Abstract
Several neurodevelopmental disorders are marked by atypical Methyl-CpG-binding protein 2 (MeCP2) expression or function; however, the role of MeCP2 is complex and not entirely clear. Interestingly, there are sex differences in some of these disorders, and it appears that MeCP2 has sex-specific roles during development. Specifically, recent data indicate that a transient reduction in MeCP2 within developing amygdala reduces juvenile social play behavior in males to female-typical levels. These data suggest that MeCP2 within the amygdala is involved in programming lasting sex differences in social behavior. In the present study, we infused MeCP2 or control siRNA into the amygdala of male and female rats during the first three days of postnatal life in order to assess the impact of a transient reduction in MeCP2 on arginine vasopressin (AVP), a neural marker that is expressed differentially between males and females and is linked to a number of social behaviors. The expression of AVP, as well as several other genes, was measured in two-week old and adult animals. Two-week old males expressed more AVP and galanin mRNA in the amygdala than females, and a transient reduction in MeCP2 eliminated this sex difference by reducing the expression of both gene products in males. A transient reduction in MeCP2 also decreased androgen receptor (AR) mRNA in two-week old males. In adulthood, control males had more AVP-immunoreactive (AVP-ir) cells than females in the centromedial amygdala (CMA), bed nucleus of the striaterminalis (BST) and in the fibers that project from these cells to the lateral septum (LS). A transient reduction in MeCP2 eliminated this sex difference. Interestingly, there were no lasting differences in galanin or AR levels in adulthood. Reducing MeCP2 levels during development did not alter estrogen receptorα, neurofilament or Foxg1. We conclude that a transient reduction in MeCP2 expression in the developing male amygdala has a transient impact on galanin and AR expression but a lasting impact on AVP expression, highlighting the importance of MeCP2 in organizing sex differences in the amygdala.
Key words: epigenetics, MeCP2, amygdala, sexual differentiation, development, arginine vasopressin, galanin
Introduction
Methyl-CpG-binding protein 2 (MeCP2) is encoded by an X-linked gene, MECP2, that is critical for normal brain function, as disruptions in MeCP2 are associated with several neurological disorders.1 Typically, MeCP2 plays an important role in gene repression by binding to methylated DNA2,3 and recruiting co-repressor proteins to reduce gene transcription.2,4,5 However, recent evidence suggests that MeCP2 can also have a number of other functions, such as activating gene transcription,6 modifying RNA splicing,7 and affecting neural differentiation/maturation and synaptic plasticity.8–10 Mutations of MECP2 cause Rett syndrome, an X-linked disorder that is diagnosed more often in females. Reductions in MeCP2 expression or function can also occur in autism, which is more prevalent in males. Therefore, in order to understand sex differences in some neurodevelopmental disorders, it becomes important to elucidate the function of MeCP2 in the context of sexual differentiation of the brain.
Recent data indicate that MeCP2 is important for typical functioning of mature neurons. For example, re-expression of MeCP2 in juvenile or adult mice restores many of the deficits induced by MeCP2 deletion.11,12 Likewise, using an inducible MeCP2 knockout model, reductions in MeCP2 later in life can recapitulate many of the deficits seen in the MeCP2 knockout.13 Together, these data suggest that some of the neurological outcomes induced by MeCP2 disruption may not be programmed during development, but rather are a consequence of loss of adult MeCP2 function. However, the importance of MeCP2 during development in organizing lasting changes in behavior has also been recently established. Specifically, a transient reduction in MeCP2 within the developing amygdala during the first three days of postnatal life reduces the levels of juvenile social play in males to female-typical levels.14 Given the intriguing finding that typical male juvenile social behavior is disrupted by decreased MeCP2 expression in the developing amygdala, we wanted to examine if MeCP2 disrupts sex differences in gene expression within the amygdala. Juvenile male rats play at higher frequencies than females, and this sex difference is organized by testosterone action within the developing male amygdala.15,16 Testosterone is metabolized into two principle ligands: estradiol, which binds to estrogen receptor (ER) and the androgen, dihydrotestosterone, which binds to androgen receptor (AR),17 and these receptors are critical for organizing many sex differences in the brain and behavior. It is unclear, however, what genes are altered by reduced MeCP2 expression within the developing amygdala to effect this change in social behavior in juvenile male rats. AVP expression within the bed nucleus of the striaterminalis (BST) and the centromedial amygdala (CMA) is known to be involved in a number of social behaviors,18–20 including social play behavior.21 Additionally, it has been recently suggested that MeCP2 can regulate vasopressin (AVP) expression within the hypothalamus.22 These data suggest AVP as a potential candidate when examining genes that are regulated by MeCP2 and influence social behavior.
AVP expression within the BST and CMA is highly sexually dimorphic, as adult males express 2–3 times more AVP in these areas.23 Moreover, this sex difference is organized during early postnatal life by steroid hormone actions on ER and AR.24–26 Therefore, we hypothesized that transiently reducing MeCP2 during this time period would have a lasting impact on AVP expression levels within the amygdala, possibly through its action on ER or AR.27,28 Also, as AVP is co-expressed in a subset of galanin expressing neurons,29 we also examined galanin expression.
Results
Experiment 1: A transient reduction in MeCP2 reduced AVP, galanin and androgen receptor (AR) mRNA at PN14.
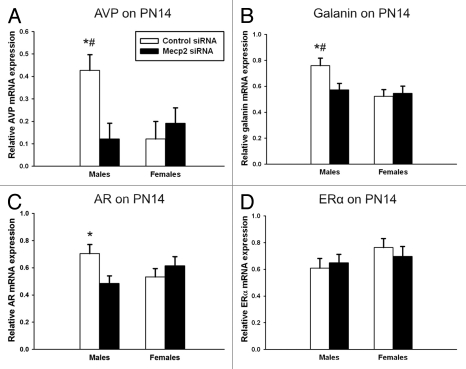
AVP mRNA in the amygdala. As AVP expression is sexually dimorphic and is relevant to a number of social behaviors, we examined the impact of neonatal MeCP2 siRNA on AVP mRNA expression in the amygdala two weeks after infusion. We found an interaction between sex and treatment on relative AVP mRNA expression [F(1,15) = 6.82, p = 0.02, Fig. 1A]. Post hoc comparisons indicate that control males expressed higher levels of AVP mRNA in the amygdala than both control females, (p = 0.011), and MeCP2 siRNA-treated males (p = 0.007).
