbated brain injury similar to EDA+/+ mice (Figure 1).
Conclusions
In summary, our study unveils a previously unknown role of EDA+-FN in promoting inflammation and brain injury in mice following ischemia/reperfusion. The mechanism is likely related in part to TLR4 signaling as illustrated in Figure 6. Our findings suggest that the elevated levels of plasma EDA+-FN observed in chronic inflammatory conditions could worsen injury and outcome in patients following acute ischemic injury. These findings shed new light on the pathophysiology of ischemic stroke, and suggest new potential therapeutic approaches to this important cause of human morbidity and mortality.
Supplementary Material
Acknowledgments
We thank Katina Wilson for assistance in analyzing blood gases.
Sources of Funding This work was supported by ASH Scholar Award from the American Society of Hematology to A.K.C., and National Heart, Lung and Blood Institute of the National Institutes of Health grants HL063943 and NS024621 to S.R.L., and HL076539 to D. G. M.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 3.ffrench-Constant C. Alternative splicing of fibronectin--many different proteins but few different functions. Experimental cell research. 1995;221:261–271. doi: 10.1006/excr.1995.1374. [DOI] [PubMed] [Google Scholar]
- 4.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin eiiia domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 5.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, et al. Regulated splicing of the fibronectin eda exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satoi S, Kitade H, Hiramatsu Y, Kwon AH, Takahashi H, Sekiguchi K, et al. Increased extra domain-a containing fibronectin and hepatic dysfunction during septic response: An in vivo and in vitro study. Shock. 2000;13:492–496. doi: 10.1097/00024382-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Castellanos M, Leira R, Serena J, Blanco M, Pedraza S, Castillo J, et al. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35:1671–1676. doi: 10.1161/01.STR.0000131656.47979.39. [DOI] [PubMed] [Google Scholar]
- 8.Peters JH, Maunder RJ, Woolf AD, Cochrane CG, Ginsberg MH. Elevated plasma levels of ed1+ (“cellular”) fibronectin in patients with vascular injury. J Lab Clin Med. 1989;113:586–597. [PubMed] [Google Scholar]
- 9.Kanters SD, Banga JD, Algra A, Frijns RC, Beutler JJ, Fijnheer R. Plasma levels of cellular fibronectin in diabetes. Diabetes Care. 2001;24:323–327. doi: 10.2337/diacare.24.2.323. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan AK, Kisucka J, Cozzi MR, Walsh MT, Moretti FA, Battiston M, et al. Prothrombotic effects of fibronectin isoforms containing the eda domain. Arterioscler Thromb Vasc Biol. 2008;28:296–301. doi: 10.1161/ATVBAHA.107.149146. [DOI] [PubMed] [Google Scholar]
- 11.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The eiiia segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 12.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain a of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 13.Lasarte JJ, Casares N, Gorraiz M, Hervas-Stubbs S, Arribillaga L, Mansilla C, et al. The extra domain a from fibronectin targets antigens to tlr4-expressing cells and induces cytotoxic t cell responses in vivo. J Immunol. 2007;178:748–756. doi: 10.4049/jimmunol.178.2.748. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre JS, Levesque T, Picard S, Pare G, Gravel A, Flamand L, et al. Extra domain a of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of toll-like receptor 4. Arthritis Rheum. 2011;63:1527–1533. doi: 10.1002/art.30308. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 16.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: Putative role for cytokines, adhesion molecules and inos in brain response to ischemia. Brain pathology. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. Tak-242 selectively suppresses toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Sha T, Sunamoto M, Kitazaki T, Sato J, Ii M, Iizawa Y. Therapeutic effects of tak-242, a novel selective toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571:231–239. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, et al. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18584–18589. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. Nf-kappab is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 22.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: Putative role for cytokines, adhesion molecules and inos in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 24.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.