Abstract
Background & Aims
Macrophages mediate the epithelial response to Helicobacter pylori and are involved in the development of gastritis. Sonic Hedgehog (Shh) regulates gastric epithelial differentiation and function, but little is known about its immunoregulatory role in the stomach. We investigated whether gastric Shh acts as a macrophage chemoattractant during the innate immune response to H pylori infection.
Methods
Mice with parietal cell-specific deletion of Shh (PC-ShhKO) and control mice were infected with H pylori. Levels of gastric Shh, cytokines, and chemokines were assayed by quantitative reverse-transcriptase PCR or by a Luminex®-based multiplex assay, 2, 7, or 180 days after infection. Circulating concentrations of Shh were measured by ELISA. Bone marrow chimera experiments were performed with mice that have myeloid cell-specific deletion of the Hedgehog signal transduction protein smoothened (LysMCre/SmoKO). Macrophage recruitment was measured in gastric tissue and peripheral blood by fluorescence-activated cell sorting analysis.
Results
Control mice infected with H pylori for 6 months developed an inflammatory response characterized by infiltration of CD4+ T cells and increased levels of interferon-γ and interleukin (IL)-1β in the stomach. PC-ShhKO mice did not develop gastritis, even after 6 months of infection with H pylori. Control mice had increased concentrations of Shh, accompanied by the recruitment of CD11b+F4/80+Ly6Chigh macrophages 2 days after infection. Control mice that received bone marrow transplants from control mice had an influx of macrophages to the gastric mucosa in response to H pylori infection; this was not observed in H pylori-infected control mice that received bone marrow transplants from LysMCre/SmoKO mice.
Conclusion
H pylori induces release of Shh from the stomach; Shh acts as a macrophage chemoattractant during initiation of gastritis.
Keywords: gastric cancer, bacteria, signaling, monocyte
INTRODUCTION
Gastritis, typically caused by H. pylori, is the most consistent lesion leading to gastric cancer. The inflammatory response associated with gastritis is characterized by the expression of a number of proinflammatory cytokines such as IFNγ, TNFα, IL-1β and IL-8 1–4. During the innate immune response (“the first line of defense”) release of IL-8, or murine counterparts Macrophage Inflammatory Protein (MIP2) and CXCL1/KC 5, 6, are necessary for lymphocyte and neutrophil migration 7–11. The rapid influx of macrophages 48 hours postinfection 5, 12 is essential for the innate response to H. pylori-derived signals from the epithelium 9, that are crucial to the development of gastritis 13. Yet, H. pylori evades the host immune response via a mechanism that is largely unknown, and the result is a state of persistent bacterial colonization and inflammation that can eventually lead to the development of cancer.
Another parameter that is identified as a factor to disease progression during H. pylori infection is the Sonic Hedgehog (Shh) signaling pathway, but knowledge of the role of Hedgehog signaling in the adult stomach is limited. In the normal stomach, Sonic Hedgehog (Shh) regulates gastric epithelial cell differentiation and function 14–16. In addition to its role in epithelial cell differentiation, Shh also regulates T cell differentiation and proliferation and cytokine production via the activation of Ptch1 receptors and transcription target Gli1 17–20, but the immunoregulatory role of Hedgehog signaling in the stomach is unknown.
The dysregulation of Shh during H. pylori-induced gastritis is reported as either a global increase or decrease in expression during the innate to adaptive immune response. At the chronic inflammatory stage the inhibitory effect of IL-1β on the parietal cell results in loss of Shh expression 21. However, during the initial stages of infection Shh is up-regulated in inflamed tissues of the gastrointestinal tract including that of H. pylori infection 22 for reasons unknown. Given that Shh plays a role in the development of the immune response 18, 19, 23 we hypothesize that Shh is a H. pylori-induced epithelial factor essential to drive the innate immune response. Indeed, components or the Hedgehog signaling pathway including Ptch receptor and transduction protein smoothened (Smo) are expressed by macrophages 17, 19, 23. Moreover, Shh has been shown to behave as a chemoattractant for human monocytes 24. Here we demonstrate for the first time that Shh, acutely induced during H. pylori infection, may play an immunoregulatory role during the gastric immune response by acting as a macrophage chemoattractant.
MATERIALS AND METHODS
Animal Models
A mouse model expressing a parietal cell-specific deletion of Shh (fromerly known as HKCre/ShhKO, referred to as PC-ShhKO in the current studies) was generated as previously published 14. Age-matched Shh loxP (homozygous for the loxP sites without the Cre transgene) and HKCre littermates were used as controls.
PC-ShhKO mice were crossed with gastrin deficient mice (GKO) to generate double knockout PC-ShhKO /GastrinKO mice. Gastrin deficiency was confirmed by radioimmunoassay and PCR. Mice were genotyped according to previously published PCR primers and conditions 25.
A mouse model expressing a myeloid cell-specific deletion of Smoothened (LysMCre/SmoKO) was generated using transgenic mice bearing loxP sites flanking exon 1 of the Smo gene (Smo loxP) and mice expressing a Cre recombinase transgene from the Lysozyme M locus (LysMCre). Genotyping was based on polymerase chain reaction primers and protocols described in Clausen et al. 26 and Long et al. 27. Age-matched Smo loxP (homozygous for the loxP sites without the Cre transgene) and LysMCre littermates were used as controls. All mice were 8 weeks of age when inoculated. All mouse studies were approved by the University of Cincinnati Institutional Animal Care and Use Committee (IACUC) that maintains an American Association of Assessment and Accreditation of Laboratory Animal Care (AAALAC) facility.
Omeprazole and antibiotic treatments, Helicobacter pylori culture conditions and quantification, Quantitative real-time RT-PCR (qRT-PCR), Fluorescence-activated cell sorting (FACS), Gastrin radioimmunoassay, Luminex®-based multiplex assay, Western blot analysis, ShhN ELISA and Histological evaluation
See Supplemental Materials for detailed methods
Statistical Analyses
The significance of the results was tested by a one-way or two-way ANOVA using commercially available software (GraphPad Prism, GraphPad Software, San Diego, CA). A P value <0.05 was considered significant.
