Abstract
Acute amphetamine administration activates glycogen synthase kinase-3 (GSK3) by reducing its inhibitory serine-phosphorylation in mouse striatum and cerebral cortex. This results from Akt inactivation and is required for certain behavioral effects of amphetamine, such as increased locomotor activity. Here we tested if regulation of Akt and GSK3 were similarly affected by longer-term administration of amphetamine, as well as of methylphenidate, since each of these is administered chronically in patients with attention deficit hyperactivity disorder (ADHD). Akt is activated by post-translational phosphorylation on Thr308, and modulated by Ser473 phosphorylation, whereas phosphorylation on Ser21/9 inhibits the two GSK3 isoforms, GSK3α and GSK3β. After eight days of amphetamine or methylphenidate treatment, striatal Akt and GSK3 were dephosphorylated similar to reported changes after acute amphetamine treatment. Oppositely, in the cerebral cortex and hippocampus Akt and GSK3 phosphorylation increased after eight days of amphetamine or methylphenidate treatment. These opposite brain region changes in Akt and GSK3 phosphorylation matched opposite changes in the association of Akt with β-arrestin and GSK3, which after eight days of amphetamine treatment were increased in the striatum and decreased in the cerebral cortex. Thus, whereas the acute dephosphorylating effect of stimulants on Akt and GSK3 in the striatum was maintained, the response switched in the cerebral cortex after eight days of amphetamine or methylphenidate treatment to cause increased phosphorylation of Akt and GSK3. These results demonstrate that prolonged administration of stimulants causes brain region-selective differences in the regulation of Akt and GSK3.
Keywords: amphetamine, attention deficit hyperactivity disorder, glycogen synthase kinase-3, methylphenidate, stimulants
1. Introduction
Alterations in dopaminergic neurotransmission have been associated with a number of central nervous system diseases, such as attention deficit hyperactivity disorder (ADHD), schizophrenia, and Parkinson’s disease [1,2]. Therapeutics for these diseases target the dopaminergic system, with stimulants increasing dopaminergic signaling, and antipsychotics blocking dopaminergic receptors. It has long been known that intracellular signaling by the D1 and D2 families of dopamine receptors is transduced by causing synthesis of the second messenger cyclic AMP to increase and decrease, respectively. More recently dopamine receptors have been found to signal through mechanisms involving the adaptor protein β-arrestin [3-5]. In particular, a D2 receptor pathway involving β-arrestin and modulation of the activities of glycogen synthase kinase-3 (GSK3) and Akt has been described that is an important mediator of some behavioral actions of amphetamine.
GSK3 is a serine/threonine kinase initially identified as a regulator of glycogen synthase, but now known to be involved in many cellular processes, including gene expression, microtubule organization, development, and cell survival [6-8]. The activity of the two GSK3 isoforms, GSK3α and GSK3β, is predominantly regulated by inhibitory phosphorylation on Ser21-GSK3α and Ser9-GSK3β. This post-translational modification is often mediated by Akt [9,10], which is activated by phosphorylation of Thr308-Akt and modulated by phosphorylation on Ser473-Akt. Stimulation of D2 receptors modulates the regulatory phosphorylation of GSK3 and Akt by altering a β-arrestin-2/Akt/PP2A/GSK3 complex [11,12]. Activation of D2 receptors promotes β-arrestin association with GSK3, Akt and PP2A, facilitating PP2A-mediated dephosphorylation and inactivation of Akt, resulting in dephosphorylation and activation of GSK3. Thus, D2 receptor activation after amphetamine treatment of mice decreased activating Thr308-Akt phosphorylation and decreased inhibitory phosphorylation of Ser21-GSK3α and Ser9-GSK3β, and the latter was shown to be required for amphetamine-induced locomotor hyperactivity [12,13]. Recently, O’Brien et al. [14] found that GSK3 stabilizes this complex, enhancing PP2A-mediated dephosphorylation of Akt.
