Abstract
Prostate cancer is the second leading cause of cancer deaths among men. The availability of animal models that represent the events and factors that exist in the natural history and biology of human prostate cancer is essential in dealing with prostate cancer. In recent decades and presently, emphasis has been directed at the development and employment of prostate cancer induced in transgenic mice. However, the important consistent hallmark characteristic and event of decrease in zinc and citrate and downregulation of ZIP1 zinc transporter in prostate malignancy has not been studied or identified in any animal model. We investigated the status of these parameters in TRAMP tumors as compared with human prostate cancer. The results show that citrate levels are markedly decreased in the developing and advancing stages of malignancy in TRAMP. Zinc levels are also decreased and ZIP1 transporter is lost in TRAMP tumors. In vitro studies show that zinc treatment of TRAMP C2 cells exhibits cytotoxic effects. Collectively, these results mimic the ZIP1, zinc, and citrate status and relationship that exist in human prostate cancer. This is the first report that establishes the existence of the human prostate zinc/citrate hallmark characteristic and relationship in an animal model. It now appears that the TRAMP model will be suitable for studies relating to the implications and role of zinc- and citraterelated metabolism in the development and progression of human prostate cancer.
Keywords: prostate cancer, TRAMP, zinc, citrate, ZIP1 transporter, genetic/metabolic transformation
Introduction
Advancements in the understanding and elucidation of the factors and mechanisms involved in prostate carcinogenesis require the availability and employment of experimental models. This also applies to other important issues such as the development and testing of potential chemotherapeutic agents for the treatment and prevention of the development and progression of malignancy. Such experimental models include in vitro studies with cell lines and in vivo studies with animals. The latter includes transgenic animals in which induced genetic alterations result in the development of prostate cancer. The difficulty and problem is that no model exists that is fully representative of the events and conditions that exist in the natural history of the development and progression of human prostate cancer. This imposes limitations on the appropriate use and translational application of information derived from such models.
It is essential that the model be well-characterized in relation to the known events and factors involved in the development and progression of human prostate cancer. Among such known events and factors is the zinc and citrate relationship that exists in human prostate cancer. It is well established that zinc and citrate levels are virtually always markedly decreased in prostate cancer as compared with normal and benign prostate (for reviews see ref. 1–4). This relationship currently, in our view, is the most consistent and persistent “hallmark” characteristic of human prostate cancer, which distinguishes prostate cancer from normal and benign prostate. Additionally, recent clinical and experimental studies have established that ZIP1 zinc uptake transporter is downregulated in prostate cancer in association with the decrease in zinc. The ZIP1/zinc/citrate decrease constitutes a “genetic/metabolic” transformation of normal prostate cells to malignant cells in the development of prostate cancer.1,5 Despite this important relationship, the coupled ZIP1/zinc/citrate relationship has not been determined or established in any of the existing animal models that purport to represent human prostate cancer.
This present report focuses on the TRAMP (transgenic adenocarcinoma of the mouse prostate) model, which is presently the most employed animal model for studies relating to human prostate cancer. As noted above, no substantial information is available concerning the existence in TRAMP for the presence or absence of the zinc/citrate relationship that exists in the development and progression of human prostate cancer. This report presents initial information and characterization of the Zip1/zinc/citrate relationship in TRAMP, and describes its translational representation of human prostate cancer. It is important to note that the components of this study were independently conducted by Dr. Kurhanewicz and colleagues at UCSF, and by Dr. Costello and colleagues at UMd; and, where appropriate, will be referred to as the “UCSF” and the “UMd” studies.
Results
The citrate metabolic profile in TRAMP vs. human prostate.