RESULTS
H. pylori-infected PC-ShhKO mice fail to develop gastritis
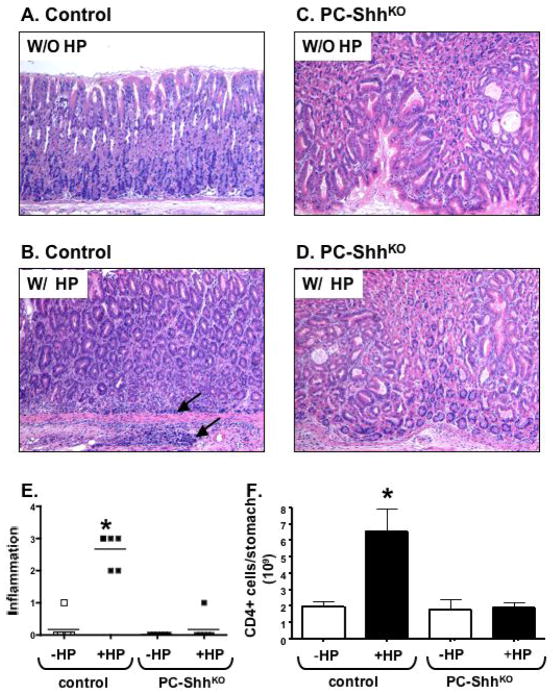
To determine the role of gastric Shh signaling in the initiation of gastritis, control and PC-ShhKO mice were infected with H. pylori and analyzed 6 months after inoculation. Histological examination revealed that H. pylori generated a significant inflammatory response in the control group (Fig. 1B) compared to the uninfected animals (Fig. 1A). Interestingly, PC-ShhKO mice did not develop an inflammatory response after 6 months of H. pylori infection (Fig. 1C, D). The characteristic foveolar hyperplasia previously reported was observed 14 (Supplemental Fig. 1B). Scores based on percentage of tissue occupied by infiltrating immune cells for control mice infected for 6 months were significantly higher than infected PC-ShhKO mice (Fig. 1E). Consistent with the histology, quantitation of the mucosal lymphocytes showed significantly greater CD4+ T cell numbers in the infected control mice after 6 months of H. pylori infection compared to the uninfected group (Fig. 1E). PC-ShhKO mice did not display elevated CD4+ T cells in response to H. pylori infection (Fig. 1F) suggesting there was an impaired host immune response to H. pylori infection.
Fig. 1. Analysis of inflammation in H. pylori infected control and PC-ShhKO mice.
Representative H&E-stained sections of inflamed stomach from control mice (HKCre or Shhflx/flx) inoculated with (A) media (W/O HP) or (B) W/ HP, and or PC-ShhKO mice inoculated with (C) media (W/O HP) or (D) W/ HP. Area of inflammatory infiltrate is shown in the stomachs of infected control mice (arrow). Images captured at 10X magnification. (E) Histological score was graded on inflammation (neutrophil and lymphocytic infiltration). A score of 1=5–25%, 2=26–50%, 3=51–75% and 4=76–100% of the total mucosa. Each data point represents the histological score given for an individual animal. CD4+ T cells were analyzed by flow cytometry and expressed as the number of cells in the gastric mucosa per mouse in uninfected and H. pylori infected control and PC-ShhKO mice. W/O HP: without H. pylori infection, W/ HP: with H. pylori infection, *P < 0.05 compared to uninfected group as analyzed by two way ANOVA, n = 5–8 animals/group.
We considered that resistance to colonization with H. pylori might account for the reduced gastritis observed in the PC-ShhKO infected mice. To examine this possibility, control and PC-ShhKO infected and uninfected mice were analyzed for the presence of H. pylori by quantitative cultures 28, 29. H. pylori bacterial colonies were not detected in the control and PC-ShhKO uninfected mice (data not shown) but only in infected control and PC-ShhKO mice (Supplemental Fig. 1C). Colonization 2, 7, and 180 days post-infection did not reveal significant differences between control and PC-ShhKO mice.
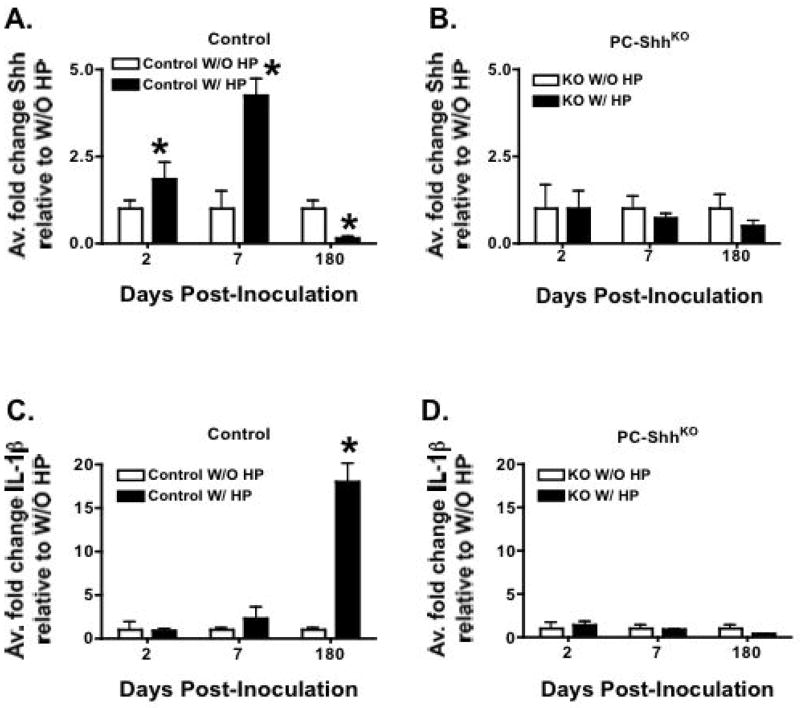
Changes in gastric Shh expression during innate and adaptive immune responses was measured by qRT-PCR in tissues collected from H. pylori-infected control and PC-ShhKO mouse stomachs 2, 7 and 180 days post-inoculation (Fig. 2A). At 2 and 7 days post-infection Shh expression was significantly elevated in the stomachs of infected control mice (Fig. 2A) compared to PC-ShhKO animals (Fig. 2B). Macrophages subsequently recruited to the site of infection secrete proinflammatory cytokine IL-1β 9. Consistent with the literature, we observed a significant increase in IL-1β within 180 days post-inoculation in stomachs of H. pylori-infected control mice (Fig. 2C). IL-1β was not elevated in the stomachs of infected PC-ShhKO mice confirming the absence of inflammation in our previously published data 14 (Fig. 2D).
