Abstract
Malignant neuroblastomas are childhood tumors that remain mostly incurable. We explored efficacy of N-(4-hydroxyphenyl) retinamide (4-HPR) and (−)-epigallocatechin-3-gallate (EGCG) in altering expression of oncogenic microRNAs (OGmiRs) and tumor suppressor miRs (TSmiRs) for controlling growth of human malignant neuroblastoma SK-N-BE2 and IMR-32 cells. Combination of 4-HPR and EGCG most significantly decreased expression of OGmiRs (miR-92, miR-93, and miR-106b) and increased expression of TSmiRs (miR-7-1, miR-34a, and miR-99a) in both cell lines. Overexpression of miR-93 and miR-7-1, respectively, decreased and increased efficacy of treatments. Thus, alterations in expression of specific OGmiRs and TSmiRs by 4-HPR and EGCG inhibited growth of malignant neuroblastomas.
Keywords: apoptosis, (−)-epigallocatechin-3-gallate, N-(4-hydroxyphenyl) retinamide, neuroblastoma, oncogenic and tumor suppressor miRs
1. Introduction
Neuroblastoma, one of the most common solid tumors in children, is derived from primitive cells of the sympathetic nervous system and it is responsible for ~15% of all childhood cancer deaths (Castelletti et al., 2010). These tumors are particularly noteworthy for extensive heterogeneity in clinical behavior, ranging from spontaneous regression to aggressive clinical course and death of the patients.
N-(4-Hydroxyphenyl) retinamide (4-HPR), a synthetic retinoid, can induce differentiation in neuroblastoma cells and 4-HPR is used in the therapy of neuroblastoma patients who have shown increase in survival rates (Reynolds et al., 1991). Plant-derived polyphenols have received extensive attention because of their anti-cancer properties such as cell-cycle arrest, DNA damage, and activation of caspases (Janardhanan et al., 2009). Epigallocatechin-3-gallate (EGCG), a major polyphenol found in green tea, is a widely studied plant-derived agent with potential anti-cancer activity (Das et al., 2006). Retinoids at a low dose can induce differentiation in neuroblastoma cells and act synergistically with flavonoids, providing novel opportunities for controlling growth of malignant neuroblastoma (Das et al., 2009). EGCG modulates the expression of the key molecules in cell-cycle progression, inhibits the inflammatory molecule nuclear factor-κB (NF-κB), and binds to Fas for activation of apoptotic cascade (Chen et al., 2003).
Many non-translated RNA sequences, such as microRNAs (miRs), may contribute to neuroblastoma pathogenesis. miRs are frequently located at fragile sites and genomic regions involved in cancers, implicating their involvement in the pathogenesis of malignant diseases like neuroblastoma (Calin et al., 2004). miRs regulate gene expression at a post-transcriptional level by either inhibiting translation of mRNAs or causing them to be degraded. They play major roles in the differentiation of neural (Miska et al., 2004) and other cancer cells, and the dysregulation of these sequences can have oncogenic or suppressor activity in different cancers. Small interfering RNA (siRNA) mediated inhibition of MYCN also alters the expression profiles of many miR loci. Transfection of one such locus, miR-184, into neuroblastoma cells was found to cause massive apoptosis (Foley et al., 2010). A genome-wide search for promoters that respond to increase in expression of MYCN reveals that increase in oncogenic miRs (OGmiRs) and decrease in tumor suppressor miRs (TSmiRs) are associated with occurrence of aggressive neuroblastomas (Shohet et al., 2011). miR-17-92 activation triggers down regulation of multiple key effectors along the tumor growth factor-beta (TGF-β) signaling cascade as well as direct inhibition of TGF-β-responsive genes (Mestdagh et al., 2010). The TSmiRs let-7 and miR-101 target MYCN and inhibit proliferation and clonogenic growth of MYCN-amplified neuroblastoma cells (Buechner et al., 2011). miR-7 and miR-214 are specifically expressed during neuroblastoma differentiation, cortical development, and embryonic stem cell differentiation, and neurite outgrowth in vitro (Chen et al., 2010). miR-34a significantly reduces tumor growth in an in vivo orthotopic murine model of neuroblastoma and it has novel effects on activation of key signaling proteins involved in apoptosis (Tivnan et al., 2011).
Elucidating the role of miRs in cancer is clearly in its infancy and, to the best of our knowledge, there are presently only a few publications on the role of miR dysregulation in human malignant neuroblastomas. It seems quite logical to think that miRs play particularly important roles in pediatric cancer, given their involvement in normal developmental processes and the fact that pediatric malignancies tend to involve perturbation of such pathways. In this investigation, we examined the differential expression of six human miRs (three OGmiRs and three TSmiRs) in two human malignant neuroblastoma cell lines (SK-N-BE2 and IMR-32) after treatment with 4-HPR and EGCG. We also determined the effects of overexpression of an OGmiR (miR-93) and a TSmiR (miR-7-1) on the treatment with 4-HPR and EGCG in SK-N-BE2 and IMR-32 cell lines.
2. Results
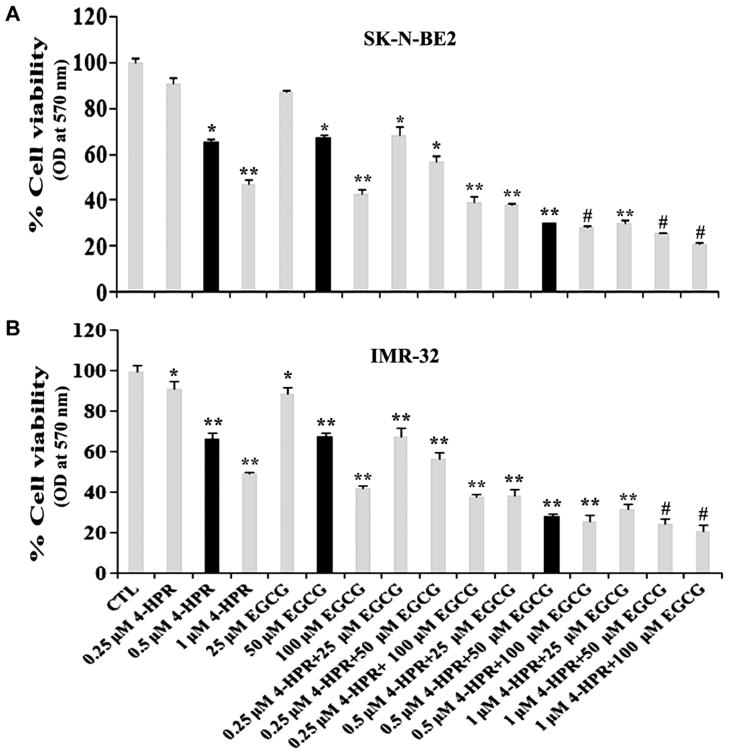
2.1. Combination of 4-HPR and EGCG reduced viability of neuroblastoma cell lines
The residual cell viability of neuroblastoma SK-N-BE2 and IMR-32 cells was determined by MTT assay after treatment with 4-HPR (0.25, 0.5, or 1 μM for 72 h) and EGCG (25, 50, or 100 μM for 24 h) as monotherapy and also as combination therapy with two drugs (Fig. 1). The MTT assay showed that although 0.5 μM 4-HPR and 50 μM EGCG alone did not cause significant decrease in cell viability but their combination effectively reduced the cell viability in both neuroblastoma cell lines (Fig. 1A & 1B). The results obtained from the MTT assay were analyzed using Compusyn software to determine the combination index (CI). The CI values for SK-N-BE2 and IMR-32 cell lines were shown in Table 1. The lowest value of the CI indicated the highly synergistic effect of the combination of two drugs. Based on the CI values (Table 1), we selected 0.5 μM 4-HRP or 50 μM EGCG as monotherapy and 0.5 μM 4-HRP and 50 μM EGCG as the combination therapy for their synergistic efficacy in SK-N-BE2 cells (CI = 0.56) as well as in IMR-32 cells (CI = 0.67) for carrying out further experiments. In all subsequent experiments, we used 0.5 μM 4-HPR + 50 μM EGCG as an effective combination therapy for inducing cell death in neuroblastoma cells and examining the alterations in molecular markers that led to this process.