These studies were conducted by the UCSF group. Figure 1 shows a ventral view of the TRAMP prostate with advanced tumors and lymph node with metastases. Figure 2 presents typical in vivo 1H MRSI spectra taken from a patient with surgically proven prostate cancer in the lateral posterior aspect of the right midgland. As previously described, 1H MRSI spectra from the normal peripheral zone contain high level of citrate and low choline and creatine.6 In contrast, the malignant tissue exhibits a significant, pathologic grade dependent decrease in citrate accompanied by increased levels of choline containing phospholipids metabolites. Figure 3 shows the results of 1H high resolution magic angle spinning (HR-MAS) spectroscopic studies of snap frozen normal murine prostate, TRAMP tumors, and human prostate cancer tissues. 1H HR-MAS spectra of normal and TRAMP mice showed the major loss of citrate in the tumors (Fig. 3A). Figure 3B shows that citrate/creatine ratios were high in ventral lobes of normal mice (mean = 19.4 ± 3.8), and were significantly reduced in the ventral lobes of early TRAMP tumors (mean = 6.0 ± 4.5). The citrate was further decreased to barely detectable levels in advanced TRAMP tumors (mean = 0.33 ± 0.46, p = 0.012) and lymph node metastases (mean = 0.08 ± 0.06, p = 0.006). It is evident that citrate is continuously decreased as prostate tumor develops and advances, which is similar to human primary site malignancy. The loss of citrate in the TRAMP metastatic lesions is also consistent with the decreased concentration of zinc in human prostate lymph node metastasis as Marberger et al. had reported.7
Figure 1.
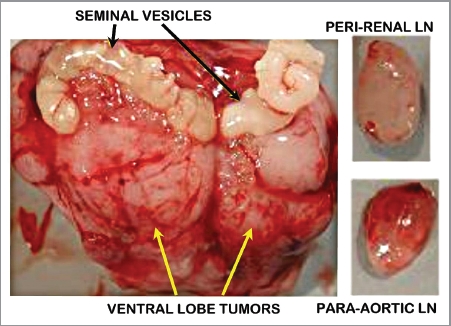
Tramp prostate tumors and metastasis. (A) Large prostate ventral lobe tumors; normal seminal vesicles. (B) Metastatic lesions in lymph nodes (LN).
Figure 2.
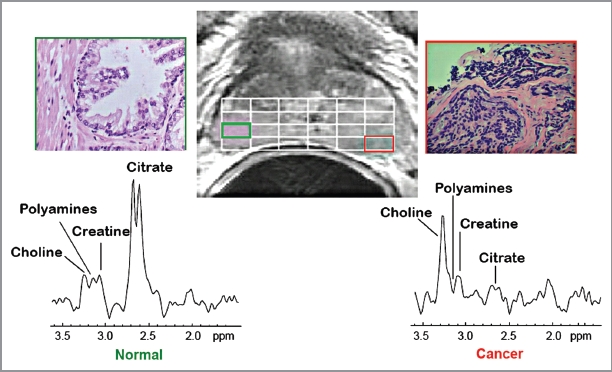
In vivo 3T 1H MRSI spectra taken from a region of normal peripheral zone and spectra taken from a region of prostate cancer and corresponding H&E stained sections identified on the corresponding T2 weighted MR image (center). 1H MRSI provides arrays of contiguous 1H MR spectra from throughout the prostate (white grid overlaid on MR image) and the spectra were selected to be localized within regions of healthy and cancerous tissues based on concordance of step-section histology of the resected prostate and T2 weighted MRI. Citrate is dramatically reduced in the Gleason 4+4 cancer as compared with healthy peripheral zone.
Figure 3.
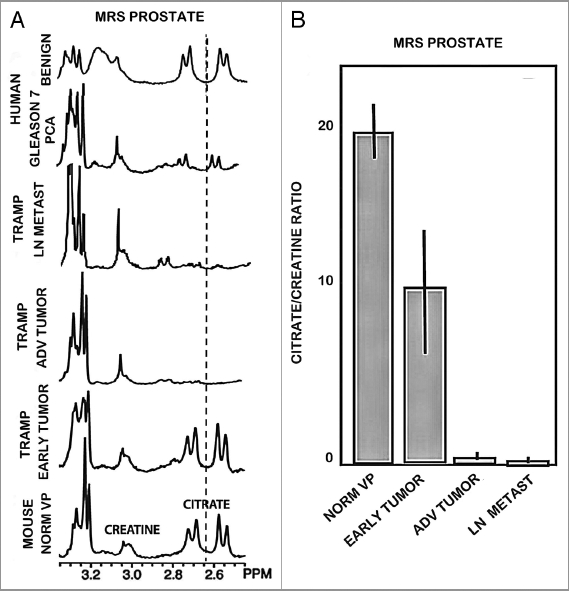
(A) Ex vivo 11.7T 1H HR-MAS spectra tissues taken from the normal murine prostatic ventral lobe, early stage TRAMP tumor, late stage TRAMP tumor, healthy human peripheral zone tissue and Gleason 4+3 prostate cancer and a metastatic lymph node in a TRAMP mouse. Note the appearance of citrate (4 peaks) is different than in the in vivo 1H MRSI spectra (two overlapping peaks). This is due to the increased chemical shift dispersion that occurs at 11.7T vs. 3T. (B) Quantification (Citrate/creatine peak area ratio) of the reduction in citrate on progression from normal to late stage and metastatic prostate cancer.