Fig. 2. Shh and IL-1β expression in control and PC-ShhKO H. pylori-infected mouse stomachs.
RNA was extracted from stomachs of uninfected (open bars, W/O HP) and H. pylori-infected (closed bars, W/ HP) control and PC-ShhKO (KO) mice 2, 7 and 180 days post-inoculation. Quantitative RT-PCR was performed and average fold change in gene expression is shown for Shh from RNA collected from (A) control and (B) PC-ShhKO; IL-1β from RNA collected from (C) control and (D) PC-ShhKO; Data is shown as the mean + SEM, n = 4 mice/group, *P<0.05 compared to uninfected group (W/O HP).
The H. pylori infected mucosa eventually exhibits recruitment of TH1 cells characterized by interferon gamma (IFNγ-expressing T lymphocytes as part of adaptive immunity 1 and this was confirmed at 180 days post-inoculation in infected control mice (Supplemental Fig. 2A) compared to the PC-ShhKO group (Supplemental Fig. 2B). Although the biological equivalents of human IL-8 for inflammatory responses in rodent H. pylori models have yet to be formally defined, the murine CXC chemokine Macrophage Inflammatory Protein (MIP2, neutrophil chemotactic chemokine) is a likely candidate 5, 6. MIP2 was significantly elevated at 2, 7 and 180 days post-inoculation in the stomachs of H. pylori-infected control mice (Supplemental Fig. 2C), while increased MIP2 expression was not observed in the stomachs of PC-ShhKO mice infected with H. pylori (Supplemental Fig. 2D). Another fundamental difference observed between control and PC-ShhKO mice was the expression of IL-12, an important cytokine for the initiation of the TH1 immune response 30. In the infected stomachs of control mice there was a significant increase in IL-12 mRNA expression within 2 and 7 days post-inoculation (Supplemental Fig. 2E) but not in the stomachs of H. pylori infected PC-ShhKO animals (Supplemental Fig. 2F). Collectively, these data identify fundamental differences in Shh, cytokine and chemokine expression early after infection (7 days post-inoculation) compared to chronic H. pylori colonization (180 days post-inoculation).
Shh induced by H. pylori infection occurs via an acid-independent mechanism
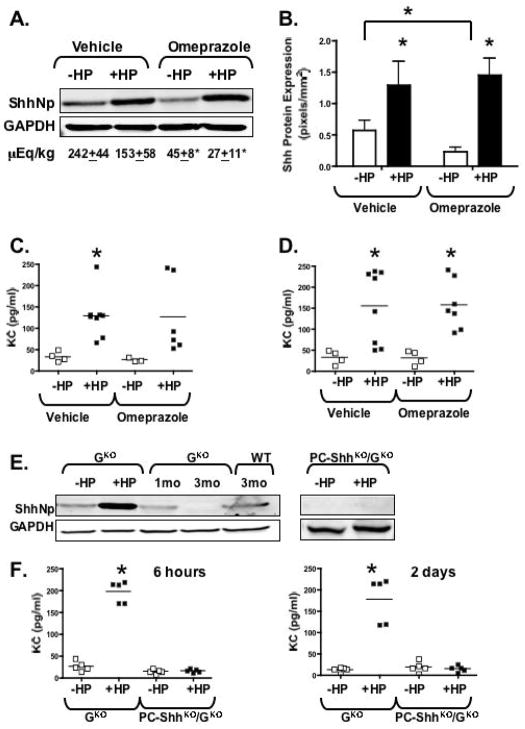
In the gastric epithelium Shh expression and processing is induced via an acid-dependent mechanism regulated by gastrin 16, 31, 32. The PC-ShhKO mice are known to be both hypochlorhydric and hypergastrinemic 14. To demonstrate that the lack of gastritis was related to loss of Shh rather than a consequence of the hypochlorhydria control mice were treated with H+, K+-ATPase proton pump inhibitor omeprazole prior to H. pylori infection and changes in Shh protein expression measured 6 hours post-inoculation (Fig. 3A, B). In controls treated with vehicle there was a significant increase in Shh expression in response to H. pylori infection within 6 hours of inoculation (Fig. 3A, B). Omeprazole treatment alone significantly reduced baseline Shh protein levels in the stomachs of controls treated with the inhibitor (Fig. 3A, B). Nevertheless even under these hypochlorhydric conditions H. pylori induced Shh expression 6 hours post-infection (Fig. 3A, B).
Fig. 3. Shh protein expression in response to acute H. pylori infection in hypochlorhydric omeprazole-treated controls and gastrin-deficient mice.
(A) Representative western blot of Shh protein (ShhNp, 19kDa processed Shh) expression in stomach homogenates collected from vehicle- or omeprazole-treated mice without H. pylori (−HP) or with H. pylori (+HP) 6 hours post-infection. Acid levels measured as μEq/kg of H+ are shown. (B) Quantification of Shh protein expression. Data are shown as means ± SEM for 3 individual experiments and expressed as Shh (pixels/mm2), *P < 0.05 compared to uninfected mice. Tissue KC concentrations measured by Luminex® multiplex assay in stomach collected from vehicle- or omeprazole-treated mice without H. pylori (−HP) or with H. pylori (+HP) 6 hours (C) and 2 days (D) post-infection. (E) Representative western blot of ShhNp expression in gastrin-deficient (GKO) and PC-ShhKO/GKO mice without H. pylori (−HP) or with H. pylori (+HP) 6 hours post-infection. Also shown are changes in Shh protein expression in 1 and 3 month old GKO and 3 month old wild type (WT) mice. (F) Tissue KC concentrations measured by Luminex® multiplex assay in stomach collected from gastrin-deficient (GKO) and PC-ShhKO/GKO mice without H. pylori (−HP) or with H. pylori (+HP) 6 hours and 2 days post-infection. n = 4–7 per group, *P < 0.05 compared to uninfected mice analyzed by two-way ANOVA.