Although the acute effects of amphetamine on signaling to Akt and GSK3 have been well-characterized, little is known about these responses following longer-term administration of stimulants, a regimen used in patients undergoing stimulant-based therapies. In the present study, we examined the effects of eight days of treatment with amphetamine or methylphenidate on the regulation of GSK3 and Akt. The results show that prolonged amphetamine or methylphenidate treatment decrease phosphorylation of GSK3 and Akt in the striatum, as reported for acute stimulant treatment, but have the opposite effect in the cerebral cortex.
2. Materials and methods
2.1. Animals
This study used adult, male C57Bl/6J mice, ~3 months of age. All mice were housed and treated in accordance with National Institutes of Health guidelines, and procedures with mice were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Amphetamine and methylphenidate (Sigma-Aldrich, St. Louis, MO) were dissolved in saline and injected intraperitoneally (i.p.). Corresponding vehicle solutions were administered to control mice.
2.2. Tissue Preparation and Western Blots
Mice were decapitated and brains were rapidly removed and frozen. Brain regions were dissected on ice and homogenized in ice-cold lysis buffer containing 10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% NP-40, 10 mg/ml leupeptin, 10 mg/ml aprotinin, 5 mg/ml pepstatin, 1 mM phenylmethanesulfonyl fluoride, 1 mM sodium vanadate, 50 mM sodium fluoride, and 100 nM okadaic acid. The lysates were centrifuged at 20,000×g for 15 min to remove insoluble debris. Protein concentrations in the supernatants were determined in triplicate using the Bradford protein assay. Extracts were mixed with Laemmli sample buffer (2% SDS) and placed in a boiling water bath for 5 min. Proteins were resolved in SDS-polyacrylamide gels, and transferred to nitrocellulose. Blots were probed with antibodies to phospho-Ser9-GSK3β, phospho-Ser21-GSK3α (Cell Signaling, Danvers, MA), total GSK3α/β (Upstate Biotechnology Inc., Lake Placid, NY), phospho-Ser473-Akt (Cell Signaling), phospho-Thr308-Akt (Cell Signaling), or total Akt (Cell Signaling). Immunoblots were developed using horseradish peroxidase-conjugated goat anti-mouse, or goat anti-rabbit IgG, followed by detection with enhanced chemiluminescence, and the protein bands were quantitated with a densitometer.
2.3. Immunoprecipitation
Brain region lysates were incubated with anti-Akt (Cell Signaling) overnight at 4°C with gentle agitation, followed by incubation with protein G sepharose beads (Amersham) for 2 h at 4°C. The immune complexes were washed three times with PBS. Immunoprecipitants were immunoblotted for GSK3β (BD Transduction Laboratories, San Jose, CA) or β-arrestin (Cell Signaling).
2.4. Statistical Analysis
Data was analyzed by unpaired t-test, two-way ANOVA, and Bonferroni post tests. Values are expressed as mean ± SEM.
3. Results
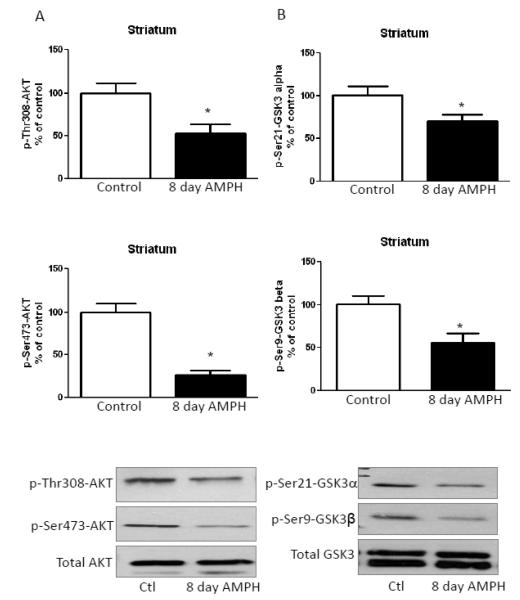
3.1. Long-term amphetamine treatment reduces phosphorylation of Akt and GSK3 in the striatum
Using brain regions isolated from mice treated daily for eight days with 2 mg/kg amphetamine, we assessed changes in the phosphorylation of Akt and GSK3 90 min after the final drug administration. In the striatum, chronic amphetamine treatment significantly decreased Thr308-Akt and Ser473-Akt phosphorylation, but did not change total Akt levels (Fig. 1A), similar to reported changes after acute amphetamine administration [13]. Long-term amphetamine treatment also decreased phosphorylation of Ser21-GSK3α and Ser9-GSK3β phosphorylation in the striatum without changing total GSK3 levels (Fig. 1B), as occurred after acute amphetamine administration [13]. Thus, in the striatum the regulation of Akt and GSK3 phosphorylation was equivalent after administration of amphetamine acutely or for eight days.