Zinc and ZIP1 in TRAMP animals.
These studies were conducted by the UMd group on tissue samples from the UCSF studies described in the preceding section. In the only report concerning zinc levels in TRAMP that we could find, Ghosh et al.8 reported that zinc levels were decreased in TRAMP tumors. We performed dithizone (DTZ) staining of tissue sections of advanced TRAMP tumor vs. normal mouse prostate. Figure 4A shows the presence of dark Zn-DTZ pigment which reflects a relatively high level of zinc predominantly in the acini glandular epithelium. In contrast, the absence of Zn-DTZ staining reflects a significant loss of cellular zinc in the tumor cells. Figure 4B shows our results with human prostate sections using the same DTZ staining procedure and Figure 4C shows the results of Gyorkey et al.9 with DTZ staining of human prostate. In both cases DTZ detects the loss of zinc in human malignancy, which verifies the DTZ detection of decreased zinc in TRAMP tumors.
Figure 4.
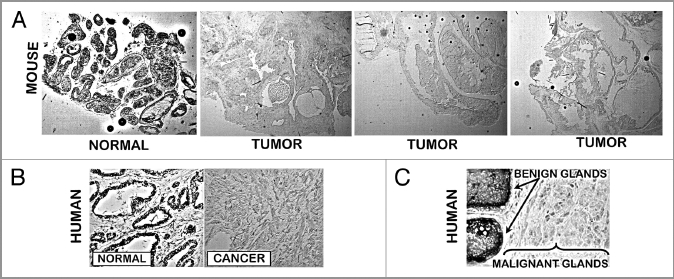
Dithizone zinc stain of prostate tissue sections. (A) Normal mouse prostate showing Zn-DTZ dark pigment formation due to presence of zinc. TRAMP tumors exhibit loss of zinc staining. (B) Our DTZ stain showing high Zn in normal peripheral zone glandular epithelium vs loss of Zn in peripheral zone adenocarcinoma. (C) DTZ staining of human prostate section which shows high Zn-DTZ in benign glandular epithelium and loss of zinc in malignancy (Gyorkey et al.9).
Since it is well established that ZIP1 transporter downregulation is a major cause of zinc decrease in human prostate malignancy, we determined its status in TRAMP. As shown in Figure 5A, ZIP1 transporter is abundant in the normal mouse prostate glandular epithelium. It is especially noteworthy that the transporter is localized at the basilar membrane, which is consistent with its functioning for zinc uptake from interstitial fluid derived from blood plasma. Figure 5A also shows ZIP1 transporter that is localized at the apical membrane. This could possibly function for the re-uptake of zinc from prostatic fluid so as to conserve and retain the cellular level of zinc. Most importantly, ZIP1 transporter is absent in the TRAMP tumor. As is evident, there is no detectable ZIP1 at the plasma membrane or elsewhere in the TRAMP tumor cells. Figure 5B (taken from Franklin et al.10) shows the basal and apical membrane localization of ZIP1 in the normal human glandular epithelium and the loss of transporter in malignancy. Thus the TRAMP model closely mimics the ZIP1 status in normal and malignant human prostate.
Figure 5.