Once in the stomach H. pylori activates an influx of macrophages and neutrophils. IL-8 expression by gastric epithelium following contact with H. pylori plays a major role in the initial host immune response, that acts as a strong chemotactic and activating factor for neutrophils, which in turn contribute to initiate and expand the inflammatory cascade 33. Thus, changes in murine IL-8 analogue KC in response to H. pylori under hypochlorhydric conditions were measured (Fig. 3C). There was a significant increase in KC tissue concentrations in response to H. pylori 6 hours post-infection in both vehicle and omeprazole treated mice (Fig. 3C) that were maintained 2 days post-infection (Fig. 3D).
We have also previously demonstrated that hypochlorhydria due to the lack of gastrin does not protect the gastrin-deficient (GKO) mouse model from the development of gastritis, but rather causes chronic inflammation in response to bacterial overgrowth 25, 29. However, we have demonstrated that in the same GKO mouse model a loss of Shh is associated with chronic inflammation and hypochlorhydria 32. To address this discrepancy Shh expression was analyzed in GKO mice at 1 month of age prior to the development of gastritis (Fig. 3E). Shh expression was induced in response to H. pylori 6 hours post-infection in 1 month-old GKO mice (Fig. 3E) and this correlated with a significant increase in KC tissue concentrations measured 6 hours and 2 days post-infection (Fig. 3F). Compared to wild type (WT) controls, by 3 months of age Shh expression was lost in GKO mice (Fig. 3E) that correlated with an increase in parietal cell atrophy and inflammation (Supplemental Fig. 3B). Thus, we suggest that the early induction of Shh in response to H. pylori infection drives the initiation of the inflammatory response observed in the hypochlorhydric GKO mice.
PC-ShhKO exhibit severe hypergastrinemia that contributes to foveolar hyperplasia 14. To identify whether the lack of gastritis in the PC-ShhKO could be attributed to the epithelial abnormalities associated with hypergastrinemia, PC-ShhKO mice were crossed onto a gastrin-deficient background 25 (PC-ShhKO/GKO) and changes in Shh expression measured in response to H. pylori infection by immunoblot (Fig. 3E). Loss of circulating gastrin concentrations in the PC-ShhKOGKO mice was measured by a gastrin radioimmunoassay (Supplemental Fig. 3A). H&E stains used for histological scores showed that PC-ShhKO/GKO mice (Supplemental Fig. 3D) did not exhibit the epithelial abnormalities observed in the PC-ShhKO group (Supplemental Fig. 3C). Gastric sections from infected and uninfected PC-ShhKO/GKO animals were scored for inflammation and foveolar hyperplasia after 6 months infection and demonstrated no significant differences (Supplemental Fig. 3E, F). Lack of Shh induction in response to H. pylori in the PC-ShhKO/GKO mice was observed (Fig. 3E), and analysis for KC (Fig. 3F) revealed a loss of immune induction in the double PC-ShhKO/GKO knockout but not in the GKO mice, indicating that hypergastrinemia does not account for the deficient immune response to H. pylori in PC-ShhKO mice. Collectively, these data also suggest that during the initial stages of H. pylori infection (within 6 hours) Shh is induced by an acid-independent mechanism, and that the immune response remains intact under conditions of hypochlorhydria and hypergastrinemia.
Gastric Shh acts as a macrophage chemoattractant in response to H. pylori infection
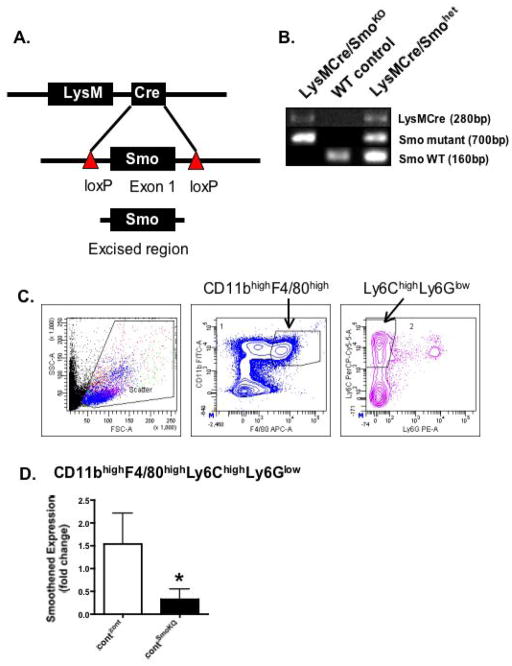
Macrophages rapidly invade the stomach 48 hours post-infection in response to H. pylori 5, 12 and are essential innate responders 9 that are crucial to the development of gastritis 13. To identify the plausible mechanism between Shh signaling and the initiation of the immune response to H. pylori infection, a mouse model expressing a deletion of Smo within the myeloid cell lineage was developed (LysMCre/SmoKO) (Fig. 4A, B). Macrophages were sorted by FACS from blood collected from control and LysMCre/SmoKO mice based on the F480+/CD11b+/Ly6Chi/Ly6Gneg gating as shown in the representative flow cytometric contour plots of Fig. 4C. Smo expression was analyzed by qRT-PCR and showed a significant reduction in expression within macrophges isolated from the LysMCre/SmoKO mice (Fig. 4D).
Fig. 4. Generation and verification of mice expressing a deletion of Smoothened in the myeloid cell lineage (LysMCre/SmoKO mice).
(A) A mouse model expressing a myeloid cell-specific deletion of Smoothened (LysMCre/SmoKO) was generated using transgenic mice bearing loxP sites flanking exon 1 of the Smo gene (Smo loxP) and mice expressing a Cre recombinase transgene from the Lysozyme M locus (LysMCre). (B) Genotyping was based on polymerase chain reaction primers specific for Cre, mutant Smo and wild type Smo (Smo WT). Shown are the fragments amplified from LysMCre/SmoKO, WT control and LysMCre/Smohet (heterozygous for WT and mutant Smo) mice. (C) Representative flow cytometric contour plots of the gating scheme for CD11bhighF4/80highLy6ChighLy6Glow macrophages in peripheral blood collected from control recipients transplanted with control donor (contcont) or LysMCre/SmoKO donor (contSmoKO) bone marrow cells. (D) RNA was extracted from FACS sorted macrophages and analyzed for expression of smoothened. Shown is the fold change relative to the contcont group.