FIGURE 1. Amphetamine treatment for eight days reduces phosphorylation of Akt and GSK3 in the striatum.
A. Phosphorylation of Thr308-Akt and Ser473-Akt was reduced in the striatum of mice treated with 2 mg/kg amphetamine for eight days. B. Phosphorylation of Ser21-GSK3α and Ser9-GSK3β was reduced in mouse striatum after eight days of amphetamine treatment. Values are presented as percent of matched control mice. Means ± SEM. n=3. *, p≤0.05.
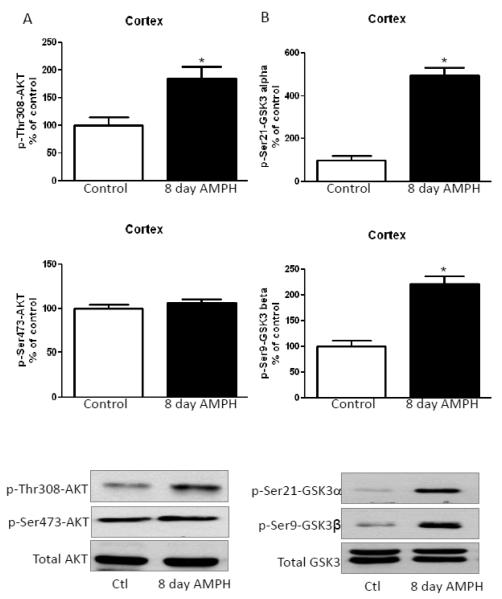
3.2. Long-term amphetamine treatment increases phosphorylation of Akt and GSK3 in the cerebral cortex and hippocampus
The cerebral cortices of chronic amphetamine-treated mice were analyzed 90 min after the final treatment. Chronic amphetamine treatment significantly increased the phosphorylation of Thr308-Akt, but there was no significant effect on Ser473-Akt phosphorylation or total Akt levels (Fig. 2A). Ser21-GSK3α and Ser9-GSK3β phosphorylation was also significantly increased after chronic amphetamine treatment (Fig. 2B). Thus, in the cerebral cortex the regulation of Akt and GSK3 phosphorylation was opposite after administration of amphetamine for eight days to the reported decreased phosphorylation occurring after acute amphetamine treatment [13].
FIGURE 2. Amphetamine treatment for eight days increases phosphorylation of Akt and GSK3 in the cerebral cortex.
A. Phosphorylation of Thr308-Akt was increased in the cerebral cortex of mice treated for eight days with 2 mg/kg amphetamine. B. Phosphorylation of Ser21-GSK3α and Ser9-GSK3β in the cerebral cortex was increased after amphetamine treatment for eight days. Values are presented as percent of matched control mice. Means ± SEM. n=3. *, p≤0.05.
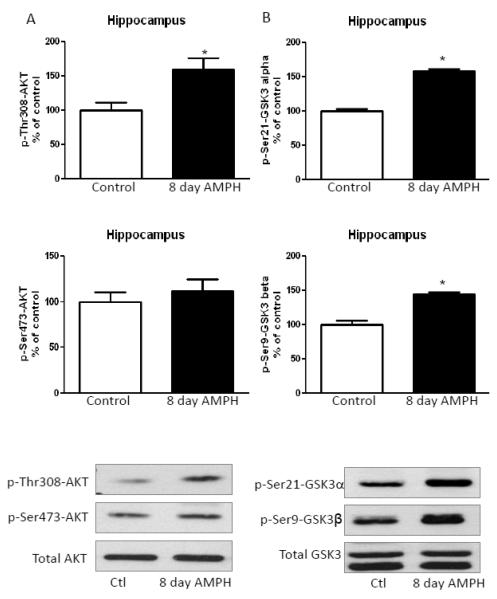
As in the cerebral cortex, in the hippocampus of mice treated with amphetamine for eight days there was a significant increase in Thr308-Akt phosphorylation, but no change in Ser473-Akt phosphorylation or total Akt levels (Fig. 3A). Significant increases in the serine-phosphorylation of GSK3α and GSK3β were also apparent in the hippocampus after chronic amphetamine treatment (Fig. 3B).