Figure 1.
Relative mRNA expression in the amygdala of PN14 animals infused with MeCP2 or control siRNA on PN0-2. (A) Post hoc tests indicate that control males expressed more relative AVP mRNA than control females (*p = 0.011) and MeCP2 siRNA-treated males (#p = 0.007). (B) Post hoc tests indicate that control males expressed more relative galanin mRNA than control females (*p = 0.005) and MeCP2 siRNA-treated males (#p = 0.008). (C) Post hoc tests indicate that control males expressed more relative AR mRNA than MeCP2 siRNA-treated males (*p = 0.002). (D) There was no effect on relative ERα mRNA.
Galanin mRNA in the amygdala. As AVP is co-expressed in a subset of galanin cells, we examined the effect of neonatal MeCP2 siRNA on galanin mRNA expression in the amygdala two weeks after infusion. Interestingly, there was a main effect of sex [F(1,18) = 4.98, p = 0.039] and an interaction between sex and treatment [F(1,18) = 5.41, p = 0.032] on relative galanin mRNA expression (Fig. 1B). Post hoc comparisons indicate that control males expressed higher galanin mRNA levels in the amygdala than both control females (p = 0.005) and MeCP2 siRNA-treated males (p = 0.008).
AR and ERα mRNA in the amygdala. As AVP expression is organized during development by steroid action on ER and AR, we examined the effect of neonatal MeCP2 siRNA on AR and ERα mRNA expression in the amygdala two weeks after infusion. There was a main effect of treatment [F(1,19) = 8.1, p = 0.01] and an interaction between sex and treatment [F(1,19) = 4.5, p < 0.05] on relative AR mRNA (Fig. 1C). Post hoc comparisons indicate that control males expressed higher levels of AR mRNA in the amygdala than MeCP2 siRNA-treated males (p = 0.002). In contrast, there was no effect of sex [F(1,19) = 2.17, p = 0.16], treatment [F(1,19) = 0.03, p = 0.86], or an interaction [F(1,19) = 0.59, p = 0.45] on relative ERα mRNA in the amygdala (Fig. 1D).
Neurofilament, Foxg1 and MeCP2, mRNA in the amygdala. In order assess the possibility that the changes observed in gene expression were not simply the impact of overall transcriptional dysregulation, we examined the effect of neonatal MeCP2 siRNA on neurofilament, light polypeptide (NF-L), MeCP2 and Foxg1 mRNA expression in the amygdala. NF-L is a neuron-specific cytoskeletal gene,30 and Foxg1 is a gene that encodes for a transcription factor that is important for brain development and has been recently identified to be deleted or inactivated in Rett syndrome.31,32 MeCP2 mRNA was also assessed in order to ensure that expression levels returned to normal two weeks following the infusions on PN0–2, as previously shown in reference 14. There was no effect of neonatal MeCP2 siRNA on the expression of any of these genes. There was no effect of sex [F(1,18) = 0.07, p = 0.79] or treatment [F(1,18) = 0.71, p = 0.41] on relative NF-L mRNA in the amygdala. There was also no effect of sex [F(1,19) = 0.57, p = 0.46] or treatment [F(1,19) = 0.03, p = 0.86] on relative MeCP2 mRNA in the amygdala. Finally, there was no effect of sex [F(1,18) = 0.09, p = 0.77] or treatment [F(1,18) = 0.32, p = 0.58] on relative Foxg1 mRNA in the amygdala.
AVP mRNA in the hypothalamus. In order to assess the possibility that the infusion of MeCP2 siRNA may have spread to the hypothalamus and altered AVP gene expression in that brain region, we examined the effect of neonatal infusion of MeCP2 siRNA into the amygdala on AVP mRNA expression in the hypothalamus. There was no effect of sex [F(1,18) = 0.001, p = 0.97] or treatment [F(1,18) = 0.33, p = 0.57] on relative AVP mRNA in the hypothalamus.
Experiment 2: Neonatal infusions of MeCP2 siRNA reduced AVP, but not galanin or AR-ir, in adulthood.
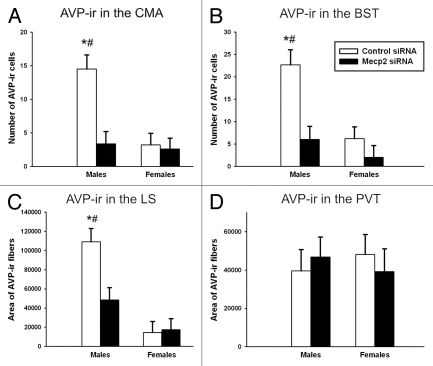
AVP-ir cell number and fiber density in the CMA, BST and lateral septum. In order to assess if the impact of neonatal MeCP2 siRNA on AVP expression in males was maintained into adulthood; we examined AVP-ir cell number in adulthood. Our data replicate previous findings of a sex difference in AVP-ir cell number, with males have higher AVP-ir cell number than females. More importantly, we find a lasting effect of transient reduction in MeCP2 on AVP-ir cells. That is, MeCP2 siRNA-treated males had lower AVP-ir cell number than control males. An interaction between sex and siRNA treatment was found in number of AVP-ir cells within the CMA [F(1,29) = 8.34, p = 0.007, Figs. 2A and 4A], BST [F(1,30) = 4.58, p = 0.04, Fig. 2B], and one of the projection sites of these cells, the lateral septum (LS) [F(1,31) = 6.55, p = 0.016, Fig. 2C]. Post hoc comparisons indicate that control males expressed more AVP-ir cells than control females in adulthood within the CMA (p < 0.001) and BST (p < 0.001), as well as more AVP-ir fibers in the LS (p < 0.001). Additionally, control males had more AVP-ir cells compared with MeCP2 siRNA-treated males within the CMA (p < 0.001) and BST (p < 0.001), as well more AVP-ir fibers in the LS (p = 0.003). As in experiment 1, there were no differences between MeCP2 siRNA-treated females and control females in any brain region examined. As expected, the number of AVP-ir cells within the paraventricular thalamic nucleus (PVT), a control region as this area is innervated by AVP cells that are not sexually dimorphic, did not differ by sex [F(1,29) = 0.006, p = 0.97] or treatment [F(1,29) = 0.002, p = 0.94, Fig. 2D].
Figure 2.