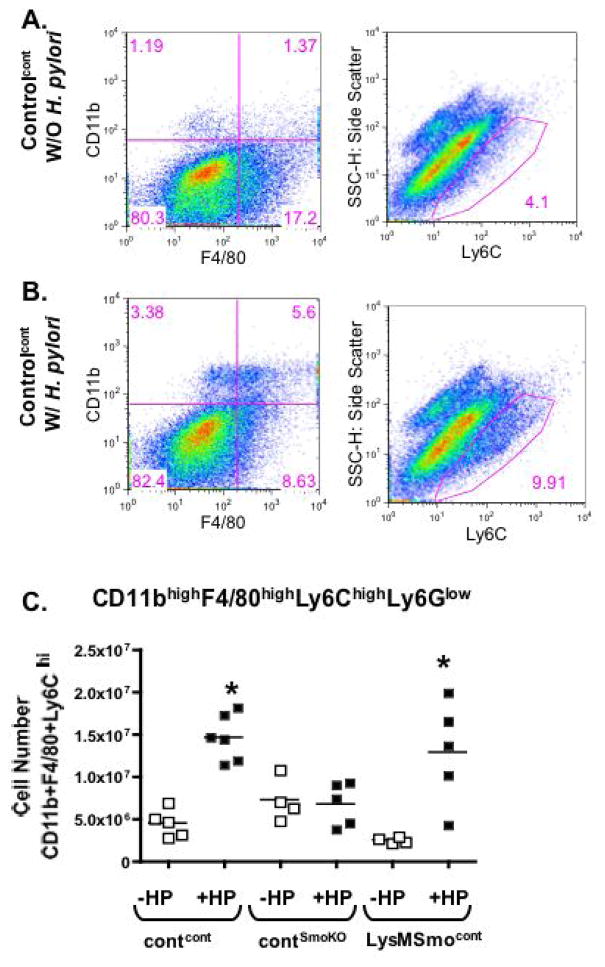
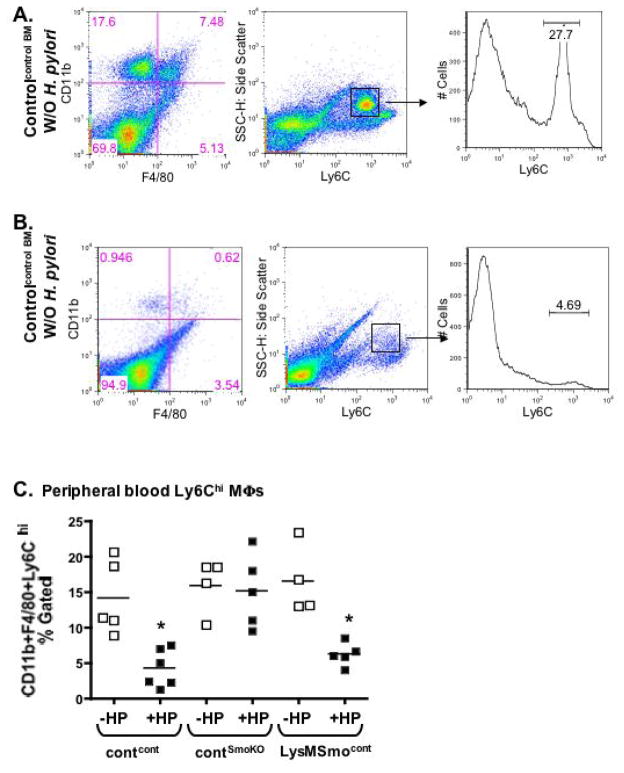
To determine the role of gastric epithelial Shh as a macrophage chemoattractant during H. pylori infection, bone marrow chimera experiments using LysMCre/SmoKO donor cells were performed (Fig. 5). Representative flow cytometric dot plots shown in Fig. 5A, B demonstrate a significant increase in gastric macrophages in control recipients transplanted with control bone marrow (Contcont) 2 days post-H. pylori infection compared to the uninfected group (Fig. 5A, B). The number of gastric F480+/CD11b+/Ly6Chi/Ly6Gneg macrophages for all experimental groups is shown in Fig. 5C. Fig. 5C demonstrates the lack of macrophage influx in response to H. pylori infection in stomachs collected from control recipients transplanted with LysMCre/SmoKO donor bone marrow. In contrast, macrophage recruitment in response to infection was restored in LysMCre/SmoKO recipient mice transplanted with control donor bone marrow cells (Fig. 5C). Consistent with the macrophage influx observed in contcont and LysMSmocont recipients in response to H. pylori infection, there was a significant decrease in the number of F480+/CD11b+/Ly6Chi/Ly6Gneg macrophages in the periphery that was not observed in the contSmoKO group (Fig. 6A–C).
Fig. 5. Changes in gastric macrophage number in response to H. pylori infection.
Flow cytometric dot plots showing changes in CD11bhighF4/80highLy6ChighLy6Glow cell distribution in gastric tissue control recipients transplanted with control donor (contcont) (A) without H. pylori (W/O H. pylori) or (B) with H. pylori (W/ H. pylori) infection 2 days post-inoculation. (C) Quantification of gastric CD11bhighF4/80highLy6ChighLy6Glow cells collected from control recipients transplanted with control donor (contcont) or LysMCre/SmoKO donor (contSmoKO) bone marrow cells and LysMCre/SmoKO recipients transplanted with control bone marrow cells (LysMSmocont) without H. pylori (−HP) or with H. pylori (+HP) infection. N = 4–8 per group, *P < 0.05 compared to uninfected analyzed by one-way ANOVA.
Fig. 6. Changes in peripheral macrophage number in response to H. pylori infection.