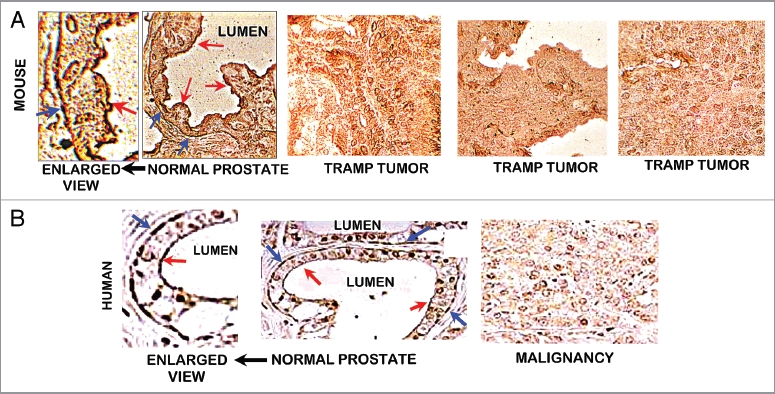
(A) ZIP1 transporter in normal mouse prostate and TRAMP tumors. ZIP1 IHC reveals the presence of the transporter in normal prostate acinus. The transporter is localized at the glandular epithelium basal membrane (blue arrows), and at the apical membrane (red arrows). There is no detectable transporter in the TRAMP tumors. (B) ZIP1 transporter in human prostate (taken from Franklin et al.10). Shows same ZIP1 relationship as in (A).
ZIP1 and zinc in the TRAMP C2 cell line.
These studies were conducted by the UMd group. Some ZIP1 and zinc relationships in the TRAMP C2 cell line, which was derived from a heterogeneous 32 week TRAMP tumor,11 were determined. We first determined if ZIP1 transporter was evident in these cells. Western blot analysis (Fig. 6) reveals that ZIP1 transporter exists in association with the plasma membrane. Therefore, it is evident that the C2 malignant cell line does not represent the status that exists in the tumor from which it was derived, but is representative of the in situ normal glandular epithelial cells. This same relationship exists in the human prostate malignant cell lines such as PC-3 and LNCaP cells.12,13 The re-appearance of ZIP1 in the malignant cell lines is evidence that the in situ loss of transporter is not due to deletion of the gene or due to a permanent loss of gene expression, but is likely due to epigenetic silencing that depends on the in situ prostate conditions.
Figure 6.
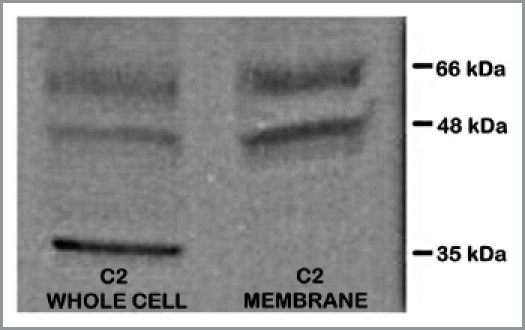
ZIP1 transporter western blot analysis of TRAMP C2 cells. 48 and 66 kDa bands are membrane-complexed forms of the transporter protein. 35 kDa is likely the free cytosolic protein.
Since the presence of the transporter indicated that the C2 cells would likely take up and accumulate zinc from medium, we performed corresponding studies to determine the effects of zinc treatment on the cells. Figure 7A shows that the C2 cells accumulate zinc when exposed to physiological concentration of zinc. Figure 7B reveals that zinc accumulation inhibits C2 cell proliferation and induces apoptosis (Fig. 7C), which demonstrate that increased cellular zinc is cytotoxic in C2 cells. These are zinc treatment effects, which are also observed in human prostate cells.1,2,14
Figure 7.
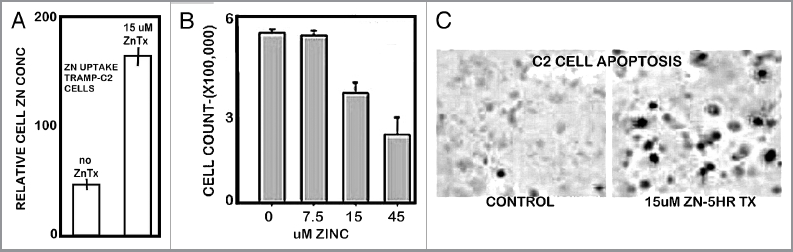
Effects of zinc treatment on TRAMP C2 cells. (A) Effect on cellular accumulation of zinc. (B) Effect on cell proliferation. (C) Effect on apoptosis.