AVP-ir in adult animals infused with MeCP2 or control siRNA on PN0–2. (A) Post hoc comparisons indicate that control males expressed more AVP-ir cells than control females (*p < 0.001) and MeCP2 siRNA-treated males (#p < 0.001) in the CMA. (B) Post hoc comparisons indicate that control males expressed more AVP-ir cells than control females (*p < 0.001) and MeCP2 siRNA-treated males (#p < 0.001) in the BST. (C) Post hoc comparisons indicate that control males expressed a larger area of AVP-ir fibers than control females (*p < 0.001) and MeCP2 siRNA-treated males (#p = 0.003) in the LS. (D) There was no effect of MeCP2 siRNA treatment on AVP-ir fiber area in the paraventricular nucleus of the thalamus (PVT).
Figure 4.
Photomicrographs of adults infused with MeCP2 or control siRNA on PN0–2. (A) AVP-ir cells in the CMA at 4x (left) and 10x (right). (B) galanin-ir cells in the CMA at 4x (left) and10x (right). White dashed box represents the area analyzed.
Galanin-ir cell number in the CMA and BST. In order to assess if the impact of neonatal MeCP2 siRNA on galanin expression in males was maintained into adulthood; we examined galanin-ir cell number in adulthood. Interestingly, there was no lasting effect of a transient reduction in MeCP2 on galanin-ir cells in adulthood. There was no effect of sex [F(1,31) = 1.23, p = 0.81] or treatment [F(1,31) = 0.06, p = 0.28] on the number of galanin-ir cells in the CMA (Fig. 3A). Likewise, there was no effect of sex [F(1,32) = 0.13, p = 0.78] or treatment [F(1,32) = 0.08, p = 0.73] on the number of galanin-ir cells in the BST.
Figure 3.
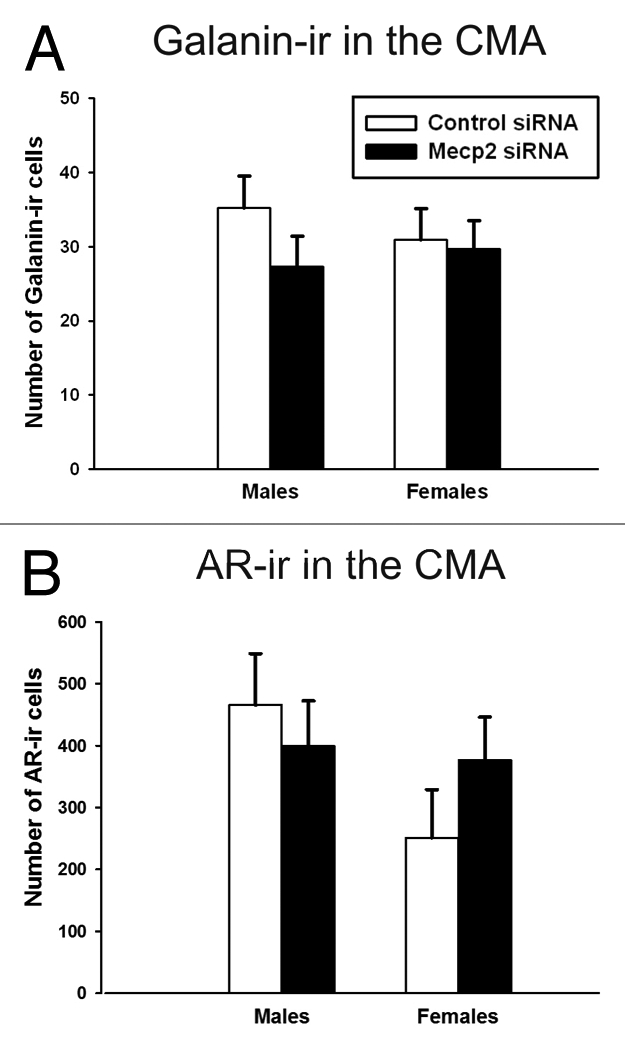
Immunoreactive cells in the CMA of adults infused with MeCP2 or control siRNA on PN0–2. (A) There was no effect of sex (p = 0.81) or treatment (p = 0.28) on the number of galanin-ir cells. (B) There was no effect of sex (p = 0.78) or treatment (p = 0.73) on the number of AR-ir cells.
AR-ir cell number in the CMA. In order to assess if the impact of neonatal MeCP2 siRNA on AR expression in males was maintained into adulthood, we examined AR-ir cell number in adulthood. Interestingly, there was no lasting effect of a transient reduction in MeCP2 on AR in adults. There was no effect of sex [F(1,30) = 2.4, p = 0.13] or treatment [F(1,30) = 0.15, p = 0.70] on the number of AR-ir cells in the CMA (Fig. 3B).
Testosterone EIA. We measured testosterone concentrations in adult animals that had been gonadectomized and implanted with testosterone-filled implants in order to ensure that levels did not differ between sex or treatment groups and were in the physiological range for adult males. As expected, there was no effect of sex [F(1,29) = 0.002, p = 0.96] or treatment [F(1,29) = 0.423, p = 0.52] on serum testosterone in castrated and testosterone-implanted adults (data not shown). Average testosterone levels were 1.31 ± 0.31 ng/mL in control females, 1.29 ± 0.35 ng/mL in control males, 1.08 ± 0.27 ng/mL in MeCP2 siRNA-treated females and 1.49 ± 0.25 ng/mL in MeCP2 siRNA-treated males. These levels fall within the range of typical adult male levels of circulating testosterone.33
Experiment 3: An infusion of SKF into the amygdala did not activate c-Fos in the BST.
As we observed a decrease in AVP protein levels in the BST, we wanted to determine if our infusion spread from the amygdala to the BST. To test this, we infused SKF 38393, a dopamine D1-like receptor agonist, into the developing amygdala and examined c-Fos-ir cell number. As SKF 38,393 increases c-Fos protein levels, it provides a functional marker of altered cell activity and should help us determine the spread of the infusion. Within the amygdala, animals treated with any dose SKF had more c-Fos-ir cells than saline animals [t(9) = 2.99, p = 0.02] and this effect was dose-dependent [r2 = 0.43, F(1,9) = 6.88, p = 0.03]. However, there was no effect of SKF infusion into the amygdala on c-Fos-ir cells in the BST [t(7) = 5.17, p = 0.62]. While c-Fos-ir cells were detected around the site of infusion, there was no apparent spread of SKF 38,393 medial to the optic tract, such as in the hypothalamus. These data, as well as a lack of alterations in AVP expression within the hypothalamus, suggest that there was little spread outside the amygdala.