Flow cytometric dot plots showing changes in CD11bhighF4/80highLy6ChighLy6Glow cell distribution in peripheral blood collected from control recipients transplanted with control donor (contcont) (A) without H. pylori (W/O H. pylori) or (B) with H. pylori (W/ H. pylori) infection 2 days post-inoculation. (C) Quantification of peripheral CD11bhighF4/80highLy6ChighLy6Glow cells collected from control recipients transplanted with control donor (contcont) or LysMCre/SmoKO donor (contSmoKO) bone marrow cells and LysMCre/SmoKO recipients transplanted with control bone marrow cells (LysMSmocont) without H. pylori (−HP) or with H. pylori (+HP) infection. n = 4–8 per group, *P < 0.05 compared to uninfected analyzed by one-way ANOVA.
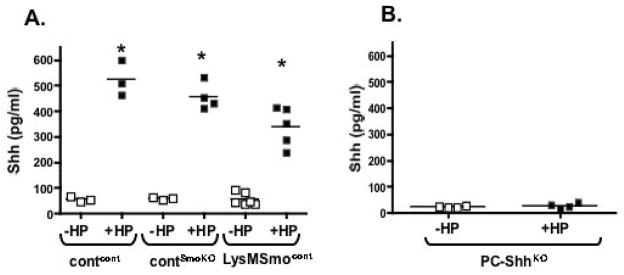
Shh signaling ligand (ShhN) was measured in plasma collected from contcont, contSmoKO and LysMSmocont mice 2 days post-infection (Fig. 7A). Circulating Shh concentrations were significantly elevated in all infected experimental groups (Fig. 7A). In contrast, increased Shh plasma concentrations were not observed in PC-ShhKO mice 2 days post-infection (Fig. 7B) thus showing that Shh is acutely induced and secreted from the gastric epithelium in response to H. pylori infection.
Fig. 7. Circulating Shh concentrations in control and PC-ShhKO mice.
Circulating Shh concentrations were measured by ELISA using plasma collected from (A) control recipients transplanted with control donor (contcont) or LysMCre/SmoKO donor (contSmoKO) bone marrow cells and LysMCre/SmoKO recipients transplanted with control bone marrow cells (LysMSmocont) or (B) PC-ShhKO mice without H. pylori (−HP) or with H. pylori (+HP) infection. *P < 0.05 compared to uninfected group as analyzed by one-way ANOVA, n = 3–6 animals/group.
DISCUSSION
Hedgehog signaling components Ptch, Smo, and Gli are all expressed in macrophages 19, 23. Furthermore, pathway analysis of LPS-stimulated macrophages revealed Hedgehog signaling genes were up-regulated within 24 hours demonstrating a capacity for stimulated macrophages to be Hedgehog-responsive 34. Bone marrow chimera experiments using LysMCre/SmoKO mice demonstrated the role of gastric Shh as a macrophage chemoattractant. First, the targeted deletion of Hedgehog signaling within the myeloid cell lineage, that included macrophages, resulted in an inability for H. pylori to induce macrophage recruitment to the site of infection. This phenotype was rescued by transplanting control donor bone marrow cells into LysMCre/SmoKO mice. Second, the secretion of Shh into the peripheral circulation was observed in response to gastric H. pylori infection, while circulating Shh concentrations did not change between uninfected and infected PC-ShhKO mice. Increased circulating Shh concentrations in response to gastric infection may suggest the basolateral secretion of Hedgehog from the epithelium into the periphery. Both in vivo and in vitro studies have demonstrated the basolateral secretion of Shh from the epithelium. For example, during the development of the Drosophila imaginal discs, monolayered epithelial invaginations that grow to assemble the adult exoskeleton, apical Shh protein is internalized and routed to the basolateral membrane where it is released to form a long-range gradient 35. In the gastric epithelium Shh appears to be expressed at the basolateral membrane of parietal cells and is secreted both apically and basolaterally from cultured a gastric cancer cell line 16. In addition, Shh is freely diffusible and involved in long-range signaling by packaging into cholesterol moieties 36 and is also shed in microparticles from stimulated T-cells 37. Based on such studies we may suggest that Shh secreted basolaterally from the stomach epithelium into the circulation may act on immune cells in the blood such as monocytes/macrophages, thus supporting the role of Shh as a chemoattractant.
Shh expression was biphasic in H. pylori-infected control mice, where Shh mRNA expression was induced within 6 hours of inoculation and subsequently decreased at 180 days. In addition, the fact that the initial induction of Shh was not observed in the PC-ShhKO mice suggests that the stimulatory response to H. pylori is predominantly targeted to the parietal cells. Given that PC-ShhKO mice did not develop gastric inflammation, even after 6 months of H. pylori infection, suggests that the initial induction in Shh observed in response to infection might be a critical event that initiated the inflammatory response. The initial induction detected here corroborates previous studies that report an early up-regulation of Shh in inflamed tissues of the gastrointestinal tract including that induced by H. pylori infection 22. After 180 days of infection, Shh expression decreased in the stomachs of control mice, coinciding with an increase in pro-inflammatory cytokines IFNγ and IL-1β. Macrophages recruited to the site of infection are matured by the local environment and secrete pro-inflammatory cytokines such as TNFα and IL-1β 9. Our findings are consistent with a recent study demonstrating an inhibitory effect of IL-1β on Shh expression and secretion in the stomach that is mediated by the disruption of normal parietal cell function 21. However, the ‘real-time’ regulation of Shh is difficult to observe in an animal model of H. pylori infection. Our data may suggest that Shh expression is not completely suppressed during infection and may be part of a circuitry that contains a positive and negative feedback loop which could lead to long-term oscillations in inflammatory regulation (Marwaha, Schumacher, Zavros and Eghbalnia, unpublished data).
In the stomach Shh expression and processing is regulated by an acid-dependent mechanism 16, 31, 32. However, we observed that under hypochlorhydric conditions Shh expression was induced in response to H. pylori infection as early as 6 hours post-infection. A study using the gastrin-deficient mouse model reported a loss of Shh associated with chronic inflammation and hypochlorydria 3. Indeed a similar mechanism of loss of Shh occurs in gastrin-deficient mice, in which Shh is induced early on to induce the development of the immune response that occurs in these mice. PC-ShhKO exhibit severe hypergastrinemia that contributes to foveolar hyperplasia 14. To identify whether the lack of gastritis in the PC-ShhKO was attributed to the epithelial abnormalities associated with hypergastrinemia, PC-ShhKO mice were crossed onto a gastrin-deficient background (PC-ShhKO/GKO). PC-ShhKO/GKO mice did not develop foveolar hyperplasia by 4 months of age when compared to the PC-ShhKO group. Although PC-ShhKO/GKO mice were equivalently colonized with H. pylori compared to the gastrin-deficient mice, PC-ShhKO/GKO animals lacked an immune response to infection similar to the PC-ShhKO group. We concluded from these studies that during innate immunity in response to H. pylori infection, Shh is induced via an acid-independent mechanism. To begin to decipher the mechanism by which Shh is induced in response to H. pylori infection we may turn our attention to the NFκB signaling pathway. Shh is a known target gene of NFκB during tumor growth in pancreas 38 and in human gastric cancer cell lines NFκB induces Shh gene expression and signaling 39. Whether NFκB induces Shh during innate immunity in the stomach is still unclear.