Fig. 1.
Changes in residual cell viability following the treatments. Cells were treated with 4-HPR for 72 h, EGCG for 24 h, and also pretreated with 4-HPR for 48 h and then treated with EGCG for 24 h. After the treatments, changes in cell viability were determined by the MTT assay. All experiments were conducted in triplicates and the results were analyzed for statistical significance.
Table 1.
Combination index (CI) of the drugs in SK-N-BE2 and IMR-32 cells
| SK-N-BE2 cells | IMR-32 cells | ||||
|---|---|---|---|---|---|
| 4-HPR (μM) | EGCG (μM) | CI values | 4-HPR (μM) | EGCG (μM) | CI values |
| 0.25 | 25 | 0.816 | 0.25 | 25 | 0.904 |
| 0.25 | 50 | 0.794 | 0.25 | 50 | 1.046 |
| 0.25 | 100 | 0.764 | 0.25 | 100 | 1.141 |
| 0.5 | 25 | 0.566 | 0.5 | 25 | 0.685 |
| 0.5 | 50 | 0.562 | 0.5 | 50 | 0.668 |
| 0.5 | 100 | 0.714 | 0.5 | 100 | 0.982 |
| 1.0 | 25 | 0.835 | 1.0 | 25 | 0.928 |
| 1.0 | 50 | 0.871 | 1.0 | 50 | 0.906 |
| 1.0 | 100 | 1.004 | 1.0 | 100 | 1.090 |
The CI values for neuroblastoma SK-N-BE2 and IMR-32 cells after treatment with 4-HPR + EGCG (0.25 + 25, 0.25 + 50, 0.25 + 100, 0.5 + 25, 0.5 + 50, 0.5 + 100, 1.0 +25, 1.0 + 50, and 1.0 + 100 μM). Conventionally, CI > 1 indicates antagonism, CI = 1 indicates additive effect, and CI < 1 indicates synergism. The lowest CI value correlates with the highest synergistic effect of the combination of two drugs.
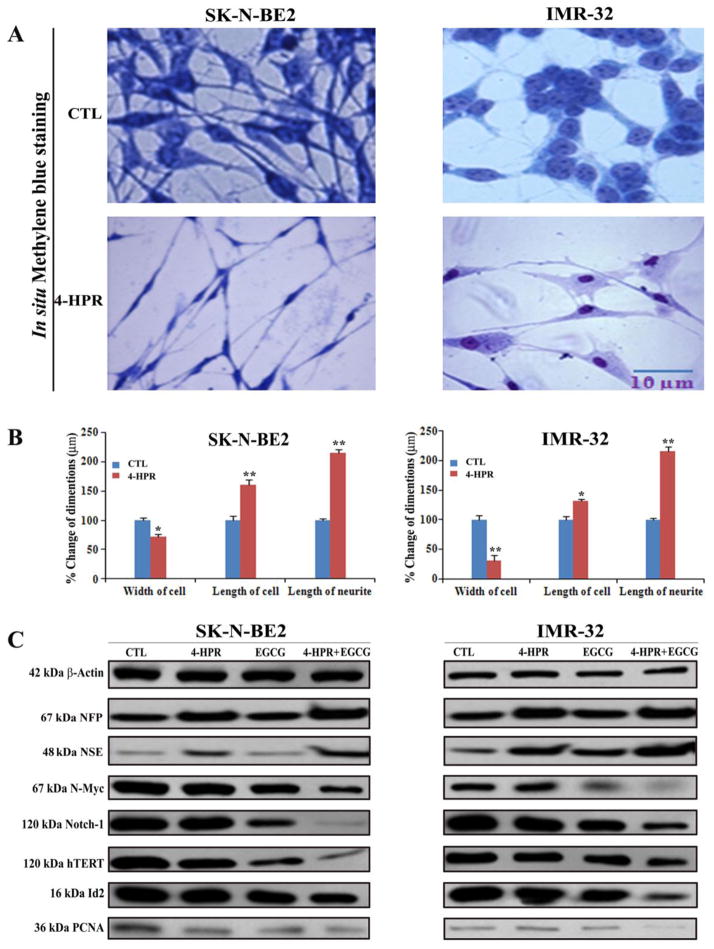
2.2. Morphological and biochemical features of 4-HPR induced neuronal differentiation in SK-N-BE2 and IMR-32 cells
Treatment with 0.5 μM 4-HPR for 72 h induced neuronal differentiation in both SK-N-BE2 and IMR-32 cell lines (Fig. 2). The morphological features of neuronal differentiation were assessed following in situ methylene blue staining (Fig. 2A). Microscopic observations (n = 20) revealed that 4-HPR treatment induced small and retracted cell bodies with thin, elongated, and branched neurite extensions, while the untreated (control) neuroblastoma cells maintained wide cell body with short cytoplasmic processes (Fig. 2A). The measurements of morphological features confirmed that width of the cell, length of the cell, and neurite extensions were significantly increased in 4-HPR treated cells, compared with control cells (Fig. 2B). Expression of many signaling molecules [N-Myc, Notch-1, hTERT (catalytic subunit of human telomerase), Id2, and PCNA (proliferation cell nuclear antigen)] associated with neuronal dedifferentiation was decreased dramatically following treatment with 4-HPR in both cell lines (Fig. 2C). Induction of neuronal differentiation due to treatment with 4-HPR decreased the expression of hTERT and PCNA leading to inhibition of cell proliferation.
Fig. 2.
Treatment with 4-HPR decreased cell proliferation and induced morphological and biochemical features of neuronal differentiation. (A) Decrease in cell proliferation and increase in morphological features of neuronal differentiation. Cells were treated with 0.5 μM 4-HPR for 72 h. Appearance of morphological features of neuronal differentiation included small, thin, and retracted cell bodies with elongated and branched processes. (B) Measurement of dimensions of neuronal differentiation (width of cell, length of cell, and length of neurite extension). (C) Representative Western blots to show changes in expression of biochemical markers of neuronal differentiation and cell proliferation. Treatments: control (CTL), 0.5 μM 4-HPR for 72 h, 50 μM EGCG for 24 h, and 0.5 μM 4-HPR for 48 h (pretreatment) + 50 μM EGCG for 24 h. Expression of β-actin was used as a loading control. All experiments were conducted in triplicates.