Discussion
These data indicate that MeCP2 participates in the developmental organization of the AVP system in a sex-specific manner. In two-week old and adult male rats, a transient reduction in MeCP2 during neonatal development reduced AVP levels within the amygdala, a region critical for social and emotional behavior. We found that two-week old control males expressed more AVP mRNA within the amygdala compared with females, and a transient reduction in MeCP2 within the amygdala during the first three days of postnatal life decreased AVP mRNA expression to female levels. A reduction in MeCP2 during development had no effect on female AVP expression. In addition, these effects are lasting into adulthood, suggesting that MeCP2 is involved in the organization of male-typical AVP expression. That is, adult males had more AVP-ir cells within the CMA, BST and fibers within the lateral septum compared with females; a transient reduction in MeCP2 within the developing male amygdala eliminates this sex difference. The functional significance of reduced AVP, including its possible influence on juvenile social play behavior, is not clear; however, these data indicate that MeCP2 is important in the organization of AVP within the amygdala in a sex-specific manner.
As AVP-ir cells in the CMA project to the lateral septum, we expect a decrease in AVP-ir fibers in this area. However, it is less clear how the infusion of MeCP2 siRNA into the amygdala decreased AVP-ir cell number in the BST. It is possible that the infusion could have spread to the BST and directly altered AVP-ir cell number; however, we have previously confirmed that the MeCP2 siRNA infusion into the amygdala does not reduce MeCP2 levels in the hypothalamus.14 Furthermore, we found that an injection of SKF 38,393 into the amygdala increases c-Fos-ir labeling in the amygdala but not the BST, even though systemic SKF 38,393 administration can increase c-Fos-ir label in both the amygdala and BST.34 These data provide an indirect yet functional assay of spread to the BST from infusion site in the amygdala. More importantly, previous data have shown that the BST and CMA are interconnected, and lesions of the BST disrupt AVP expression in the amygdala.35 Based on this information, it is not surprising that a disruption in the developing amygdala would result in a similar disruption in the BST. We also show that there was no influence of reduced MeCP2 on AVP mRNA within the hypothalamus in two week-old animals. As this area is rich in AVP, but not interconnected with the amygdala, this suggests that the disruption within the BST may not have been due to the spread of siRNA.
AVP in the CMA and BST is co-expressed with galanin,29 and it is thought that AVP expression is induced in a subset of these neurons in a sexually dimorphic manner.36,37 While some studies have reported no sex difference in galanin,38 others have reported a relatively small sex difference in galanin in both rats36 and mice.39 In the present study, two-week old males expressed more galanin mRNA than females in the amygdala; however, there was no sex difference detected in the number of galanin-ir cells in either the CMA or the BST in adulthood. Galanin is regulated by circulating testosterone levels,40 and the adult animals in this study received testosterone-filled implants; whereas, the two-week old animals did not. As there is a sex difference in endogenous testosterone levels around this time-point,41,42 this could contribute to a sex difference in galanin mRNA expression in the two-week old animals. Interestingly, it was recently reported that young adult males express higher levels of galanin than females, and this difference declines with age as levels of galanin decrease in males.43 Therefore, it is possible that the sex difference in galanin levels within rodents similarly declines with age. It is also possible that sex differences in galanin expression are easier to detect with quantitative PCR. Importantly, a transient reduction in MeCP2 within the developing amygdala decreased galanin mRNA expression within the amygdala in two-week old males, but not females, and this effect was not lasting into adulthood. These data suggest that MeCP2 plays an important role in programming AVP expression into adulthood; however, the influence of MeCP2 on galanin appears transient.
The mechanism by which a transient reduction in MeCP2 disrupts the expression of these peptides is not clear. Research has shown that MeCP2 binds to the AVP promoter region in the paraventricular nucleus of the hypothalamus to repress gene transcription.22 Although this is not in agreement with the present findings that a reduction in MeCP2 decreases AVP expression in the BST and CMA; this could point toward distinct functions of MeCP2 in different brain regions or at different time points, depending on the activity of other co-regulatory proteins. Although MeCP2 is typically thought to mediate a decrease in gene transcription, it can also increase gene transcription through its interaction with cAMP response element-binding protein 1 (CREB1).6 Importantly, sex differences in cAMP pathways are thought to be critical for the organization of some sex differences. For example, CREB-binding protein (CBP) and phosphorylated CREB are both higher in males in several brain areas44,45 and CBP is necessary for the organization of some sex differences in behaviors.44 These data suggest that higher levels of CREB activity in males within sexually dimorphic brain regions may facilitate MeCP2-induced gene transcription and organize sex differences. Indeed, studies have shown that CREB binds to the AVP promoter region to activate gene transcription in vitro.46 In contrast to its role in the repression of AVP within the paraventricular nucleus of the hypothalamus, where the AVP is relatively steroid insensitive, MeCP2 may interact with CREB within the developing amygdala to increase steroid-dependant AVP gene transcription. Therefore, a reduction in MeCP2 during development could disrupt the organization of steroid hormone-dependant AVP expression within the amygdala.
Although a correlation between AVP expression and juvenile social play behavior has previously been observed in reference 21, it is unclear whether a reduction in AVP expression could explain the reduction in juvenile social play behavior recently seen in the males by Kurian et al. As a reduction in MeCP2 in the developing male amygdala results in lasting disruptions in both juvenile social play behavior and AVP expression, MeCP2 may be critical for the organization of the male-typical amygdala and its related behaviors. Like many sexually dimorphic systems, juvenile social play behavior and AVP expression are organized largely by testosterone exposure in early life.15,16,25,47 A reduction in MeCP2 disrupts sex differences in both social play and AVP expression, suggesting that MeCP2 may be important for steroid hormone activity. Therefore, reducing neonatal MeCP2 may have a lasting impact through altering the function or expression of steroid receptors. Our data suggest that this is the case for AR, as a reduction in MeCP2 during the first three days of postnatal life decreases its expression in two week-old males. The reduction in AR expression may play a role in the decrease in AVP expression in MeCP2-siRNA treated males. This is in agreement with data indicating that neonatal treatment with dihydrotestosterone, which binds to AR, contributes to male-like AVP mRNA expression in adult rat brain.24 As AR is critical in the development of social play behavior,15,16,48 a reduction in AR expression during development could also contribute to the lasting decrease in juvenile social play behavior in males.