Fundamental differences in the cytokine and chemokine profiles were observed between control and PC-ShhKO H. pylori-infected mice that may have contributed to the lack of inflammation observed with loss of local Shh. Control animals displayed up-regulation of MIP2 following H. pylori infection, a response absent in PC-ShhKO infected mice. MIP2 may promote leukocyte recruitment in mice infected with H. pylori 40. Thus, loss of the regulatory mechanism for MIP2 expression, as observed in the infected stomachs of PC-ShhKO mice, would be expected to disrupt the initiation of the immune response. Another fundamental difference observed between control and PC-ShhKO mice was with regard to IL-12 cytokine expression. IL-12 is commonly produced by activated or mature dendritic cells and is a cytokine important in initiating the TH1 immune response 30. This cytokine response was absent in PC-ShhKO and PC-ShhKO/GKO infected mice. In fact baseline levels of IL-12 in PC-ShhKO mouse stomachs relative to those measured in controls were significantly suppressed (Marwaha, Schumacher, Zavros and Eghbalnia, unpublished data). During the early stages of infection, H pylori causes an inflammatory reaction involving both polymorphonuclear and mononuclear cells 41 and increased levels of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-8, and IL- 6 33, 42. One would thus expect a disruption in the initiation of the immune response in the absence of cytokine and chemokine signaling 13 similar to that observed in the PC-ShhKO mice. Our current knowledge of the immunoregulatory role of Hedgehog is extended by demonstrating that Shh signaling is crucial for macrophage infiltration to the stomach during the early stages of H. pylori infection that is necessary for development of the gastric immune response. Here we discuss that the initial induction of Shh is crucial for macrophage recruitment and subsequently the initiation and establishment of the immune response. Therefore loss of Shh, as seen during gastritis, results in the disruption of a sufficient immune mechanism that may be instrumental to eradicate bacterial infection, thus allowing H. pylori-induced chronic inflammation.
Acknowledgments
This work was supported by NIH 1R01DK083402-01 grant (Y. Zavros) and in part by the Digestive Health Center Cincinnati Children’s Medical Health Center (DHC: Bench to Bedside Research in Pediatric Digestive Disease) CHTF/SUB DK078392. We would like to acknowledge the assistance of Monica DeLay manager of the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center, supported in part by NIH AR-47363. All flow cytometric data were acquired using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center, supported in part by NIH AR-47363. We also acknowledge the assistance of Dr. Marsah Wils-Karp and Alyssa Sproles of The Cincinnati Cytokine/Chemokine/Mediator Measurement Core at the Digestive Health Center Cincinnati Children’s Medical Health Center. We would like to thank Dr. Kathryn A. Eaton (Department of Microbiology and Immunology, University of Michigan) for her discussion and suggestions.
Abbreviations
- Shh
Sonic Hedgehog
- Ptch 1
Patched receptor 1
- Smo
Smoothened
- Gli1
Glioma-associated Oncogene homolog 1
- H. pylori
Helicobacter pylori
Footnotes
Financial Disclosures: The authors have nothing to disclose.
AUTHOR CONTRIBUTION:
Michael A. Schumacher: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
Jessica M. Donnelly: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content
Amy C. Engevik: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content
Chang Xiao: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content
Li Yang: technical, or material support; critical revision of the manuscript
Susan Kenny: technical, or material support; critical revision of the manuscript for important intellectual content
Andrea Varro: technical, or material support; critical revision of the manuscript for important intellectual content
Frédéric Hollande: technical, or material support; critical revision of the manuscript for important intellectual content
Linda Samuelson: technical, or material support; critical revision of the manuscript for important intellectual content
Yana Zavros: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mattapallil JJ, Dandekar S, Canfield DR, Solnick JV. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology. 2000;118:307–15. doi: 10.1016/s0016-5085(00)70213-7. [DOI] [PubMed] [Google Scholar]
- 2.Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol. 2001;166:7456–61. doi: 10.4049/jimmunol.166.12.7456. [DOI] [PubMed] [Google Scholar]
- 3.Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–66. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 4.Zavros Y, Rathinavelu S, Kao JY, Todisco A, Del Valle J, Weinstock JV, Low MJ, Merchant JL. Treatment of Helicobacter gastritis with IL-4 requires somatostatin. Proc Natl Acad Sci U S A. 2003;100:12944–9. doi: 10.1073/pnas.2135193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Cover TL. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol Med Microbiol. 2007;51:577–586. doi: 10.1111/j.1574-695X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero RL, Avé P, Ndiaye D, Bambou JC, Huerre MR, Philpott DJ, Mémet S. NF-kappaB activation during acute Helicobacter pylori infection in mice. Infect Immun. 2008;76:551–561. doi: 10.1128/IAI.01107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson KAR, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:237–259. doi: 10.1016/j.bpg.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003;48:257–65. doi: 10.1023/a:1021963007225. [DOI] [PubMed] [Google Scholar]
- 9.Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gatroenterology. 2007;133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Mutoh H, Sashikawa M, Hayakawa H, Sugano K. Monocyte chemoattractant protein-1 is generated via TGF-beta by myofibroblasts in gastric intestinal metaplasia and carcinoma without H. pylori infection. Cancer Sci. 2010;101:1783–1789. doi: 10.1111/j.1349-7006.2010.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe T, Arakawa T, Fukuda T, Higuchi K, Kobayashi K. Role of neutrophils in a rat model of gastric ulcer recurrence caused by interleukin-1 beta. Am J Pathol. 1997;150:971–979. [PMC free article] [PubMed] [Google Scholar]