FIGURE 3. Amphetamine treatment for eight days increases phosphorylation of Akt and reduces phosphorylation of GSK3 in the hippocampus.
A. Hippocampal Thr308-Akt phosphorylation was increased after 2 mg/kg amphetamine treatment for eight days. B. Hippocampal Ser21-GSK3α and Ser9-GSK3β phosphorylation was increased after administration of amphetamine for eight days. Values are presented as percent of matched control mice. Means ± SEM. n=3. *, p≤0.05.
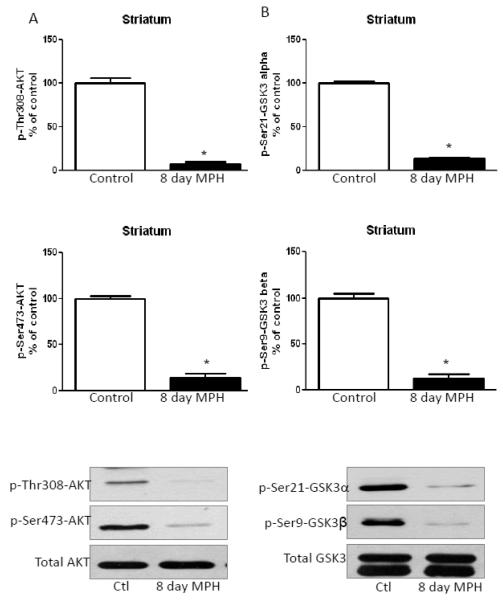
3.3. Long-term methylphenidate treatment mimics amphetamine-induced dephosphorylation of Akt and GSK3 in the striatum
Mice were treated with 20 mg/kg methylphenidate daily for eight days. Similar to chronic amphetamine treatment, chronic treatment with methylphenidate significantly decreased Thr308-Akt and Ser473-Akt phosphorylation without affecting total Akt levels (Fig. 4A). Striatal phospho-Ser21-GSK3α and phospho-Ser9-GSK3β were also decreased after eight days of methylphenidate treatment, and there was no effect on total GSK3 levels (Fig. 4B).
FIGURE 4. Methylphenidate treatment for eight days mimics amphetamine-induced alterations in phosphorylation of Akt and GSK3 in the striatum.
A. Treatment with 20 mg/kg methylphenidate for eight days reduced striatal phosphorylation of Thr308-Akt and Ser473-Akt. B. Phospho-Ser21-GSK3α and phospho-Ser9-GSK3β were reduced in striatum after methylphenidate treatment for eight days. Values are presented as percent of matched control mice. Means ± SEM. n=3. *, p≤0.05.