As MeCP2 has been shown to be involved promoting neuronal differentiation of neural precursor cells,9 it is possible that a reduction in MeCP2 altered cell differentiation or cell death; however, previous findings from other labs indicate that sex differences in the levels of AVP are not due to cell death, but rather most likely due to phenotypical differentiation.49 Furthermore, our data indicate several other genes are unaffected by siRNA treatment, including neurofilament, Foxg1, ERα and MeCP2. As all AVP cells are co-expressed with galanin and AR, and these co-expressed genes were not altered in adults, these data suggest that AVP differences are unlikely due to cell death, and consistent with the idea MeCP2 plays a role in the organization of sexually-dimorphic AVP cells.
While numerous studies have confirmed that MeCP2 disruptions occur in Rett syndrome, as well as several other neurodevelopmental disorders, less is known about what genes MeCP2 may regulate within socially-relevant brain regions. As a transient reduction in MeCP2 levels has lasting consequences on AVP, but not galanin or AR expression, MeCP2 may be important for organizing certain neuronal phenotypes. Our data suggest that reductions in MeCP2 may impact male juvenile social behavior by disrupting the organization of AVP within the developing amygdala. The consequences of a lasting decrease in AVP expression in males on other social and cognitive behaviors remain to be fully explored. Interestingly, the programming of AVP expression was not altered in females, suggesting a greater resistance to neonatal disruptions of MeCP2 expression than males. It is important to note that while many of the consequences of MeCP2 loss can be reversed by postnatal restoration of MeCP2 expression,11,12 the present study demonstrates that a reduction in MeCP2 during a critical time point in brain development can have lasting consequences.
Method
General.
Subjects. Sprague-Dawley rats supplied by Charles River Labs (Wilmington, MA) were bred in our animal facility. Dams were checked daily to determine the day of birth, and were allowed to deliver normally. The rats were housed under a 12:12 light/dark cycle with food and water available ad libitum. This research was approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Infusions into the amygdala. Infusions were done with a stereotaxic device modified for neonatal rats, and coordinates from center and bregma suture lines were empirically determined for direct infusion to the amygdala (1 mm lateral, 2 mm caudal and 5.5 mm ventral from the pial surface at our nose cone angle). Under cold anesthesia, the amygdala was bilaterally infused with either MeCP2 or control siRNA (experiments 1 and 2) or SKF 38,393 (experiment 3). Further details are described below. After infusions, pups were allowed to recover under a warm lamp before being returned to the dam.
Statistical analysis. All statistical comparisons were performed using SigmaStat statistical software version 3.5 (Systat Software, Inc.). Statistical comparisons for experiments 1 and 2 were performed using a two-way ANOVA and post hoc tests were conducted using a Fisher LSD when a significant interaction was found. Statistical comparisons for experiment 3 were performed using a Student's t-test and linear regression. Outliers were determined using GraphPad Software, Inc., (www.graphpad.com). Effects with a p value of < 0.05 were considered statistically significant.
Experiment 1: Impact of neonatal infusions of MeCP2 siRNA on mRNA expression on PN14.
Subjects and general procedures. Twenty-four pups were collected from four dams on postnatal day (PN) 0 (day of birth). Neonatal pups were pooled and assigned to a treatment group (five male control siRNA, seven male MeCP2 siRNA, seven female control siRNA and five female MeCP2 siRNA). Neonatal pups were then sorted to form litters consisting of mixed sex and treatment groups and then placed back with a dam. Pups were infused with MeCP2 or control siRNA on PN0–2 and then remained with the dam until sacrificed two weeks later on PN14.
MeCP2 siRNA infusions. MeCP2 (sc-35893, Santa Cruz Biotechnology) and control siRNA (sc-37007, Santa Cruz Biotechnology) were resuspended to 100 µM in the supplied siRNA diluent with Lipofectamine LTX reagent (15338100, Invitrogen). The amygdala was bilaterally infused with 1 µL (100 pmol) of either MeCP2 or control siRNA. Each animal received three infusions 24 h apart (i.e., on PN0, PN1 and PN2). We have previously shown that this amount of MeCP2 siRNA induced a transient and localized reduction in MeCP2 expression within the amygdala.14
Tissue processing. Animals were sacrificed by rapid decapitation on PN14. Brains were extracted and the amygdala and hypothalamus were dissected and snap frozen in isopentane on dry ice. Total RNA was isolated using the AllPrep DNA/RNA Mini Kit (80204, Qiagen). RNA concentrations were determined using a Qubit Fluorometer (Invitrogen) and cDNA was generated using ImProm-II™ Reverse Transcription System (A3800, Promega) in an EppendorfMasterCycler Personal PCR machine. The samples were stored at −80°C.
Real-time polymerase chain reaction. RT-PCR. Amplifications were done using a Stratagene Mx3000PTM real-time PCR system using GoTaq® Colorless Master Mix (M7132, Promega), SYBR green and ROX as a reference dye. Primers designed to target AVP, galanin, AR, ERα, NF-L, Foxg1 MeCP2, Ywhaz and HPRT1 were run in duplicate (Table 1). HPRT1 and Ywhaz were used as normalizing genes to control for subtle variations in sample concentrations. The amplification protocol was as follows: an initial denaturing step at 95°C for 2 min, followed by 40 cycles of a 95°C melting step for 30 sec, 60°C annealing step for 30 sec, and a 72°C elongation step for 30 sec. Following amplification, a dissociation melt curve analysis was performed to ensure the purity of PCR products. Data were analyzed with the following program term settings based on Invitrogen recommendations: (1) amplified based threshold, (2) adaptive baseline set v1.00 to v3.00 algorithm and (3) smoothing moving average with amplification averaging three points. Relative cDNA levels were calculated using the ΔΔCT method.50
Table 1.