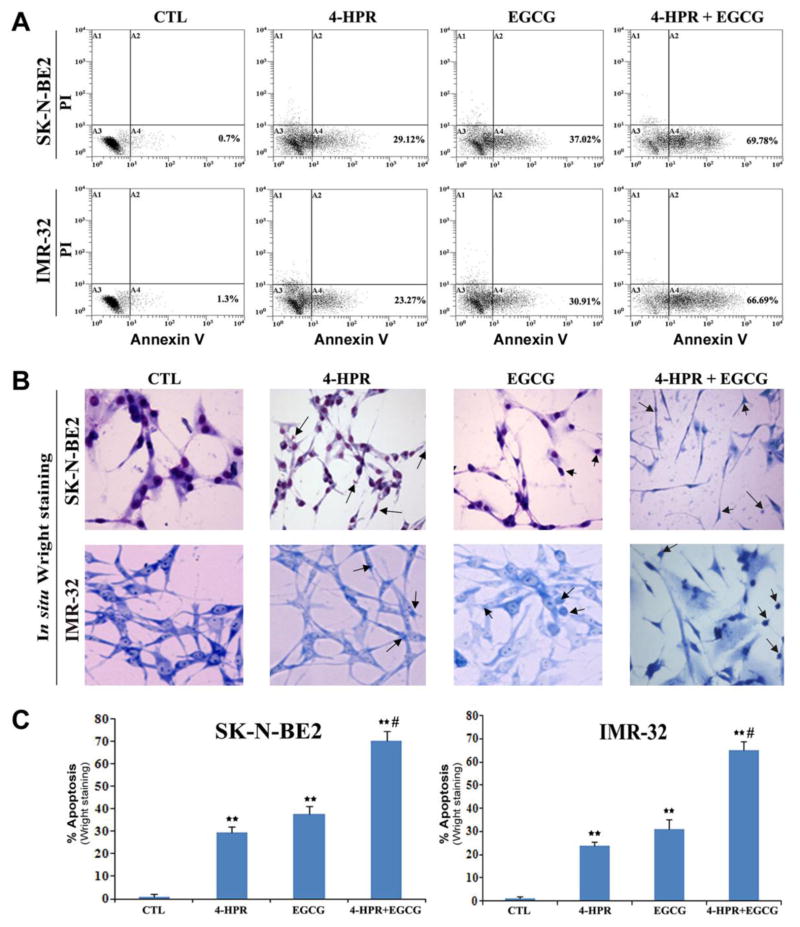
2.3. Induction of biochemical and morphological features of apoptosis after combination therapy
Prominent biochemical and morphological features of apoptosis were first examined using flow cytometric analysis and in situ Wright staining, respectively (Fig. 3). An increase in population of Annexin V positive cells in A4 area indicated occurrence of apoptosis, as shown in both SK-N-BE2 and IMR-32 cells following treatment with 4-HPR and EGCG alone or in combination (Fig. 3A). Based on flow cytometric analysis of Annexin V positive cells, we determined the percentages of apoptosis in SK-N-BE2 and IMR-32 cells after the treatments (Fig. 3A). Compared with control SK-N-BE2 or IMR-32 cells, combination of 4-HPR and EGCG very significantly increased the percentages of the Annexin V positive populations in both cell lines (Fig. 3A). The increase in Annexin V positive cells after the treatment was a prominent biochemical feature of apoptosis in neuroblastoma cells. In situ Wright staining showed the morphological changes in apoptotic cells (Fig. 3B). Characteristic morphological features of apoptotic cells included shrinkage of cell volume, chromatin condensation, and membrane-bound apoptotic bodies that appeared prominently following treatment of cells with combination of 4-HPR and EGCG (Fig. 3B). In situ Wright staining was used for counting the cells (n = 300) and determining the amounts of apoptosis (Fig. 3C).
Fig. 3.
Determination of apoptosis in cells after the treatments. Treatments: control (CTL), 0.5 μM 4-HPR for 72 h, 50 μM EGCG for 24 h, and 0.5 μM 4-HPR for 48 h (pretreatment) + 50 μM EGCG for 24 h. (A) Annexin V-FITC/PI double staining and flow cytometric analysis of apoptotic populations after the treatments. Combination of 4-HPR and EGCG induced significant population of cells in A4 area, indicating apoptotic death. (B) In situ Wright staining to examine morphological features of apoptosis. (C) Determination of percentages of apoptosis based on morphological features revealed by Wright Staining.
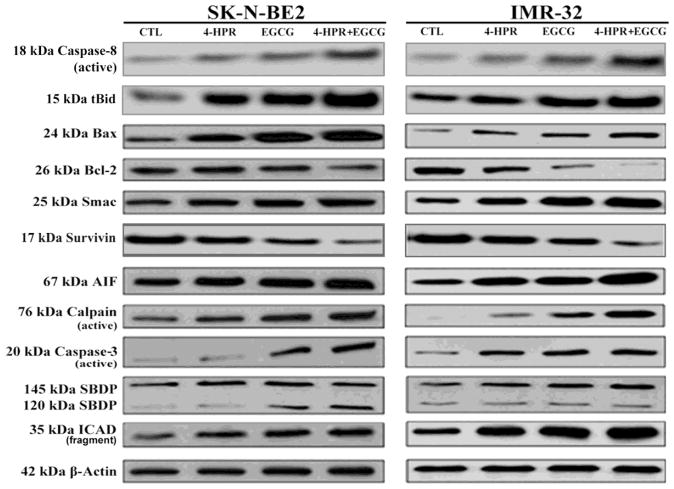
2.4. Combination of 4-HPR and EGCG activated extrinsic and intrinsic apoptotic cascades
Morphological features of apoptosis induced by treatment with 4-HPR and EGCG were correlated with the expression of key signaling molecules involved in the activation of both extrinsic and intrinsic pathways to increase proteolytic activities for apoptosis in SK-N-BE2 and IMR-32 cells (Fig. 4). Activation of caspase-8 and cleavage of Bid to truncated Bid (tBid) after treatment with 4-HPR and EGCG indicated the induction of the receptor mediated extrinsic pathway of apoptosis. Both drugs also induced the mitochondria mediated intrinsic pathway of apoptosis as evidenced from an increase in expression of Bax (pro-apoptotic protein) and decrease in expression of Bcl-2 (anti-apoptotic protein) that could result in an increase in Bax:Bcl-2 ratio in both cell lines (Fig. 4). An increase in Bax:Bcl-2 ratio is known to alter mitochondrial permeability to release cytochrome c along with Smac and apoptosis-inducing factor (AIF) into the cytosol, triggering activation of intrinsic apoptotic cascades. Smac neutralizes a set of endogenous inhibitor-of-apoptosis proteins like survivin so as to favor activation of the final executioner caspase-3. AIF is a caspase-independent death effector, which is released from mitochondrial inter membrane space and translocated to the nucleus to cause chromatin condensation and cleavage of the genomic DNA. AIF is essential for a caspase-independent pathway of apoptosis. After activation, calpain and caspase-3 generated the calpain-specific 145 kDa sprectrin break down product (SBDP) and the caspase-3-specific 120 kDa SBDP, respectively (Fig. 4). Active caspase-3 could release caspase-activated DNase (CAD) by cleaving and inactivating the inhibitor of caspase-activated DNase (ICAD). We found that the combination of 4-HPR and EGCG very efficiently caused activation of caspase-3 and cleavage of ICAD in both cell lines.