3.4. Long-term methylphenidate treatment mimics amphetamine in increasing the phosphorylation of Akt and GSK3 in the cerebral cortex and hippocampus
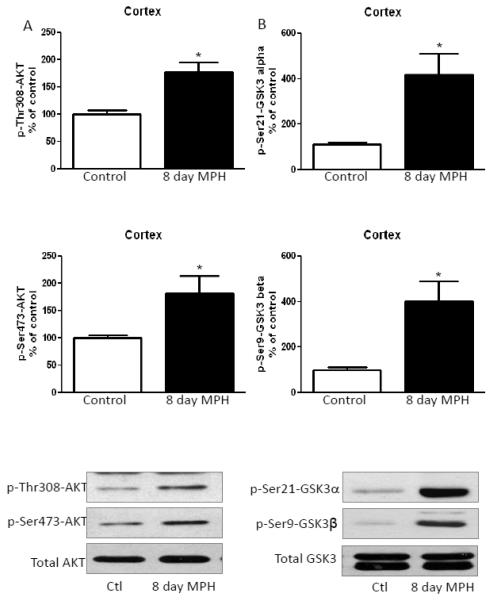
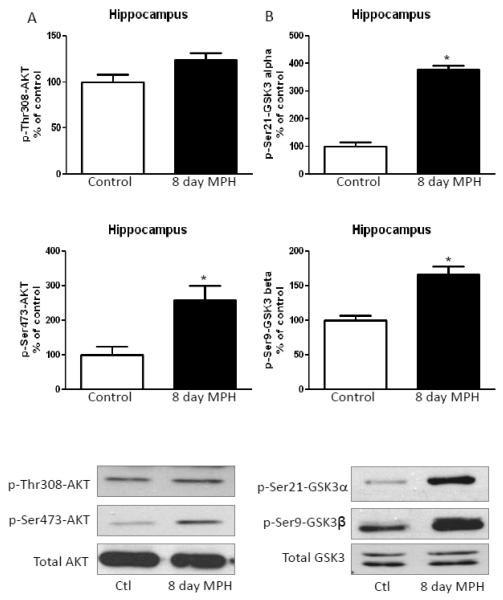
In contrast to the striatum, in the cerebral cortex, eight days of methylphenidate treatment increased phospho-Thr308-Akt and phospho-Ser473-Akt (Fig. 5A), similarly to that of chronic amphetamine treated mice, but in contrast to acute amphetamine effects. Ser21-GSK3α and Ser9-GSK3β phosphorylation in the cerebral cortex also were increased after chronic methylphenidate treatment (Fig. 5B). Chronic treatment with methylphenidate increased hippocampal Ser473-Akt phosphorylation, but only caused a slight, statistically insignificant, increase in Thr308-Akt phosphorylation (Fig. 6A). Methylphenidate treatment for eight days also increased the phosphorylation of both GSK3 isoforms in the hippocampus (Fig. 6B), without affecting total GSK3 levels.
FIGURE 5. Methylphenidate treatment for eight days increases phosphorylation of Akt and reduces phosphorylation of GSK3 in the cerebral cortex.
A. Phospho-Thr308-Akt and phospho-Ser473-Akt were increased in the cerebral cortex after 20 mg/kg methylphenidate treatment for eight days. B. Phospho-Ser21-GSK3α and phospho-Ser9-GSK3β were increased in the cerebral cortex after methylphenidate treatment for eight days. Values are presented as percent of matched control mice. Means ± SEM. n=3. *, p≤0.05.
FIGURE 6. Methylphenidate treatment for eight days increases phosphorylation of Akt and reduces phosphorylation of GSK3 in the hippocampus.
A. Phospho-Ser473-Akt was increased in hippocampus from mice treated with 20 mg/kg methylphenidate for eight days. B. Phospho-Ser21-GSK3α and phospho-Ser9-GSK3β were increased in the cerebral cortex after methylphenidate treatment for eight days. Values are presented as percent of matched control mice. Means ± SEM. n=3. *, p≤0.05.