Discussion
The results of this study demonstrate ZIP1/zinc/citrate relationships in the TRAMP animal, which show important similarities to human prostate cancer. Since the early Mawson and Fisher report in 195215 and Huggins report in 1946,16 innumerable studies consistently demonstrated that zinc levels and citrate levels are markedly decreased (∼60–90%) in prostate cancer as compared with normal and benign prostate.1–4 For example normal prostate peripheral zone (where most malignancies arise and develop) contains approximately 3,000 nmols zinc/gm tissue, as compared with 600 nmols/gm in cancer. The citrate levels are approximately 13,000 and 1,000 for normal vs. cancer. One rarely, if ever, finds malignant loci that retain the characteristically high zinc or citrate levels of normal prostate. We regard this relationship as the most consistent “hallmark” distinguishing characteristic of prostate cancer that currently exists. More recent studies have established that ZIP1, the functional zinc uptake transporter in normal prostate glandular epithelium, is downregulated in adenocarcinoma, which is associated with the loss of zinc in the malignant cells. Of importance is the demonstration that this concurrent loss of ZIP1, zinc, and citrate occurs in premalignant and early stage development of malignancy and persists in the progression of malignancy.10,17–19 Since the cellular zinc level that exists in the highly specialized normal peripheral zone glandular epithelial cells is cytotoxic in the malignant cells, the silencing of ZIP1 gene expression results in loss of cellular zinc accumulation, which eliminates the adverse effects on malignancy.
These relationships provide the basis for our concept of prostate carcinogenesis as represented in Figure 8. In addition to eliminating the cytotoxic effects, the loss of zinc also transforms the citrate-producing normal cells to citrate-oxidizing malignant cells, which provides the bioenergetic and synthetic metabolic requirements of prostate malignancy.1,5,20 The similar ZIP1/zinc/citrate changes in TRAMP emphasize and importance of the genetic/metabolic transformation in human prostate cancer development. If further studies corroborate and extend this similarity in TRAMP, an important model will exist to investigate the many unknown factors, mechanisms and signaling pathways associated with the “genetic/metabolic” transformation in prostate carcinogenesis.
Figure 8.
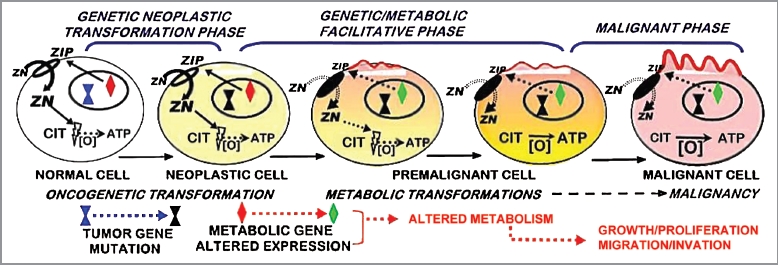
The ZIP1/zinc/citrate genetic/metabolic transformation concept of prostate carcinogenesis.
It is highly unlikely that the TRAMP model, or any animal model, will perfectly mimic the natural history, biology, and pathology of human prostate cancer. One must be selective in employing any model to study specific relationships that will be appropriately translated to human cancer. It is critical that the relationship, factor, or mechanism under investigation exists in a model that has been shown to be representative of the status in human prostate cancer. Our focus on the ZIP1/zinc/ citrate relationship is most relevant because it is currently, in our view, the most consistent and clinically-established relationship that distinguishes prostate cancer from normal and benign prostate. The now-apparent similar implications of ZIP1/zinc/citrate in TRAMP and human prostate cancer add to the evidence of the important and consistent role of zinc in the development and progression of prostate cancer.
We consider this report to be a beginning in the investigation of TRAMP as an animal model for genetic/metabolic relationships of human prostate cancer. There are many issues that remain to be established in TRAMP, and we are continuing with such studies. Nevertheless, it is now becoming evident that TRAMP could provide an excellent model of human prostate cancer for in vivo studies, such as: the differing in situ factors that protect the normal prostate cells vs. the malignant cells from cytotoxic effects of zinc, the development and employment of zinc chemotherapeutic agents for the treatment and prevention of prostate cancer, the genetic, signaling pathways, and events involved in the “genetic/metabolic” transformation in the carcinogenesis process (Fig. 8), and other important related issues. It will also be important to conduct similar studies regarding the implications of the ZIP1/zinc/citrate relationship in other animal models that purport to represent human prostate cancer.
Materials and Methods
UMSC studies.