Fig. 4.
Activation of molecular components of the extrinsic and intrinsic pathways of apoptosis. Treatments: control (CTL), 0.5 μM 4-HPR for 72 h, 50 μM EGCG for 24 h, and 0.5 μM 4-HPR for 48 h (pretreatment) + 50 μM EGCG for 24 h. Representative Western blots to show activation and level of molecules involved in extrinsic and intrinsic pathways of apoptosis. Expression of β-actin was used as a loading control. All experiments were conducted in triplicates.
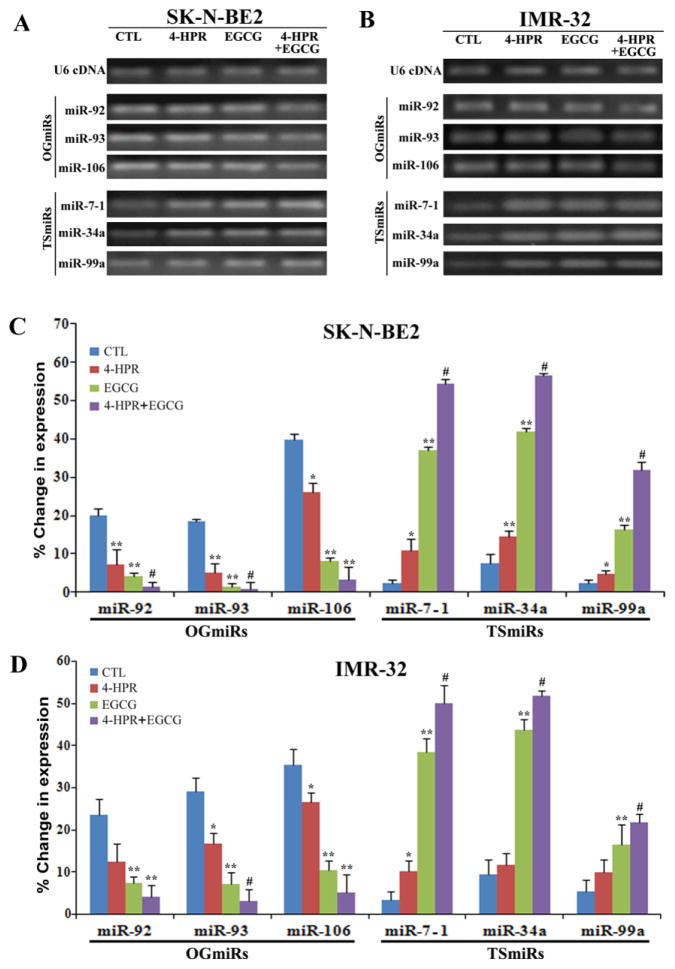
2.5. Alterations in expression of miRs in neuroblastoma cells following treatments
We studied the alterations in expression of three OGmiRs (miR-92, miR-93, and miR-106b) and three TSmiRs (miR-7-1, miR-34a, and miR-99a) in SK-N-BE2 and IMR-32 cells after treatments with 4-HPR or/and EGCG (Fig. 5). First, we performed qualitative RT-PCR experiments and resolved the RT-PCR products on the 2.2 agarose gels by electrophoresis (Fig. 5A & 5B). Expression of OGmiRs was decreased whereas expression of TSmiRs was increased in both cell lines after the treatments. Then, we performed quantitative real-time RT-PCR analyses to determine the changes in expression of these miRs in SK-N-BE2 and IMR-32 cells after the treatments (Fig. 5C & 5D). Our quantitative real-time RT-PCR results confirmed that OGmiRs (miR-92, miR-93, and miR106b) were down regulated whereas TSmiRs (miR-7-1, miR-34a, and miR-99a) were upregulated in both cell lines after the treatments when compared with the untreated control cells (Fig. 5C & 5D). The relative changes in expression of these miRs were more significant in combination therapy than in monotherapy. Most drastic change was observed in the levels of expression of miR-93 (~30 fold) and miR-7-1 (~25 fold) in SK-N-BE2 cells after the combination therapy. In IMR-32 neuroblastoma cells, combination therapy caused alterations in the relative expression of miR-93 and miR-7-1 by around 10 folds and 18 folds, respectively.
Fig. 5.
cDNA amplification and quantification of the expressed pre-micro RNA after treatments with 0.5 μM 4-HPR or/and 50 μM EGCG. (A) Amplified PCR products from cDNAs of SK-N-BE2 cells were analyzed by 2.2% agarose gel electrophoresis. (B) Amplified PCR products from cDNAs of IMR-32 cells were analyzed by 2.2% agarose gel electrophoresis. (C) Quantitative real-time RT-PCR analysis for the percent changes in expression of miRs after normalizing with U6 RNA in SK-N-BE2 cells. (D) Quantitative real-time RT-PCR analysis for the percent changes in expression of miRs after normalizing with U6 RNA in IMR-32 cells.
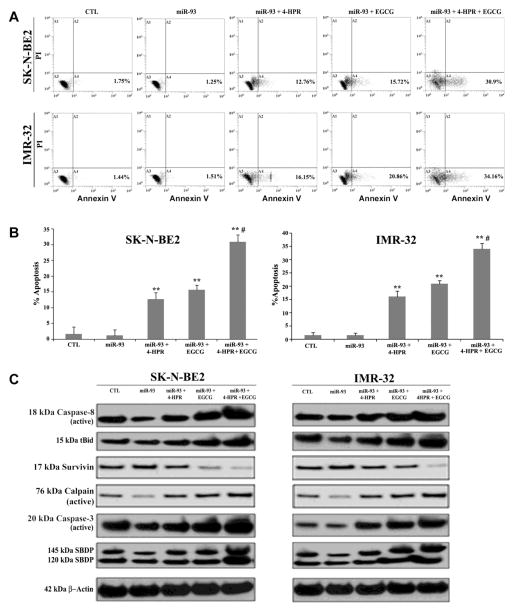
2.6. Overexpression of miR-93 reduced apoptosis whereas overexpression of miR-7-1 increased apoptosis in neuroblastoma cells
We examined the effects of overexpression of miR-93, a potent OGmiR as indicated in our previous experiments, on efficacies of 4-HPR and EGCG in SK-N-BE2 and IMR-32 cells (Fig. 6). Flow cytometric analyses of the Annexin V stained cells did not show any significant changes in amounts of apoptosis between control cells and cells transfected with miR-93 (Fig. 6A & 6B). Combination therapy with 4-HPR and EGCG caused more apoptosis than monotherapy in the miR-93 transfected cells. Obviously, the miR-93 transfected cells mounted strong resistance to the effect of monotherapy but could not completely abolish the pro-apoptotic effect of the combination therapy (Fig. 6A & 6B). Induction of apoptosis occurred via activation of caspase-8, production of tBid, down regulation of survivin, and upregulation of proteolytic activities of calpain and caspase-3 in the miR-93 transfected cells after the treatments (Fig. 6C). We found discernible increases in the apoptosis marker molecules such as active caspase-8 (27%), tBid (33%), active calpain (30%), active caspase-3 (25%), 145 kDa SBDP (33%), and 120 kDa SBDP (31%) in SK-N-BE2 cells following miR-93 transfection and combination therapy when compared with the control cells (Supplementary Fig. 1). Similar therapeutic strategy enhanced levels of expression of active caspase-8 (20%), tBid (21%), active calpain (29%), active caspase-3 (41%), 145 kDa SBDP (30%), and 120 kDa SBDP (10%) in IMR-32 cells (Supplementary Fig. 1).