3.5. Long-term stimulant treatment regulates the β-arrestin/Akt/GSK3 complex
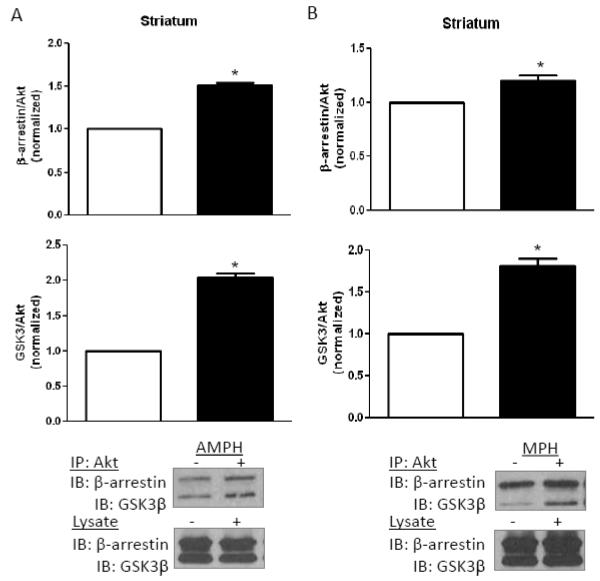
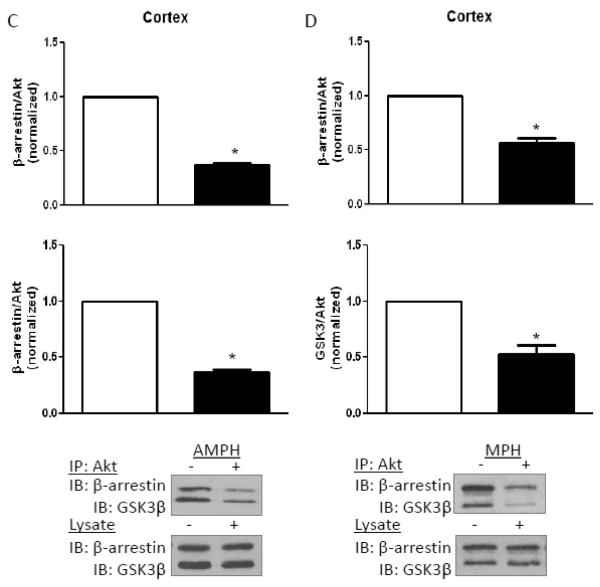
To assess the effect of chronic stimulant treatment on the complex containing Akt with β-arrestin and GSK3, which regulates Akt and GSK3 phosphorylation in response to D2 receptor stimulation, Akt was immunoprecipitated from brain region lysates after eight days of amphetamine or methylphenidate treatment. Western blot analyses revealed increased β-arrestin and GSK3β co-immunoprecipitated with Akt from the striatum after chronic amphetamine (Fig. 7A) or methylphenidate (Fig. 7B) administration. This matches the reported increased association of these proteins after acute amphetamine administration, which underlies the reduced phosphorylation of Akt and GSK3 [12,13]. However, the opposite was found in the cerebral cortex, as less β-arrestin and GSK3β co-immunoprecipitated with Akt after eight days of amphetamine (Fig. 7C) or methylphenidate (Fig. 7D) administration. These results match the opposite effects in the striatum and cerebral cortex of long term stimulant treatment on the phosphorylation of Akt and GSK3.
FIGURE 7. Regulation of the β-arrestin/Akt/GSK3 signaling complex.
Akt was immunoprecipitated, followed by immunoblotting for GSK3 and β-arrestin, from (A) striatum after eight days of amphetamine treatment, (B) striatum after eight days of methylphenidate treatment, (C) cerebral cortex after eight days of amphetamine treatment, and (D) cerebral cortex after eight days of methylphenidate treatment. Values are normalized to results from stimulant-free control mice. Means ± SEM. n=4. *, p≤0.05.
4. Discussion
Uncovering mechanisms regulating the β-arrestin/Akt/GSK3 complex that is vital to dopamine-mediated signaling may help to better understand diseases associated with altered dopaminergic function and therapeutic interventions. Emamian et al. [15] found alterations in Akt and GSK3 activity in postmortem brains from subjects with schizophrenia, raising the possibility of a link to altered dopaminergic activity. The direct link between D2 receptors and the regulation of GSK3 phosphorylation was first identified by Beaulieu et al. [13]. They demonstrated that amphetamine administration to mice decreased the activating phosphorylation of Akt and the inhibitory serine-phosphorylation of GSK3 in the striatum and cerebral cortex, and found increased GSK3 activity in dopamine transporter knockout mice [13]. The mechanism was found to be due to a complex containing β-arrestin, Akt, PP2A and GSK3, since dopamine-mediated dephosphorylation of Akt and GSK3 was blocked in β-arrestin knockout mice [11,16]. Beaulieu et al. [17] recently reviewed the extensive evidence that has accumulated demonstrating that D2 receptors mediate dopaminergic regulation of the phosphorylation of Akt and GSK3. Among the evidence are the findings that Akt and GSK3 phosphorylation were modulated by D2/D3 receptor antagonists, but not D1 receptor antagonists [13], deletion of D2 receptors, but not D1 receptors, abolished amphetamine- and apomorphine-mediated regulation of Akt/GSK3, whereas D3 receptor knockout mice displayed reduced, but not abolished, dopamine-regulated Akt phosphorylation [18], treatment with quinpirole, a D2 receptor agonist, reduced the phosphorylation of Akt and GSK3, whereas raclopride, a D2 receptor antagonist, and nafadotride, a D3 receptor antagonist, increased the phosphorylation of Akt and GSK3 in rat brain [19]. Furthermore, D2 receptor activation was also linked to Akt dephosphorylation in the developing zebrafish brain [20]. Thus, it is now well-established that D2 receptors signal to regulate the phosphorylation of Akt and GSK3 using a mechanism mediated by a β-arrestin-containing complex. Although most research on dopaminergic signaling to the β-arrestin/Akt/GSK3 complex has examined signaling after acute administration