Primers used for RT-PCR
| Gene | Accession# | Forward sequence | Reverse sequence | Efficiency | Product size |
| AVP | NM_016992.2 | TGC CTG CTA CTT CCA GAA CTG C | AGG GGA GAC ACT GTC TCA GCT C | 93–94% | 55 bp |
| Galanin | M18102 | GCG CTG GCT ACC TTC TGG | GCT CTC AGG CAG GGG TAC | 96–104% | 125 bp |
| MeCP2 | NM_022673.2 | CGT CCC CTT GCC TGA AGG TTG GA | CTT TCC AGC AGA GCG ACC AG | 92–98% | 42 bp |
| AR | NM_012502.1 | GGC AAA GGC ACT GAA GAG AC | CCC AGA GCT ACC TGC TTC AC | 96–101% | 114 bp |
| ERα | NM_012689.1 | TCC GGC ACA TGA GTA ACA AA | TGA AGA CGA TGA GCA TCC AG | 100–103% | 109 bp |
| NF-L | NM_031783 | ACC AGC GTG GGT AGC ATA AC | GAA GAG CAG TCA GAG GTG G | 96–104% | 157 bp |
| Foxg1 | NM_012560.2 | GTT CAG CTA CAA CGC GCT CAT CAT | TCA CGA AGC ACT TGT TGA GGG ACA | 93–101% | 173 bp |
| Ywhaz | NM_013011.3 | TTG AGC AGA AGA CGG AAG GT | GAA GCA TTG GGG ATC AAG AA | 95–100% | 136 bp |
| HPRT | NM_012583 | GCA GAC TTT GCT TTC CTT GG | CAA GCC TAA AAG ACA GCG G | 100–105% | 239 bp |
Experiment 2: Impact of neonatal infusions of MeCP2 siRNA on immunocytochemistry in adulthood.
Subjects and general procedures. To examine the stability of the alterations in AVP, galanin and AR expression observed on PN14, we replicated and extended the findings of experiment 1. AVP, galanin and AR immunoreactivity (ir) was examined in brains collected from adult rats that were used in a previous study examining the impact of neonatal infusions of MeCP2 siRNA on juvenile social play behavior and juvenile sociability.14 The infusion paradigm was the same as in experiment 1 above. Briefly, 40 pups from 5 dams were collected on the day of birth and assigned to a treatment group (10 male control siRNA, 10 male MeCP2 siRNA, 10 female control siRNA and 10 female MeCP2 siRNA). The amygdala was bilaterally infused with 1 µL (100 pmol) of either MeCP2 or control siRNA on PN0, PN1 and PN2. All animals were gonadectomized and then implanted with Silastic® tubing (2.5 cm long, 1.5 mm inner diameter, 2.4 mm outer diameter, Dow Corning Corp.,) filled with testosterone (Sigma-Aldrich, Inc.,) at 6 mo of age to standardize testosterone levels, as AVP expression is highly regulated by circulating levels of testosterone.
Tissue processing. At 7.5 mo of age, rats were deeply anaesthetized with iso-urane and rapidly decapitated. At least 1 ml of trunk blood was collected from each animal, immediately placed on ice and centrifuged for 10 min at 10,000 g. The serum was removed and stored in a clean tube at −20°C until used in a testosterone assay. The brains were fixed by immersion in 5% acrolein in 0.1 M Tris-buffered saline (TBS) for 24 h followed by 72 h in 30% sucrose dissolved in TBS. Following fixation, the brains were rapidly frozen in methylbutane and stored at −80°C until tissue processing. Brains were cut using a cryostat into 4 series of 40 µm sections each and stored at −20°C in a cryoprotectant solution (60% sucrose, 30% ethylene glycol, 2% polyvinylpyrrolidone) until use in immunocytochemistry.
Immunocytochemistry for AVP, galanin and AR. All steps in were performed at room temperature unless otherwise noted. All of the tissue targeted by each antibody was processed at the same time. All washes were done three times for 5 min each in either TBS or 0.3% TBS-Triton X-100 (TBS-T). For each antibody, one of every fourth section of the brain was washed and incubated for 15 min in 0.1% sodium borohydride in TBS. After washes, the tissue was placed in a blocking solution containing 20% normal goat serum and 3% hydrogen peroxide in TBS for 60 min. The tissue was incubated for 18 h with rabbit anti-vasopressin (1:10,000; 20069, Immunostar, Inc.,), rabbit anti-galanin (1:7,000; T-4333, Bachem Americas), or rabbit anti-androgen receptor, PG-21 (1:2,000; 06-680, Millipore) in a solution of 2% goat serum and 0.5% gelatin in TBS-T. After washes, the tissue was incubated for 90 min in biotinylated goat anti-rabbit IgG (1:500; BA-1000, Vector Labs) in TBS-T with 0.5% gelatin. The tissue was then washed and incubated in Vectastain Elite ABC peroxidase (PK-6101; Vector Laboratories) using half the concentration recommended by the manufacturer for 60 min. After washes, the tissue was incubated for 10 min using a Vector SG kit (SK-4700, Vector Laboratories) diluted in TBS-T with 0.5% gelatin using the concentration recommended by the manufacturer. After three final washes, the sections were mounted, air-dried and coverslipped with Permount (SP15-100, Fisher Scientific).
Immunocytochemical analysis. The following areas were chosen for analysis: the bed nucleus of the striaterminalis (BST) and the centromedial amygdala (CMA), which express steroid-responsive AVP cells; the lateral septum (LS), which receives projections from these cells; and the paraventricular thalamic nucleus (PVT), which receives AVP projections from the suprachiasmatic nucleus, a hypothalamic region that contains non-steroid sensitive AVP cells.51,52 The Atlas of Paxinos and Watson53 was used to match a bilateral plate for each animal containing the LS, corresponding to plate 18; BST, corresponding to plate 21; CMA, corresponding to plate 30; and PVT, corresponding to plate 33. These areas were inspected under bright-field illumination using an Olympus BX-61 microscope fitted with a 10x (for cell counts) or 20x (for fiber analysis) objective and an Olympus FV II digital camera. The number of AVP or galanin-ir cells in the CMA and BST was counted at 10x by an experimenter blind to treatment groups. AVP-ir fiber area in the LS and PVT and AR-ir cell number in the CMA were determined using Olympus MicroSuite (Soft Imaging Corp.). For each of these brain regions, the same sized area was analyzed for each animal. The threshold to detect foreground was set at 15% (for AVP-ir fibers) or 5% (for AR-ir cells) above the background gray value mean that was determined individually for each animal. Microscope light intensity was kept constant throughout the imaging session for each brain region analyzed to ensure consistent measurements.