Fig. 6.
Overexpression of miR-93 inhibited efficacy of 4-HPR or/and EGCG for induction of apoptosis. Cells transfected with 50 nM pre-miR-93 mimic were seeded into 6-well plates at 5×105 cells per well in triplicate. After 12 h of incubation, cells were treated with 0.5 μM 4-HPR for 72 h or/and 50 μM EGCG for last 24 h. (A) The cells were collected for analysis of apoptosis by Annexin V staining and flow cytometry. (B) The percentage of apoptotic cells is represented in a bar diagram from three independent experiments. (C) Western blot analysis was performed from the cell lysates obtained after each treatment and employing β-actin as a loading standard.
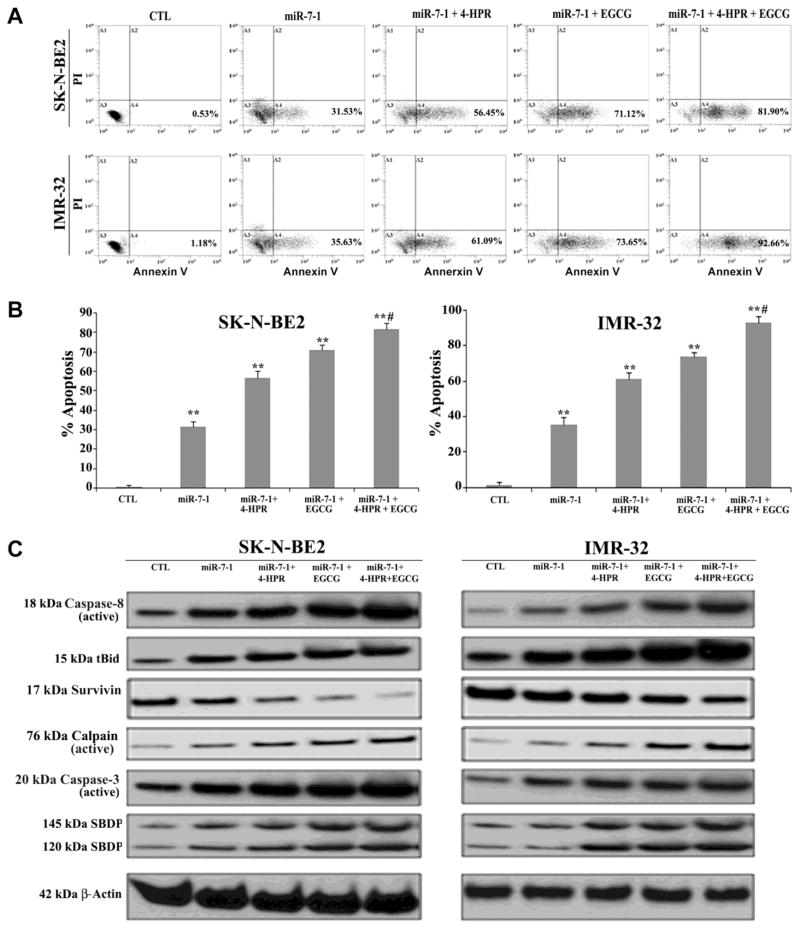
We also observed decrease in expression of survivin (35%) in both cell lines following miR-93 transfection and combination therapy (Supplementary Fig. 1). We also performed flow cytometry and Western blotting after transfection of the precursor miR-7-1 mimic in SK-N-BE2 and IMR-32 cells and subsequent drug treatments (Fig. 7). Overexpression of only miR-7-1, a potent TSmiR, in both cell lines induced significant apoptosis, which was further increased when the cells were subjected to combination therapy (Fig. 7A & 7B). Over 80% of the miR-7-1 transfected SK-N-BE2 and IMR-32 cells committed apoptotic death when treated with combination of 4-HPR and EGCG (Fig. 7B). Our Western blotting showed activation of extrinsic apoptotic pathway in the generation of active caspase-8 and tBid in both SK-N-BE2 and IMR-32 cells. Expression of survivin was decreased after drug treatment. Upregulation of calpain and caspase-3 cleaved of α-spectrin to generate calpain-specific 145 kD SBDP and caspase-3-specific 120 kD SBDP, respectively, in course of apoptosis (Fig. 7C). Quantitative estimation of the Western blots showed significant increases in the apoptosis indicator molecules such as active caspase-8 (67%), tBid (46%), active calpain (42%), active caspase-3 (52%), 145 kDa SBDP (49%), and 120 kDa SBDP (51%) in SK-N-BE2 cells following miR-7-1 transfection and combination therapy when compared with untreated control cells (Supplementary Fig. 2). Similar therapeutic strategy elevated the levels of expression of active caspase-8 (20%), tBid (21%), active calpain (29%), active caspase-3 (41%), 145 kDa SBDP (30%), and 120 kDa SBDP (10%) in IMR-32 cells when compared with untreated control cells (Supplementary Fig. 2).
Fig. 7.
Overexpression of miR-7-1 augmented 4-HPR and EGCG induced apoptosis. Cells transfected with 50 nM pre-miR-7-1 mimic were seeded into 6-well plates at 5×105 cells per well in triplicate. After 12 h of incubation, cells were treated with 0.5 μM 4-HPR for 72 h or/and 50 μM EGCG for last 24 h. (A) The cells were collected for analysis of apoptosis by Annexin V-FITC/PI double staining and flow cytometry. (B) The percentage of apoptotic cells is represented in a bar diagram from three independent experiments. (C) Western blot analysis was performed using the cell lysates after each treatment and employing β-actin as a loading standard.
3. Discussion
In this investigation, we found that combination of 4-HPR and EGCG most significantly decreased the expression of the OGmiRs (miR-92, miR-93, and miR-106b) and increased the expression of the TSmiRs (miR-7-1, miR-34a, and miR-99a) leading to induction of apoptosis in human malignant neuroblastoma SK-N-BE2 (mutant p53) and IMR-32 (wild-type p53) cells. Our studies also demonstrated that overexpression of miR-93 and miR-7-1, respectively, decreased and increased the efficacy of the treatments. Thus, alterations in expression of specific OGmiRs and TSmiRs by 4-HPR and EGCG appeared to be associated with inhibition of growth of human malignant neuroblastoma cells.