of stimulants, prolonged exposure to stimulants is experienced following many therapeutic regimens and in association with drug abuse of stimulants. Furthermore, it is well known that the long-term effects of drugs may differ from acute actions. In the present study we found that eight days of amphetamine or methylphenidate treatment had similar effects on Akt and GSK3 phosphorylation in the striatum as previously reported for acute amphetamine administration [13]. Thus, chronic amphetamine or methylphenidate treatment decreased Ser473-Akt and Thr308-Akt phosphorylation and inhibitory phosphorylation of Ser21-GSK3α and Ser9-GSK3β in the striatum of mice. These changes likely resulted from the increases in β-arrestin/Akt/GSK3 complex formation induced by stimulants in the striatum, extending previous findings that long-term, as well as acute, amphetamine administration regulates Akt and GSK3 phosphorylation by influencing this protein complex [11,12]. These findings also demonstrate that methylphenidate induces signaling in the striatum similarly to amphetamine. Chronic administration of cocaine has also been reported to increase GSK3 activity in the caudate putamen [21]. Thus, short and long-term dopaminergic stimulants clearly inactivate Akt and activate GSK3 in the striatum.
Unlike signaling in the striatum, cerebral cortical and hippocampal Akt and GSK3 phosphorylation were oppositely regulated by long term administration of stimulants compared to the striatum. Amphetamine or methylphenidate administration for eight days induced increases, rather than decreases, in activating Akt phosphorylation and inhibitory-serine phosphorylation of both GSK3 isoforms in the cerebral cortex and hippocampus. These changes were associated with disruption of the β-arrestin/Akt/GSK3 complex, suggesting that the disrupted complex underlies the increased phosphorylation of the kinases [14,16]. These findings indicate that this signaling process responds differently in the cerebral cortex than in the striatum to prolonged administration of stimulants. These results also indicate that there are further mechanisms involved in the regulation of dopamine-mediate signaling through the β-arrestin/Akt/PP2A/GSK3 protein complex in the cerebral cortex. However, little is known about mechanisms that regulate this complex. O’Brien et al. [14] used a GSK3 transgenic mouse model to demonstrate that GSK3 promotes stability of the complex, whereas inhibition of GSK3 promoted dissociation of β-arrestin from Akt. Thus, it is possible that prolonged treatment with stimulants modifies this mechanism regulating complex stability, but further studies are needed to identify mechanisms that can regulate the β-arrestin/Akt/PP2A/GSK3 protein complex that may influence responses to prolonged stimulant administration.
In addition to binding Akt and GSK3, β-arrestin is also involved in the ERK1/2 signaling pathway [22,23]. After acute amphetamine treatment, ERK1/2 activation is increased in mouse prefrontal cortex [20] and in mouse and rat striatum [25-27]. Conflicting reports have been published regarding the effects of chronic stimulant administration on ERK1/2 signaling. Zhai et al. [27] reported unaltered striatal ERK1/2 activation, as compared to control mice, after chronic amphetamine administration, while increased striatal ERK1/2 activation was reported after repeated amphetamine administration in rats [25,26]. Downstream of ERK1/2, increased CREB activation has been reported in response to acute and repeated amphetamine administration in mouse and rat striatum [25,26]. Collectively, these results indicate that there are brain region and treatment regimen alterations in responses to stimulants. These findings also confirm that β-arrestin is a critical regulatory component in determining outcomes of dopaminergic signaling, a better understanding of which may lead to better improved therapeutic interventions.