Four normal mice (12–16 weeks old) and 12 TRAMP mice (3 mice at ≤ 9 weeks, 9 mice ≥ 12 weeks, with 3 mice having lymph node metastases) were asphyxiated by CO2 and immediately dissected to remove the genitourinary tract, primary tumors, and metastatic sites. Individual prostate lobes from normal and early stage TRAMP mice were separated using a dissecting microscope to yield the ventral, dorsal, lateral, and anterior lobes and seminal vesicles. Initial studies focused on the ventral and dorsal lobes, where TRAMP tumors are known to originate. Samples were frozen in liquid nitrogen until HR-MAS analysis. High resolution magic angle spinning (HR-MAS) spectroscopy is a non-destructive technique which can provide detailed metabolic information from intact tissue samples prior to histopathology analysis.21 HR-MAS studies were performed using a 11.5T Varian UNITY spectrometer and a gHX nanoprobe. Samples were spun at 2.5 kHz at 20° C. 1D spectra were acquired using standard pulse-acquire and CPMG sequences with presaturation. 1H HR-MAS data were analyzed using MacNUTS software (Acorn NMR, Livermore). Creatine was used as an internal chemical shift (δ = 3.04 ppm) and quantization reference. Peaks were quantified using Lorentzian and Gaussian peak fitting, and metabolite ratios were compared using a Student's t-test.
UMd studies.
TRAMP tissue studies: Normal mice and TRAMP tissues were provided from the UMSC studies described above. In situ tissue zinc levels were determined by dithizone staining of slides containing tissue sections. DTZ staining was performed as described by Sternberg and Philips.22 The stained slides were examined by light microscopy using a seven headed Reichert Omega Model #4000 microscopy system equipped with a high definition, two megapixel cameras and a desktop computer for capturing and storing digital images.
The relative abundance of ZIP1 transporter was determined by IHC with ZIP1 antibody, which we developed and described in previous reports.10,23
TRAMP C2 cell studies: The TRAMP C2 cell line was obtained from Dr. Barbra Foster. The cells were cultured and maintained at 37° C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 0.005 mg/ml bovine insulin, 10 nM dihydrotestosterone, 5% fetal bovine serum, 5% Nuserum IV, 1% penicillin-streptomycin mixture with medium changes every 2–3 d. For specific experiments, the cells were plated and growth to 75–80% confluence.
For analysis of ZIP1 transporter expression, plasma membranes were prepared from C2 cells by the procedure previously described.24 Briefly whole cell lysates were centrifuged at 10,000 × g for 15 min followed by centrifugation at 110,000 × g for 30 min to collect the membrane fraction. The membrane proteins were extracted using RIPA buffer (Upstate Biotech) and the protein concentration determined using the BioRad protein assay. Proteins from whole cells lysates and the membrane fractions were separated by SDS-PAGE gel electrophoresis, transferred to nitrocellulose membranes and the membranes incubated overnight with hZIP1 antibody. Protein bands were detected by chemoluminance using horseradish peroxidase linked secondary antibody and ECL detection Reagents.
For determination of cellular Zn levels, C2 cells were grown to 75% confluence in 10 cm dishes in complete DMEM medium. On the day of the experiments, the cells were washed with PBS and the medium changed to fresh medium containing either 15 µM ZnCl2 or no zinc. Cells were incubated for 1 h, washed with PBS and 500 µl of 67% nitric acid added to the dishes. The cell lysate in nitric acid was collected and boiled for 30 min and 1.0 ml of deionized water added. Zinc in the lysates was measured by atomic absorption flame spectrometry.
For cell growth assay and assay of apoptosis cells were cultured in complete DMEM medium in 12 well plates to 80% confluence. The medium was changed to fresh medium without serum containing various concentrations of ZnCl2. Cells were cultured with and without zinc for 24 h after which the wells were washed three times with PBS, the cells collected by treatment with trypsin and counted using a Coulter Counter. To determine apoptosis, cells were cultured in 12-well plates, washed and the medium replaced with serum-free DMEM containing 15 µM ZnCl2 for 5 h. After zinc treatment the level of apoptosis was determined using the TUNEL assay according to the manufacturer's instructions and as we previously described.
