In the current issue of Cancer Biology & Therapy, Ko et al.1 present striking new data establishing for the first time that glutamine in the tumor microenvironment has dual and opposing effects on cellular activity based both on cell type and on positional context.
Glutamine is a key amino acid involved in tumor growth and cancer metabolism, and is involved in both anabolic and catabolic reactions. Glutamine plays a role in anaplerosis, which is defined as the replenishment of intermediate molecules in the tricarboxylic acid cycle (TCA cycle), and allows for the synthesis of amino acids, nucleotides and antioxidants. In addition, glutamine catabolism leads to generation of ammonia which is a diffusible inducer of autophagy.2,3 The conventional model of cancer cell metabolism states that cancer cells have impaired mitochondrial oxidative phosphorylation and high utilization of glutamine, resulting in increased tricarboxylic acid (TCA) activity and low oxidative phosphorylation and low ATP production.4 How glutamine promotes tumor growth and biomass generation without a net increase in ATP generation is not understood. Also, it is unclear how induction of autophagy can induce tumor growth (Fig. 1A).
Figure 1.
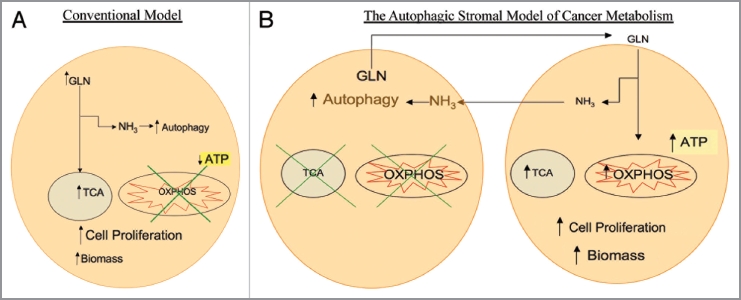
(A) Conventional model of cancer metabolism. Cancer cells utilize glutamine (GLN) to replenish tricarboxylic acid (TCA) cycle intermediates, a process termed anaplerosis, and to increase cellular biomass. The catabolism of glutamine generates ammonia (NH3), inducing autophagy. It has been postulated that cancer cells have impaired oxidative phosphorylation (OXPHOS) with low ATP generation with high autophagy which promotes cell proliferation. (B) The autophagic stromal model of cancer metabolism as proposed by the authors. Cancer associated stromal cells possess high levels of autophagy and impaired mitochondrial activity, leading to release of catabolites including glutamine which are taken up by cancer cells. Glutamine in the cancer cells not only leads to high ammonia generation that drives autophagy in stromal cells, but also increases the mitochondrial TCA cycle and oxidative phosphorylation, leading to high levels of ATP production, increased biomass and proliferation of cancer cells. This reciprocal feed-forward communication supports both tumor progression and continued activation of the surrounding stroma, compromising cancer therapy.
The study by Ko et al. provides novel insights into glutamine's tumorigenic properties and the role of autophagy. The authors demonstrate that while glutamine increases mitochondrial mass in breast cancer epithelial cancer cells growing in the tumor microenvironment, it has opposing effects on the fibroblasts in proximity to the epithelial cancer cells. Glutamine decreased the mitochondrial mass of the tumor associated fibroblasts as compared with its effects on fibroblasts cultured alone. The authors further establish that the mitochondrial biogenesis in epithelial cancer cells is linked to increased autophagy in the stromal compartment, and that the mitochondrial biogenesis can be abolished using the autophagy inhibitor chloroquine. They also demonstrate that metabolic coupling exists between epithelial cancer cells and tumor associated fibroblasts and that the epithelial cells have increased levels of transporters and enzymes involved in glutamine catabolism such as SLC6A14, glutaminase and glutamate dehydrogenase. Glutamine catabolism then leads to the generation of ammonia, a potent inducer of autophagy. Fibroblasts have decreased levels of Caveolin-1, which is a marker of mitochondrial dysfunction and fibroblast autophagy. Most importantly perhaps, high glutamine levels and the presence of stroma act synergistically to induce resistance to apoptosis of breast epithelial cancer cells under baseline conditions and after treatment with tamoxifen.
A novel paradigm of tumor metabolism termed “the autophagic stromal model” has been proposed by Lisanti and colleagues.5 This model posits that the tumor-associated stroma is highly autophagic and catabolic with low mitochondrial activity. Conversely the model predicts that cancer cells support very high mitochondrial metabolic activity and are resistant to autophagy and cell death due to heterotypic transfer of catabolites from the stroma to the transformed epithelium. This type of asymmetry promotes tumor growth, genomic instability and drug resistance.
The Ko article provides key new mechanistic insights into the “autophagic stromal model” establishing that two of glutamine's well described functions occur within the same tumor but in a divided fashion. Autophagy in the stroma leads to mitochondrial biogenesis in the epithelial cell compartment (Fig. 1B). This article also adds to a growing body of literature studying tumor metabolism and its effects on antineoplastic drug resistance by studying tumors as an organ with different compartments.6,7 Decades of tumor metabolism research has studied cancer cell lines cultured in isolation and without taking into account the role of the tumor microenvironment. Tumors, however, are not composed of cancer cells without an associated stroma. With the advent of in vitro cancer models with multiple cell types mimicking the stroma, a much better understanding of tumor metabolism is now emerging.
In conclusion, these ground-breaking studies now demonstrate that glutamine increases mitochondrial mass in breast cancer epithelial cells while decreasing mitochondrial content in the stroma, increasing stromal autophagy. The glutamine produced in the autophagic stroma protects breast cancer cells from apoptosis under both baseline conditions and during treatment with tamoxifen. The establishment of glutamine's differential metabolic effects on breast cancer epithelial cells vs. the stroma clearly illustrates the importance of metabolic coupling between the different tumor compartments. Future studies aimed at defining the mechanisms that modulate the molecular regulation of metabolic coupling will be crucial to advancing our understanding of cancer initiation and progression as well as the development of more effective cancer therapies.
Acknowledgments
The work was supported by CA129003 (CA), a the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [FSM (576200/2008-5, 473670/2008-9)], and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [FSM (14916)] and AI-076248 (HBT).
References
- 1.Ko YH, Lin Z, Flomenberg N, Pestell R, Howell A, Sotgia F, et al. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: Implications for preventing chemotherapy resistance. Cancer Biol Ther. 2011 doi: 10.4161/cbt.12.12.18671. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. 2010;3:ra31. doi: 10.1126/scisignal.2000911. [DOI] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Outschoorn UE, Whitaker-Menezes D, Pavlides S, Chiavarina B, Bonuccelli G, Casey T, et al. The autophagic tumor stroma model of cancer or “battery-operated tumor growth”: A simple solution to the autophagy paradox. Cell Cycle. 2010;9:4297–4306. doi: 10.4161/cc.9.21.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]