5. Conclusions
Overall, these results highlight differential regulation of signaling responses in different brain regions after prolonged administration of stimulants. They also confirm the importance of the β-arrestin/Akt/GSK3 complex in dopamine-mediated signaling. These findings suggest the presence of a signaling response switch in the cerebral cortex that alters the response of the β-arrestin, Akt, GSK3 complex following long-term stimulant treatment.
HIGHLIGHTS.
Eight day methylphenidate or amphetamine treatments reduced Akt and GSK3 phosphorylation in mouse striatum
Eight day methylphenidate or amphetamine treatments increased Akt and GSK3 phosphorylation in mouse cerebral cortex
Eight day methylphenidate or amphetamine treatments increased β-arrestin/Akt/GSK3 complex assembly in mouse striatum
Eight day methylphenidate or amphetamine treatments decreased β-arrestin/Akt/GSK3 complex assembly in mouse cerebral cortex
Acknowledgements
This research was supported by grants from the NIH (MH38752; MH092970).
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- GSK3
glycogen synthase kinase-3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carlsson A. Science. 2001;294:1021. doi: 10.1126/science.1066969. [DOI] [PubMed] [Google Scholar]
- 2.Gainetdenov RR, Caron MG. Annu. Rev. Pharmacol. Toxicol. 2003;43:261. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu JM, Gainetdinov RR, Caron MG. Annu. Rev. Pharmacol. Toxicol. 2009;49:327. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 4.DeFea KA. Cell Signal. 2011;23:621. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Shenoy SK, Lefkowitz RJ. Trends Pharmacol. Sci. 2011;32:521. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimes CA, Jope RS. Prog. Neurobiol. 2001;65:391. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 7.Frame S, Cohen P. Biochem. J. 2001;359:1. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doble BW, Woodgett JR. J. Cell Sci. 2003;116:1175. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stambolic V, Woodgett JR. Trends Cell Biol. 2006;16:461. doi: 10.1016/j.tcb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Jope RS, Johnson GV. Trends Biochem. Sci. 2004;29:95. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. Cell. 2008;132:125. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu JM, Del’guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR. Front. Mol. Neurosci. 2011;4:38. doi: 10.3389/fnmol.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5099. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien WT, Huang J, Buccafusca R, Garskof J, Valvezan AJ, Berry GT, Klein PS. J. Clin. Invest. 2011;121:3756. doi: 10.1172/JCI45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gorgos JA. Nat. Genet. 2004;36:131. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 16.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. Cell. 2005;122:261. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu JM, Del’Guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR. Front. Mol. Neurosci. 2011;4:38. doi: 10.3389/fnmol.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. J. Neurosci. 2007;27:881. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton LP, Rushlow WJ. Int. J. Neuropsychopharmacol. 2011:1. doi: 10.1017/S146114571100109X. [DOI] [PubMed] [Google Scholar]
- 20.Souza BR, Romano-Silva MA, Tropepe V. J. Neurosci. 2011;31:5512. doi: 10.1523/JNEUROSCI.5548-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller JS, Tallarida RJ, Unterwald EM. Neuropharmacol. 2009;56:1116. doi: 10.1016/j.neuropharm.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFea KA, Zalevsky J, Thoma MS, Déry O, Mullins RD, Bunnett NW. J. Cell Biol. 2000;148:1267. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roudabush LM, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2449. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascoli V, Valjent E, Corbillé AG, Corvol JC, Tassin JP, Girault JA, Hervé D. Mol. Pharmacol. 2005;68:421. doi: 10.1124/mol.105.011809. [DOI] [PubMed] [Google Scholar]
- 25.McGinty JF, Shi XD, Schwendt M, Saylor A, Toda S. J. Neurochem. 2008;104:1440. doi: 10.1111/j.1471-4159.2008.05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi X, McGinty JF. J. Neurochem. 2007;103:706. doi: 10.1111/j.1471-4159.2007.04760.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhai H, Li Y, Wang X, Lu L. Cell Mol. Neurobiol. 2008;28:157. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]