Quantification of serum testosterone levels. An enzyme immunoassay (EIA) for testosterone (582701, Cayman Chemical Company) was run on the serum collected from the animals. This EIA is based on the competition between testosterone and a testosterone-acetylcholinesterase conjugate for a limited number of testosterone-specific binding sites. Testosterone standards, serum samples diluted 1:8, and the necessary controls were loaded onto a 96-well plate coated with mouse anti-rabbit IgG. Testosterone-specificacetylcholinesterase (ACHe) tracer and rabbit anti-testosterone antiserum were added to all wells on the plate, except several of the control wells. The plate was incubated for 2 h at room temperature on an orbital shaker to allow for competitive binding. After this incubation, the plate was washed 5 times with a wash buffer. The concentration of testosterone was determined by measuring the enzymatic activity of ACHe with Ellman's Reagent, which contains the substrate for ACHe. The product of this enzymatic reaction absorbs at 412 nm. The plate developed in the dark for 1 h and absorbance was read on a plate reader equipped with a filter that reads wavelengths between 405 and 420 nm. The assay speciocity is 100% for testosterone, and the intra-assay coefficient was 9.0%. The detection limit of this assay was 6 pg/ml. Results were calculated using a computer spreadsheet program provided by the assay manufacturer (www.caymanchem.com/eiatools/promo/kit).
Experiment 3: Immunocytochemical assessment of infusion area.
Subjects and procedures. To determine possible spread of infusions into the amygdala, we infused varying doses of SKF 38,393 into the amygdala and examined c-Fos-ir cells in the amygdala and BST. SKF 38,393 is a D1-receptor agonist that induces c-Fos protein in several brain areas,54,55 including the amygdala and BST,34 within an hour following a systemic infusion, suggesting that the number of c-Fos-ir cells in the BST would increase if the SKF 38,393 infusions spread to the BST. On PN1, female rats were bilaterally infused into the amygdala with 0, 300, 600 or 1,200 ng of SKF in 1 µL of sterile saline. Pups recovered under a warm lamp and were sacrificed 1 h after the single infusion. Brains were fixed by immersion in 5% acrolein (Sigma-Aldrich, Inc.,) in 0.1 M TBS for 24 h followed by 24 h in 20% sucrose dissolved in TBS. Following fixation, the brains were then rapidly frozen in methylbutane on dry ice and stored at −80°C until tissue processing. Brains were cut into 50 µm sections using a cryostat and stored in cryoprotectant at −20°C. Immunocytochemistry was performed following the procedure described in experiment 1 using a rabbit anti-c-Fos primary antibody (1:2,000; sc-52, Santa Cruz Biotechnology) in a solution of 2% goat serum and 0.5% gelatin in TBS-T. The number of c-Fos-ir cells was measured with a 10x objective in the amygdala and BST (plates 30 and 21 in the atlas of Paxinos and Watson, respectively) using Olympus MicroSuite (Soft Imaging Corp.), as described above.
Acknowledgments
This research was funded by start-up funds from the Psychology Department, the Graduate School and the College of Letters and Sciences at the University of Wisconsin-Madison and National Institutes of Health (NIH) Grant R01MH072956 to A.P.A., National Science Foundation (NSF) Grant IOS-1122074 to C.J.A. Special thanks Kathleen Krol and Heather Jessen for technical assistance.
Abbreviations
- MeCP2
methyl-CpG-binding protein 2
- AVP
arginine vasopressin
- CREB1
cAMP response element-binding protein 1
- CBP
CREB-binding protein
- PN
postnatal day
- CMA
centromedial amygdala
- BST
bed nucleus of the striaterminalis
- LS
lateral septum
- PVN
paraventricular nucleus of the hypothalamus
- PVT
paraventricular thalamic nucleus
- ER
estrogen receptor
- AR
androgen receptor
- NF-L
neurofilament light polypeptide
- Foxg1
forkhead box protein G1
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- Ywhaz
tyrosine-3-monooxygenase/tryptophan-5-monooxygenase activation protein, zeta polypeptide
- siRNA
small interfering RNA
- -ir
immunoreactive
- ICC
immunocytochemistry
- RT-PCR
real-time polymerase chain reaction
- EIA
enzyme immunoassay
- TBS
tris-buffered saline
- ACHe
acetylcholinesterase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gonzales ML, LaSalle JM. The role of MeCP2 in brain development and neurodevelopmental disorders. Curr Psychiatry Rep. 2010;12:127–134. doi: 10.1007/s11920-010-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/S0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- 3.Meehan RR, Lewis JD, Bird AP. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992;20:5085–5092. doi: 10.1093/nar/20.19.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 5.Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci USA. 2005;102:17551–17558. doi: 10.1073/pnas.0507856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung BP, Jugloff DG, Zhang G, Logan R, Brown S, Eubanks JH. The expression of methyl CpG binding factor MeCP2 correlates with cellular differentiation in the developing rat brain and in cultured cells. J Neuro biol. 2003;55:86–96. doi: 10.1002/neu.10201. [DOI] [PubMed] [Google Scholar]
- 9.Tsujimura K, Abematsu M, Kohyama J, Namihira M, Nakashima K. Neuronal differentiation of neural precursor cells is promoted by the methyl-CpG-binding protein MeCP2. Exp Neurol. 2009;219:104–111. doi: 10.1016/j.expneurol.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Na ES, Monteggia LM. The role of MeCP2 in CNS development and function. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci USA. 2007;104:1931–1936. doi: 10.073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGraw CM, Samaco RC, Zoghbi HY. Adult neural function requires MeCP2. Science. 2011;333:186. doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28:7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm Behav. 1981;15:197–213. doi: 10.1016/0018-506X(81)90028-3. [DOI] [PubMed] [Google Scholar]
- 16.Smith LK, Forgie ML, Pellis SM. The postpubertal change in the playful defense of male rats depends upon neonatal exposure to gonadal hormones. Physiol Behav. 1997;63:151–155. doi: 10.1016/S0031-9384(97)00397-1. [DOI] [PubMed] [Google Scholar]
- 17.Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the diencephalon. J Clin Endocrinol Metab. 1971;33:368–370. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- 18.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 19.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Bielsky IF, Young LJ. Oxytocin, vasopressin and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psycho neuro endocrinology. 2009;34:463–467. doi: 10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 23.De Vries GJ, al-Shamma HA. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol. 1990;21:686–693. doi: 10.1002/neu.480210503. [DOI] [PubMed] [Google Scholar]