We first determined that treatment with combination of 4-HPR and EGCG significantly reduced the cell viability. The results from our investigation showed that combination of 4-HPR and EGCG down regulated dedifferentiation factors to efficiently induce differentiation in human malignant neuroblastoma SK-N-BE2 and IMR-32 cells. Transition to neuronal phenotype was accompanied by increase in expression of NFP and NSE, and decrease in expression of N-Myc, Notch-1, hTERT, Id2, and PCNA and also showed the morphological features of neuronal differentiation. The enhancement of apoptosis following treatment with 4-HPR and EGCG in both neuroblastoma cell lines was confirmed by Annexin V-FITC/PI binding assay and Wright staining. Combination of 4-HPR and EGCG effectively blocked the survival advantages in two different neuroblastoma cell lines leading to induction of apoptosis, suggesting that this combination of drugs could be used as an effective therapeutic strategy for controlling the growth of heterogeneous populations of neuroblastoma. Efficacy of combination of 4-HPR and EGCG was correlated with the expression of key signaling molecules involved in the activation of both receptor and mitochondria mediated apoptotic pathways. Increases in expression of active caspase-8 and tBid after the treatments indicated the activation of the receptor mediated pathway of apoptosis. We examined the relative expression of Bax and Bcl-2 proteins in SK-N-BE2 and IMR-32 cells following treatments. Our data suggested that combination of 4-HPR and EGCG was much more potent than 4-HPR or EGCG alone in both neuroblastoma cell lines to upregulate Bax and down regulate Bcl-2 that could result in an increase in Bax:Bcl-2 ratio. The increase in Bax:Bcl-2 ratio could trigger the release of mitochondrial pro-apoptotic factors such as Smac, and AIF into the cytosol for apoptosis (Green et al., 1998; Shimizu et al., 1999). Smac induces apoptosis by inhibiting the inhibitor-of-apoptosis proteins like survivin to cause indirect activation of caspases (Wilkinson et al., 2004). We also found that this combination therapy adeptly resulted in mitochondrial release of the pro-apoptotic molecule AIF into the cytosol. Translocation of AIF to the nucleus can cause DNA fragmentation and thus promote the caspase-independent apoptosis (Susin et al., 1999; Daugas et al., 2000). Another striking result from our investigation was the upregulation of calpain, a cysteine protease known to play an important role in apoptosis (Das et al., 2006; Karmakar et al., 2006; Karmakar et al., 2007). Increase in Bax:Bcl-2 ratio has been known to be associated with overexpression of calpain for induction of apoptosis (Ray et al., 2000). Our results showed that combination of 4-HPR and EGCG increased proteolytic activities of calpain and caspase-3 for cleavage of α-spectrin to generate calpain-specific 145 kDa SBDP and caspase-3-specific 120 kDa SBDP, respectively, to promote apoptotic death in neuroblastoma cells. CAD is a DNase with high specific activity and it exists as an inactive enzyme when complexed with ICAD in living cells (Mitamura et al., 1998; Sakahira et al., 1998). We found that in course of apoptosis in SK-N-BE2 and IMR-32 cells, caspase-3 cleaved ICAD so as to release CAD for its translocation to the nucleus for degradation of genomic DNA.
We observed dramatic changes in expression of some miRs in neuroblastoma cells after the treatments. Expression of the specific OGmiRs (miR-92, miR-93, and miR-106b) was highly decreased whereas expression of the specific TSmiRs (miR-7-1, miR-34a, and miR-99a) was highly increased in both cell lines after the treatment with combination of 4-HPR and EGCG. Overexpression of the miR-93, an OGmiR, caused both cell lines to undergo cell proliferation and reduce apoptosis even after treatment with combination 4-HPR and EGCG. On the other hand, overexpression of miR-93, a TSmiR, in both cell lines potentiated the efficacy of the combination therapy for induction of apoptosis in both cell lines. However, the effects of miRs could differ greatly in different cell types given that miRs mediate their effects through targeting mRNAs that are differentially expressed in tumors (Chen et al., 2007). The biological effects of miRs are complex as multiple mRNAs are usually targeted by a single miR. Our results showed that overexpression of miR-7-1 followed by combination therapy highly activated both the extrinsic and intrinsic pathways leading to activation of proteolytic activities of calpain and caspase-3 for apoptosis in SK-N-BE2 and IMR-32 cells.
In conclusion, we have identified miR-93 and miR-7-1 as the OGmiR and TSmiR, respectively, in neuroblastoma SK-N-BE2 and IMR-32 cells. Identification of miR-7-1 as a potent TSmiR is a significant finding for development of new therapeutic strategy for neuroblastoma. The use of miR mediated gene therapy for neuroblastoma may provide a potentially alternative avenue for treatment, which may circumvent current issues of drug resistance and adverse side effects. Our current study shows that overexpression of miR-7-1 followed by treatment with combination of 4-HPR and EGCG can be a promising therapeutic strategy for controlling the growth of different malignant neuroblastoma cells.
4. Materials and methods
4.1. Cell culture and treatments
Neuroblastoma SK-N-BE2 and IMR-32 cell lines were procured from the American Type Culture Collection (ATCC, Manassas, VA, USA). Both cell lines were grown in 1xDMEM medium, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (GIBCO/BRL, Grand Island, NY, USA). All experiments were performed growing the cells in a humidified atmosphere containing 5% CO2 at 37°C. 4-HPR and EGCG (Sigma Chemical, St. Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO) to make a stock solution and aliquots of stock solution were stored in the dark at −70°C. To avoid light sensitivity of 4-HPR, all treatments involving it were performed under subdued lighting for 72 h. All experiments included control cultures, which contained the same volume of DMSO that was used in the 4-HPR treatment. The concentration of DMSO in each experiment was always less than or equal to 0.01%, which did not affect cells. Treatment of SK-N-BE2 and IMR-32 cells with EGCG was carried out for 24 h at 37°C. Following the treatments, cells were used for determination of residual cell viability, morphological features of differentiation and apoptosis, and expression of specific miRs and proteins.
4.2. Determination of cell viability using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
All cells were grown in 6-well plates. Both SK-N-BE2 and IMR-32 cells were treated with 4-HPR (0.25, 0.5, or 1 μM for 72 h), and EGCG (25, 50, or 100 μM for last 24 h) alone or their combination. The growth medium was supplemented with 10% FBS for the first 48 h and then replaced with fresh medium supplemented with 2% FBS and treated with EGCG. In case of combination, 4-HPR was still present in growth medium during the EGCG treatment. After the treatments, the medium was discarded and replaced with a fresh medium containing MTT (0.2 mg/ml) and cells were incubated for 3 h. Then, DMSO (200 μl) was added to each well to dissolve the formazan crystals and absorbance was measured at 570 nm with background subtraction at 630 nm. Cell viability was presented as percentage of viable cells in total population (Table 1).
4.3. Detection of morphological features of differentiation
Dose-response studies were carried out to optimize the concentration of 4-HPR for inducing differentiation in both neuroblastoma cell lines. Cells were cultured in monolayer in 9-cm diameter plates in the absence and presence of 0.5 μM 4-HPR for 72 h. At the end of the treatment, cells were washed twice with ice-cold phosphate-buffered saline (PBS), pH 7.4, before fixing the cells in ice-cold 95% ethanol. Cells were stained with 0.2% (v/v) methylene blue solution (prepared in 50% ethanol) for 30 sec and washed twice with ice-cold distilled water. The plates were dried in air before being examined under the light microscope at 20x magnification.