Acknowledgments
We thank Dr. Barbara A. Foster (Roswell Park Cancer Institute, Pharmacology and Therapeutics) for kindly providing us with the TRAMP C2 cell line. The studies cited from the UMd group were supported by NIH grants DK076783 and CA79903. The studies from the UCSF group were supported by NIH grants EB005363 and R01 CA102751.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Canc. 2006 doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporter in normal prostate function and the pathogenesis of prostate cancer. Front Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costello LC, Franklin RB. The intermediary metabolism of the prostate: A key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello LC, Franklin RB. The genetic/metabolic transformation concept of carcinogenesis. Cancer Metastasis Rev. 2011 doi: 10.1007/s10555-011-9334-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurhanewicz J, Vigneron DB. Advances in MR Spectroscopy of the Prostate. Magn Reson Imaging Clin N Am. 2008;16:697–710. doi: 10.1016/j.mric.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marberger H, Marberger E, Mann T, Lutwak-Mann C. Citric acid in human prostatic secretion and metastasizing cancer of prostate gland. BMJ. 1962;1:835–836. doi: 10.1136/bmj.1.5281.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh SK, Kim P, Zhang XA, Yun SH, Moore A, Lippard SJ, et al. A novel imaging approach for early detection of prostate cancer based on endogenous zinc sensing. Cancer Res. 2010;70:6119–6127. doi: 10.1158/0008-5472.CAN-10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Györkey F, Min KW, Huff JA, Györkey P. Zinc and magnesium in human prostate gland: normal, hyperplastic, and neoplastic. Cancer Res. 1967;27:1348–1353. [PubMed] [Google Scholar]
- 10.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, et al. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 12.Costello LC, Liu L, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem. 1999;274:17499–17504. doi: 10.1074/jbc.274.25.17499. [DOI] [PubMed] [Google Scholar]
- 13.Franklin RB, Ma J, Zou J, Guan Z, Kukoyi B, Feng P, et al. Human Zip1 is a major zinc uptake transporter for accumulation of zinc in prostate cells. J Inorg Biochem. 2003;96:435–442. doi: 10.1016/S0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin RB, Costello LC. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem. 2009;106:750–757. doi: 10.1002/jcb.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mawson CA, Fischer MI. The occurrence of zinc in the human prostate gland. Can J Med Sci. 1952;30:336–339. doi: 10.1139/cjms52-043. [DOI] [PubMed] [Google Scholar]
- 16.Huggins C. The prostate secretion. Harvey Lect. 1946;52:148–193. [Google Scholar]
- 17.Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52:316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Cortesi M, Fridman E, Volkov A, Shilstein SSh, Chechik R, Breskin A, et al. Clinical assessment of the cancer diagnostic value of prostatic zinc: A comprehensive needle-biopsy study. Prostate. 2008;68:994–1006. doi: 10.1002/pros.20766. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JF, Farid I. The role of citric acid in the physiology of the prostate. Lactic/citrate ratios in benign and malignant prostatic homogenates as an index of prostatic malignancy. J Urol. 1964;92:533–536. doi: 10.1016/S0022-5347(17)64003-5. [DOI] [PubMed] [Google Scholar]
- 20.Costello LC, Feng P, Franklin RB. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005;5:143–153. doi: 10.1016/j.mito.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson MG, Vigneron DB, Tabatabai ZL, Males RG, Schmitt L, Carroll PR, et al. Proton HR-MAS spectroscopy and quantitative pathologic analysis of MRI/3D-MRSI-targeted postsurgical prostate tissues. Magn Reson Med. 2003;50:944–954. doi: 10.1002/mrm.10614. [DOI] [PubMed] [Google Scholar]
- 22.Sternberg SS, Philips FS. Histochemical demonstration of zinc in the dorsolateral prostate of the rat:studies with oxine and dithizone. Am J Pathol. 1965;47:325–337. [PMC free article] [PubMed] [Google Scholar]
- 23.Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer. 2007;6:37. doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franklin RB, Zou J, Ma J, Costello LC. Protein kinase C alpha, epsilon and AP-1 mediate prolactin regulation of mitochondrial aspartate aminotransferase expression in the rat lateral prostate. Mol Cell Endocrinol. 2000;170:153–161. doi: 10.1016/S0303-7207(00)00321-X. [DOI] [PubMed] [Google Scholar]