- 24.Han TM, De Vries GJ. Organizational effects of testosterone, estradiol and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the striaterminalis. J Neurobiol. 2003;54:502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- 25.De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the striaterminalis of rats. J Neurosci. 1994;14:1789–1794. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Bullock NA, De Vries GJ. Sexual differentiation of vasopressin projections of the bed nucleus of the stria terminals and medial amygdaloid nucleus in rats. Endocrinology. 1993;132:2299–2306. doi: 10.1210/en.132.6.2299. [DOI] [PubMed] [Google Scholar]
- 27.Wilson ME, Westberry JM, Prewitt AK. Dynamic regulation of estrogen receptor-alpha gene expression in the brain: a role for promoter methylation? Front Neuroendocrinol. 2008;29:375–385. doi: 10.1016/j.yfrne.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MA, Kolb PE, Raskind MA. Extra-hypothalamic vasopressin neurons coexpressgalanin messenger RNA as shown by double in situ hybridization histochemistry. J Comp Neurol. 1993;329:378–384. doi: 10.1002/cne.903290308. [DOI] [PubMed] [Google Scholar]
- 30.Lee MK, Cleveland DW. Neurofilament function and dysfunction: involvement in axonal growth and neuronal disease. Curr Opin Cell Biol. 1994;6:34–40. doi: 10.1016/0955-0674(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 31.Jacob FD, Ramaswamy V, Andersen J, Bolduc FV. Atypical Rett syndrome with selective FOXG1 deletion detected by comparative genomic hybridization: case report and review of literature. Eur J Hum Genet. 2009;17:1577–1581. doi: 10.1038/ejhg.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi S, Matsumoto N, Okayama A, Suzuki N, Araki A, Okajima K, et al. FOXG1 mutations in Japanese patients with the congenital variant of Rett syndrome. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01819.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee VW, de Kretser DM, Hudson B, Wang C. Variations in serum FSH, LH and testosterone levels in male rats from birth to sexual maturity. J Reprod Fertil. 1975;42:121–126. doi: 10.530/jrf.0.0420121. [DOI] [PubMed] [Google Scholar]
- 34.Olesen KM, Auger AP. Dopaminergic activation of estrogen receptors induces fos expression within restricted regions of the neonatal female rat brain. PLoS One. 2008;3:2177. doi: 10.1371/journal.pone.0002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 36.Han TM, De Vries GJ. Neurogenesis of galanin cells in the bed nucleus of the striaterminalis and centromedial amygdala in rats: a model for sexual differentiation of neuronal phenotype. J Neuro boil. 1999;38:491–498. doi: 10.1002/(SICI)1097-4695(199903)38:4<491::AID-NEU5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Planas B, Kolb PE, Raskind MA, Miller MA. Sex difference in coexpression by galanin neurons accounts for sexual dimorphism of vasopressin in the bed nucleus of the striaterminalis. Endocrinology. 1995;136:727–733. doi: 10.1210/en.136.2.727. [DOI] [PubMed] [Google Scholar]
- 38.Planas B, Kolb PE, Raskind MA, Miller MA. Galanin in the bed nucleus of the striaterminalis and medial amygdala of the rat: lack of sexual dimorphism despite regulation of gene expression across puberty. Endocrinology. 1994;134:1999–2004. doi: 10.1210/en.134.5.1999. [DOI] [PubMed] [Google Scholar]
- 39.Rajendren G, Levenkova N, Gibson MJ. Galaninimmunoreactivity in mouse basal forebrain: sex differences and discrete projections of galanin-containing cells beyond the blood-brain barrier. Neuro endocrinology. 2000;71:27–33. doi: 10.1159/000054517. [DOI] [PubMed] [Google Scholar]
- 40.Miller MA, Kolb PE, Raskind MA. Testosterone regulates galanin gene expression in the bed nucleus of the striaterminalis. Brain Res. 1993;611:338–341. doi: 10.1016/0006-8993(93)90523-P. [DOI] [PubMed] [Google Scholar]
- 41.Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- 42.Banu SK, Govindarajulu P, Aruldhas MM. Developmental profiles of TSH, sex steroids and their receptors in the thyroid and their relevance to thyroid growth in immature rats. Steroids. 2002;67:137–144. doi: 10.1016/S0039-128X(01)00144-1. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Falgueras A, Ligtenberg L, Kruijver FP, Swaab DF. Galanin neurons in the intermediate nucleus (InM) of the human hypothalamus in relation to sex, age and gender identity. J Comp Neurol. 2011;519:3061–3084. doi: 10.1002/cne.22666. [DOI] [PubMed] [Google Scholar]
- 44.Auger AP, Perrot-Sinal TS, Auger CJ, Ekas LA, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/en.143.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auger AP, Hexter DP, McCarthy MM. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res. 2001;890:110–117. doi: 10.1016/S0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
- 46.Iwasaki Y, Oiso Y, Saito H, Majzoub JA. Positive and negative regulation of the rat vasopressin gene promoter. Endocrinology. 1997;138:5266–5274. doi: 10.1210/en.138.12.5266. [DOI] [PubMed] [Google Scholar]
- 47.Han TM, De Vries GJ. Organizational effects of testosterone, estradiol and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the striaterminalis. J Neurobiol. 2003;54:502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- 48.Meaney MJ, Stewart J, Poulin P, McEwen BS. Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology. 1983;37:85–90. doi: 10.1159/000123524. [DOI] [PubMed] [Google Scholar]
- 49.de Vries GJ, Jardon M, Reza M, Rosen GJ, Immerman E, Forger NG. Sexual differentiation of vasopressin innervation of the brain: cell death versus phenotypic differentiation. Endocrinology. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 51.DeVries GJ, Buijs RM, Van Leeuwen FW, Caffé AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- 52.de Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298:141–145. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. Atlas of Paxinos and Watson (Academic Press 1986) [Google Scholar]
- 54.Meredith JM, Auger AP, Blaustein JD. D1 dopamine receptor agonist (SKF-38393) induction of Fosimmunoreactivity in progestin receptor-containing areas of female rat brain. J Neuro endocrinol. 1997;9:385–394. doi: 10.1046/j.1365-2826.1997.00594.x. [DOI] [PubMed] [Google Scholar]
- 55.Shearman LP, Zeitzer J, Weaver DR. Widespread expression of functional D1-dopamine receptors in fetal rat brain. Brain Res Dev Brain Res. 1997;102:105–115. doi: 10.1016/S0165-3806(97)00091-6. [DOI] [PubMed] [Google Scholar]