4.4. In situ Wright staining for detection of morphological features of apoptosis
The cells were grown on 6-well plates and at the end of treatments, both adherent and floating cells were centrifuged at 2000 rpm for 5 mins to sediment them on the plates. The cells were then washed twice with PBS, pH 7.4, before Wright staining. The 6-well plates were then allowed to dry and examined under the light microscope, as we reported previously (Janardhanan et al., 2008). The morphological features of apoptotic cells included at least one of such characteristics as cell shrinkage, chromatin condensation, and membrane-bound apoptotic bodies.
4.5. Flow cytometry for determination of apoptosis
Cells were harvested after the treatments and incubation periods as discussed above, and washed with PBS (pH 7.4) twice before being fixed with 70% ethyl alcohol for 15 min on ice. Subsequently, the cells were centrifuged at a low rpm to obtain pellets and residual alcohol was aspirated. Before flow cytometric analysis, cells were resuspended in AnnexinV conjugated with fluorescein isothiocyanate (FITC) and also propidium iodide (PI) according to manufacturer’s protocol (BD Biosciences, San Jose, CA, USA) and then analyzed on an Epics XL-MCL Flow Cytometer (Beckman Coulter, Fullerton, CA, USA). The results from Annexin V-FITC/PI double staining and flow cytometry were analyzed for statistical significance.
4.6. Antibodies and Western blotting
Monoclonal primary IgG antibody against β-actin (clone AC-15) was purchased (Sigma-Aldrich, St. Louis, MO, USA) and used to monitor the equal loading of cytosolic proteins on sodium dodecyl sulfate-polyacrylamide gel electroporesis (SDS-PAGE) experiments. After the treatments, protein samples extracted from neuroblastoma cells were resolved by SDS-PAGE for Western blotting using the primary IgG antibodies against caspase-8, tBid, Bax, Bcl-2, Smac, survivin, AIF, calpain, caspase-3, and ICAD (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The horseradish peroxidase conjugated goat anti-mouse or anti-rabbit secondary IgG antibody (ICN Biomedicals, Aurora, OH, USA) was used to detect primary IgG antibody. Western blots were then incubated with enhanced chemiluminescence (ECL) detection reagents (Amersham Pharmacia, Buckinghamshire, UK) and exposed to X-OMAT AR films (Eastman Kodak, Rochester, NY, USA) for autoradiography. The autoradiograms were scanned on an EPSON Scanner using Photoshop software (Adobe Systems, Seattle, WA, USA). All experiments were performed in triplicates.
4.7. RNA and DNA extractions and reverse transcription
Total RNA and DNA were extracted from 3x106 cells of each sample after respective treatments using TRIZOL as per manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Six sets of primers were designed from the primary precursor molecule sequences of human miRs database (Griffiths-Jones, 2004; Jiang et al., 2005). Of these primers, 3 sets were designed for specific OGmiR genes and other 3 sets were designed for specific TSmiR genes (Table 2). Custom synthesized primers were procured from Eurofins MWG Operon (Huntsville, AL, USA). Primers were first validated on human genomic DNA. Then, cDNA was synthesized from total RNA using gene-specific primers following a procedure reported previously (Jiang et al., 2005). Briefly, total RNA was treated with RNase-free DNase I and 1 μg of total RNA was reverse transcribed to cDNA using gene-specific primers and Thermoscript reverse transcriptase (Invitrogen, Grand Island, NY, USA). The gene-specific primers in reverse transcription (RT) reaction included a mixture of 10 μM each of the antisense primers of all miRs and U6 RNA.
Table 2.
Primers for determining levels of expression of human miRs
| miRNA | Primer sequences |
|---|---|
| U6 | Forward: 5′-CTC GCT TCG GCA GCA CA-3′ Reverse: 5′-AAC GCT TCA CGA ATT TGC GT-3′ |
| miR-92 | Forward: 5′-TCT ACA CAG GTT GGG ATC GG-3′ Reverse: 5′-CGG GAC AAG TGC AAT ACC ATA-3′ |
| miR-93 | Forward: 5′-AAG TGC TGT TCG TGC AGG T-3′ Reverse: 5′-CTC GGG AAG TGC TAG CTC A-3′ |
| miR-106b | Forward: 5′-TAA AGT GCT GAC AGT GCA GAT AGT G-3′ Reverse: 5′-CAA GTA CCC ACA GTG CGG T-3′ |
| miR-7-1 | Forward: 5′-TGG AAG ACT AGT GAT TTT GTT GT-3′ Reverse: 5′-AGA CTG TGA TTT GTT GTC GAT T-3′ |
| miR-34a | Forward: 5′-TGG CAG TGT CTT AGC TGG TTG-3′ Reverse: 5′-GGC AGT ATA CTT GCT GAT TGC TT-3′ |
| miR-99a | Forward: 5′-TAA ACC CGT AGA TCC GAT CTT G-3′ Reverse: 5′-CCA CAG ACA CGA GCT TGT G-3′ |
4.8. Use of RT product in polymerase chain reaction (PCR)
Each RT product was used as a template in PCR reaction. Briefly, each PCR reaction (25 μl) was performed in a thermal cycler (Eppendorf mastercycler gradient) after mixing 0.5 μl miR specific primers (10 μM), 2.5 μl PCR buffer (10 μM), 0.75 μl MgCl2 (50 mM), 0.5 μl dNTPs (10 mM), and 0.2 μl Platinum Taq DNA polymerase (5 U/μl) (Invitrogen, Carlsbad, CA, USA) and 2 μl RT product. The cycling protocol consisted of an initial RT enzyme inactivation step at 95°C for 10 min followed by 35 cycles of denaturation at 95°C for 15 sec, annealing at 52°C for 30 sec, and extension at 72°C for 30 sec.
4.9. Quantitative real-time PCR
The levels of expression of the miR precursors were determined using quantitative real-time PCR as described previously (Jiang et al., 2005) with several modifications. Briefly, the master mix (3 μl) containing all of the reaction components except the primers were dispensed into a real-time PCR plate (Applied Biosystems, Foster City, CA, USA). The master mix contained 0.5 μl 10xPCR buffer, 0.7 μl 25 mM MgCl2, 0.1 μl 12.5 mM dNTPs, 0.01 μl UNG, 0.025 μl Amplitaq Gold DNA polymerase, 0.5 μl diluted cDNA (1:100), and water to 3 μl final volume. All of the PCR reagents were from the SYBR green core reagent kit (Applied Biosystems, Calrsbad, CA, USA). All miRs and U6 RNA (Table 2) were assayed in duplicate in the reaction plate. Real-time PCR was performed on an Applied Biosystems 7900HT real-time PCR instrument. The PCR was performed at 95°C for 15 sec and at 60°C for 1 min for 40 cycles followed by the thermal denaturation. The expression of each miR relative to U6 RNA was determined using the 2−ΔCT method (Livak et al., 2001). To simplify presentation of data, the relative expression values were multiplied by 102.
4.10. Transfection of SK-N-BE2 and IMR-32 cells and flow cytometric analysis
Both SK-N-BE2 and IMR-32 cells were seeded at a concentration of 5x105 cells per well in 6-well plates. On the next day, cells were transfected separately with miR-93 (an OGmiR) and miR-7-1 (a TSmiR) oligomeric RNA at 50 nM final concentration using 20 μl Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) and Opti-MEM medium following the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). After 12 h, transfection medium was replaced by fresh growth medium containing 2% FBS and nothing (control), 0.5 μM 4-HPR for 72 h, and 50 μM EGCG for last 24 h. At the end of the incubation, cells were analyzed for assessment of apoptosis by flow cytometry and Western blotting.