- 12.Asim M, Chaturvedi R, Hoge S, Lewis ND, Singh K, Barry DP, Algood HS, de Sablet T, Gobert AP, Wilson KT. Helicobacter pylori induces ERK-dependent formation of a phospho-c-Fos c-Jun activator protein-1 complex that causes apoptosis in macrophages. J Biol Chem. 2010;285:20343–20357. doi: 10.1074/jbc.M110.116988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaparakis M, Walduck AK, Price JD, Pedersen JS, van Rooijen N, Pearse MJ, Wijburg OL, Strugnell RA. Macrophages are mediators of gastritis in acute Helicobacter pylori infection in C57BL/6 mice. Infect Immun. 2008;76:2235–2239. doi: 10.1128/IAI.01481-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of Parietal Cell Expression of Sonic Hedgehog Induces Hypergastrinemia and Hyperproliferation of Surface Mucous Cells. Gastroenterology. 2010;138:550–561. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Brink GR, Hardwick JC, Nielsen C, Xu C, ten Kate FJ, Glickman J, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51:628–33. doi: 10.1136/gut.51.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+, K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol. 2008;295:G99–G111. doi: 10.1152/ajpgi.00389.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakelin SJ, Forsythe JL, Garden OJ, Howie SE. Commercially available recombinant sonic hedgehog up-regulates Ptc and modulates the cytokine and chemokine expression of human macrophages: an effect mediated by endotoxin contamination? Immunobiology. 2008;213:25–38. doi: 10.1016/j.imbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Lowrey JASG, Lindey S, Hoyne GF, Dallman MJ, Howie SE, Lamb JR. Sonic hedgehog promotes cell cycle progression in activated peripheral CD4(+) T lymphocytes. J Immunol. 2002;169:1869–1875. doi: 10.4049/jimmunol.169.4.1869. [DOI] [PubMed] [Google Scholar]
- 19.Stewart GALJ, Wakelin SJ, Fitch PM, Lindey S, Dallman MJ, Lamb JR, Howie SE. Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4+ T cells. J Immunol. 2002;169:5451–5457. doi: 10.4049/jimmunol.169.10.5451. [DOI] [PubMed] [Google Scholar]
- 20.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–527. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1beta Promotes Gastric Atrophy Through Suppression of Sonic Hedgehog. Gastrotenterology. 2010;138:562–572. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen CMWJ, van den Brink GR, Lauwers GY, Roberts DJ. Hh pathway expression in human gut tissues and in inflammatory gut diseases. Lab Invest. 2004;84:1631–1642. doi: 10.1038/labinvest.3700197. [DOI] [PubMed] [Google Scholar]
- 23.Stewart GAHG, Ahmad SA, Jarman E, Wallace WA, Harrison DJ, Haslett C, Lamb JR, Howie SE. Expression of the developmental Sonic hedgehog (Shh) signalling pathway is up-regulated in chronic lung fibrosis and the Shh receptor patched 1 is present in circulating T lymphocytes. J Pathol. 2003;199:488–495. doi: 10.1002/path.1295. [DOI] [PubMed] [Google Scholar]
- 24.Dunaeva M, Voo S, van Oosterhoud C, Waltenberger J. Sonic hedgehog is a potent chemoattractant for human monocytes: diabetes mellitus inhibits Sonic hedgehog-induced monocyte chemotaxis. Basic Res Cardiol. 2010;105:61–71. doi: 10.1007/s00395-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol. 1998;274:G561–8. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 26.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 27.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 28.Lee A, O’Rourke J, Ungria MCd, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney Strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 29.Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology. 2002;122:119–33. doi: 10.1053/gast.2002.30298. [DOI] [PubMed] [Google Scholar]
- 30.Peek RM, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Zaatari MGA, McKenzie AJ, Powe DG, Scotting PJ, Watson SA. Cyclopamine inhibition of the sonic hedgehog pathway in the stomach requires concomitant acid inhibition. Regul Pept. 2008;146:131–139. doi: 10.1016/j.regpep.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Zavros Y, Waghray Meghna, Tessier Arthur, Todisco Andrea, Gumucio Deborah L, Samuelson Linda C, Dlugosz Andrzej, Merchant Juanita L. Reduced Pepsin A Processing of Sonic Hedgehog in Parietal Cells Precedes Gastric Atrophy and Transformation. J Biol Chem. 2007;282:33265–33274. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]
- 33.Crabtree JE, Peichl P, Wyatt JI, Stachl U, Lindley IJ. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand J Gastroenterol. 1993;37:65–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang IV, Alper S, Lackford B, Rutledge H, Warg LA, Burch LH, Schwartz D. Novel regulators of the systemic response to lipopolysaccharide. Am J Respir Cell Mol Biol. 2011;45:393–402. doi: 10.1165/rcmb.2010-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callejo A, Bilioni A, Mollica E, Gorfinkiel N, Andrés G, Ibáñez C, Torroja C, Doglio L, Sierra J, Guerrero I. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc Natl Acad Sci U S A. 2011;108:12591–12598. doi: 10.1073/pnas.1106881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng X, Goetz JA, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–20. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- 37.Martínez MC, Larbret F, Zobairi F, Coulombe J, Debili N, Vainchenker W, Ruat M, Freyssinet JM. Transfer of differentiation signal by membrane microvesicles harboring hedgehog morphogens. Blood. 2006;108:3012–3020. doi: 10.1182/blood-2006-04-019109. [DOI] [PubMed] [Google Scholar]
- 38.Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009;23:21–33. doi: 10.1096/fj.08-111096. [DOI] [PubMed] [Google Scholar]
- 39.El-Zaatari MTA, Grabowska AM, Kumari R, Scotting PJ, Kaye P, Atherton J, Clarke PA, Powe DG, Watson SA. De-regulation of the sonic hedgehog pathway in the InsGas mouse model of gastric carcinogenesis. Br J Cancer. 2007;96:1855–1861. doi: 10.1038/sj.bjc.6603782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obonyo M, Guiney DG, Harwood J, Fierer J, Cole SP. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect Immun. 2002;70:3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 42.Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;62:1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]