4.11. Statistical analysis
Results obtained from different experiments were analyzed using Minitab® 15 statistical software (Minitab, State College, PA, USA) and compared using one-way analysis of variance (ANOVA) with Fisher’s post hoc test. Data were presented as mean ± standard error of mean (SEM) of separate experiments (n ≥ 3). Significant difference from control value was indicated by *P < 0.05, **P < 0.01, or #P < 0.001.
Supplementary Material
Highlights.
Combination of 4-HPR and EGCG for treatment of neuroblastoma.
Combination therapy altered expression of specific miRs in neuroblastoma.
Overxpression of oncogenic miR-93 decreased efficacy of combination therapy.
Overexpression of tumor suppressor miR-7-1 boosted efficacy of combination therapy.
Acknowledgments
This work was supported by the grant R01 NS-57811 from the National Institutes of Health (Bethesda, MD, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buechner J, Tømte E, Haug BH, Henriksen JR, Løkke C, Flægstad T, Einvik C. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105:296–303. doi: 10.1038/bjc.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelletti D, Fiaschetti G, Di Dato V, Ziegler U, Kumps C, De Preter K, Zollo M, Speleman F, Shalaby T, De Martino D, Berg T, Eggert A, Arcaro A, Grotzer MA. The quassinoid derivative NBT-272 targets both the AKT and ERK signaling pathways in embryonal tumors. Mol Cancer Ther. 2010;9:3145–3157. doi: 10.1158/1535-7163.MCT-10-0539. [DOI] [PubMed] [Google Scholar]
- Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369–1378. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- Chen H, Shalom-Feuerstein R, Riley J, Zhang SD, Tucci P, Agostini M, Aberdam D, Knight RA, Genchi G, Nicotera P, Melino G, Vasa-Nicotera M. miR-7 and miR-214 are specifically expressed during neuroblastoma differentiation, cortical development and embryonic stem cells differentiation, and control neurite outgrowth in vitro. Biochem Biophys Res Commun. 2010;394:921–927. doi: 10.1016/j.bbrc.2010.03.076. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stallings RL. Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Res. 2007;67:976–983. doi: 10.1158/0008-5472.CAN-06-3667. [DOI] [PubMed] [Google Scholar]
- Das A, Banik NL, Ray SK. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int J Cancer. 2006;119:2575–2585. doi: 10.1002/ijc.22228. [DOI] [PubMed] [Google Scholar]
- Das A, Banik NL, Ray SK. Retinoids induce differentiation and downregulate telomerase activity and N-Myc to increase sensitivity to flavonoids for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Int J Oncol. 2009;34:757–765. doi: 10.3892/ijo_00000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/s0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- Foley NH, Bray IM, Tivnan A, Bryan K, Murphy DM, Buckley PG, Ryan J, O’Meara A, O’Sullivan M, Stallings RL. MicroRNA-184 inhibits neuroblastoma cell survival through targeting the serine/threonine kinase AKT2. Mol Cancer. 2010;9:83. doi: 10.1186/1476-4598-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S. The microRNA Registry. Nucl Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhanan R, Banik NL, Ray SK. N-(4-Hydroxyphenyl) retinamide induced differentiation with repression of telomerase and cell cycle to increase interferon-gamma sensitivity for apoptosis in human glioblastoma cells. Cancer Lett. 2008;26:26–36. doi: 10.1016/j.canlet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhanan R, Banik NL, Ray SK. N-Myc down regulation induced differentiation, early cell cycle exit, and apoptosis in human malignant neuroblastoma cells having wild type or mutant p53. Biochem Pharmacol. 2009;78:1105–1114. doi: 10.1016/j.bcp.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucl Acids Res. 2005;33:5394–5403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S, Banik NL, Patel SJ, Ray SK. Combination of all-trans retinoic acid and taxol regressed glioblastoma T98G xenografts in nude mice. Apoptosis. 2007;12:2077–2087. doi: 10.1007/s10495-007-0116-2. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Weinberg MS, Banik NL, Patel SJ, Ray SK. Activation of multiple molecular mechanisms for apoptosis in human malignant glioblastoma T98G and U87MG cells treated with sulforaphane. Neuroscience. 2006;141:1265–1280. doi: 10.1016/j.neuroscience.2006.04.075. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mestdagh P, Boström AK, Impens F, Fredlund E, Van Peer G, De Antonellis P, von Stedingk K, Ghesquière B, Schulte S, Dews M, Thomas-Tikhonenko A, Schulte JH, Zollo M, Schramm A, Gevaert K, Axelson H, Speleman F, Vandesompele J. The miR-17-92 microRNA cluster regulates multiple components of the TGF-β pathway in neuroblastoma. Mol Cell. 2010;40:762–773. doi: 10.1016/j.molcel.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitamura S, Ikawa H, Mizuno N, Kaziro Y, Itoh H. Cytosolic nuclease activated by caspase-3 and inhibited by DFF-45. Biochem Biophys Res Commun. 1998;243:480–484. doi: 10.1006/bbrc.1998.8122. [DOI] [PubMed] [Google Scholar]
- Ray SK, Fidan M, Nowak MW, Wilford GG, Hogan EL, Banik NL. Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 2000;852:326–334. doi: 10.1016/s0006-8993(99)02148-4. [DOI] [PubMed] [Google Scholar]
- Reynolds CP, Kane DJ, Einhorn PA, Matthay KK, Crouse VL, Wilbur JR, Shurin SB, Seeger RC. Response of neuroblastoma to retinoic acid in vitro and in vivo. Prog Clin Biol Res. 1991;366:203–211. [PubMed] [Google Scholar]
- Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–9. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Nikaido T, Toki T, Shiozawa T, Fujii S. Clear cell carcinoma has an expression pattern of cell cycle regulatory molecules that is unique among ovarian adenocarcinomas. Cancer. 1999;85:669–677. doi: 10.1002/(sici)1097-0142(19990201)85:3<669::aid-cncr17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Shohet JM, Ghosh R, Coarfa C, Ludwig A, Benham AL, Chen Z, Patterson DM, Barbieri E, Mestdagh P, Sikorski DN, Milosavljevic A, Kim ES, Gunaratne PH. A genome-wide search for promoters that respond to increased MYCN reveals both new oncogenic and tumor suppressor microRNAs associated with aggressive neuroblastoma. Cancer Res. 2011;71:3841–3851. doi: 10.1158/0008-5472.CAN-10-4391. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Tivnan A, Tracey L, Buckley PG, Alcock LC, Davidoff AM, Stallings RL. MicroRNA-34a is a potent tumor suppressor molecule in vivo in neuroblastoma. BMC Cancer. 2011;11:33. doi: 10.1186/1471-2407-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JC, Wilkinson AS, Scott FL, Csomos RA, Salvesen GS, Duckett CS. Neutralization of Smac/Diablo by inhibitors of apoptosis (IAPs). A caspase-independent mechanism for apoptotic inhibition. J Biol Chem. 2004;279:51082–51090. doi: 10.1074/jbc.M408655200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.